Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.9104
Peer-review started: April 14, 2022
First decision: May 30, 2022
Revised: June 12, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: September 6, 2022
Processing time: 133 Days and 20.2 Hours
Biliary adenofibromas (BAFs) are rare primary hepatic neoplasms, some of which can potentially undergo malignant transformation. Here, we describe a rare case of malignant transformation of BAF.
A 51-year-old female was referred to our hospital with epigastric pain. Computed tomography showed a solitary liver mass combined with the enlargement of multiple mediastinal and cervical lymph nodes, clinically mimicking a liver carcinoma with extensive lymph node metastasis. However, core needle biopsy suggested BAF with malignant transformation. Finally, the patient underwent curative resection of the neoplasm and was recurrence-free for 12 mo.
Our case serves as an example of a rare manifestation of BAF. Our report and the previously published experience, reinforce that curative resection should be considered the primary treatment for BAFs with malignant transformation, leading to a favorable prognosis.
Core Tip: Biliary adenofibromas (BAFs) are rare primary hepatic neoplasms, some of which have potential of malignant transformation. Here we describe a rare case of malignant transformation of BAF presented with epigastric pain, whose imaging showed solitary liver mass combined with enlargement of multiple mediastinal and cervical lymph nodes, clinically mimicking a liver carcinoma with extensive lymph node metastasis. The patient was treated with curative resection of the neoplasm and has been recurrence-free for 12 mo. Our case is a rare manifestation of BAF and our experience supported that curative resection should be considered the primary treatment for BAFs with malignant transformation, which leads to a favorable prognosis.
- Citation: Wang SC, Chen YY, Cheng F, Wang HY, Wu FS, Teng LS. Malignant transformation of biliary adenofibroma combined with benign lymphadenopathy mimicking advanced liver carcinoma: A case report. World J Clin Cases 2022; 10(25): 9104-9111
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/9104.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.9104
Biliary adenofibromas (BAFs) are rare primary neoplasms of the liver first described by Tsui et al[1] in 1993, most of which are benign biliary cystic tumors[2,3]. Although BAFs are benign tumor with indolent biological behavior, some BAFs have the potential to undergo malignant transformation, leading to transition to invasive carcinoma. To date, few cases of BAFs with malignant features have been reported[4,5].
In the present report, we describe an extremely rare case of malignant transformation of BAF combined with benign lymphadenopathy, which clinically mimicked liver carcinoma with extensive lymph node metastasis. The patient underwent a complete surgical resection of the disease. Meanwhile, we provide a brief review of literature concerning malignant transformation of BAFs.
A 51-year-old female with a 2-mo history of epigastric pain.
The pain was barely noticeable initially and steadily worsened thereafter. On the initial evaluation, she had loss of appetite, a 5 kg weight loss, and denied nausea, vomiting, or jaundice. The patient had periodic episodes of fever ranging from 37.5°C to 38.6°C for about a week.
The patient had laparoscopic cholecystectomy 6 years ago. Other medical, surgical, psychosocial, and family histories were non-contributory.
The patient denied any family history.
Physical examination revealed mild epigastric tenderness that was otherwise unremarkable.
The patient had an elevated C-reactive protein (CRP) of 75.1 mg/L, while the white blood cell count was within the normal range. The tumor markers alpha-fetoprotein, carcinoembryonic antigen, and cancer antigen 19-9 were within normal ranges. Liver function was normal, and serology was negative for hepatitis B and hepatitis C virus infections.
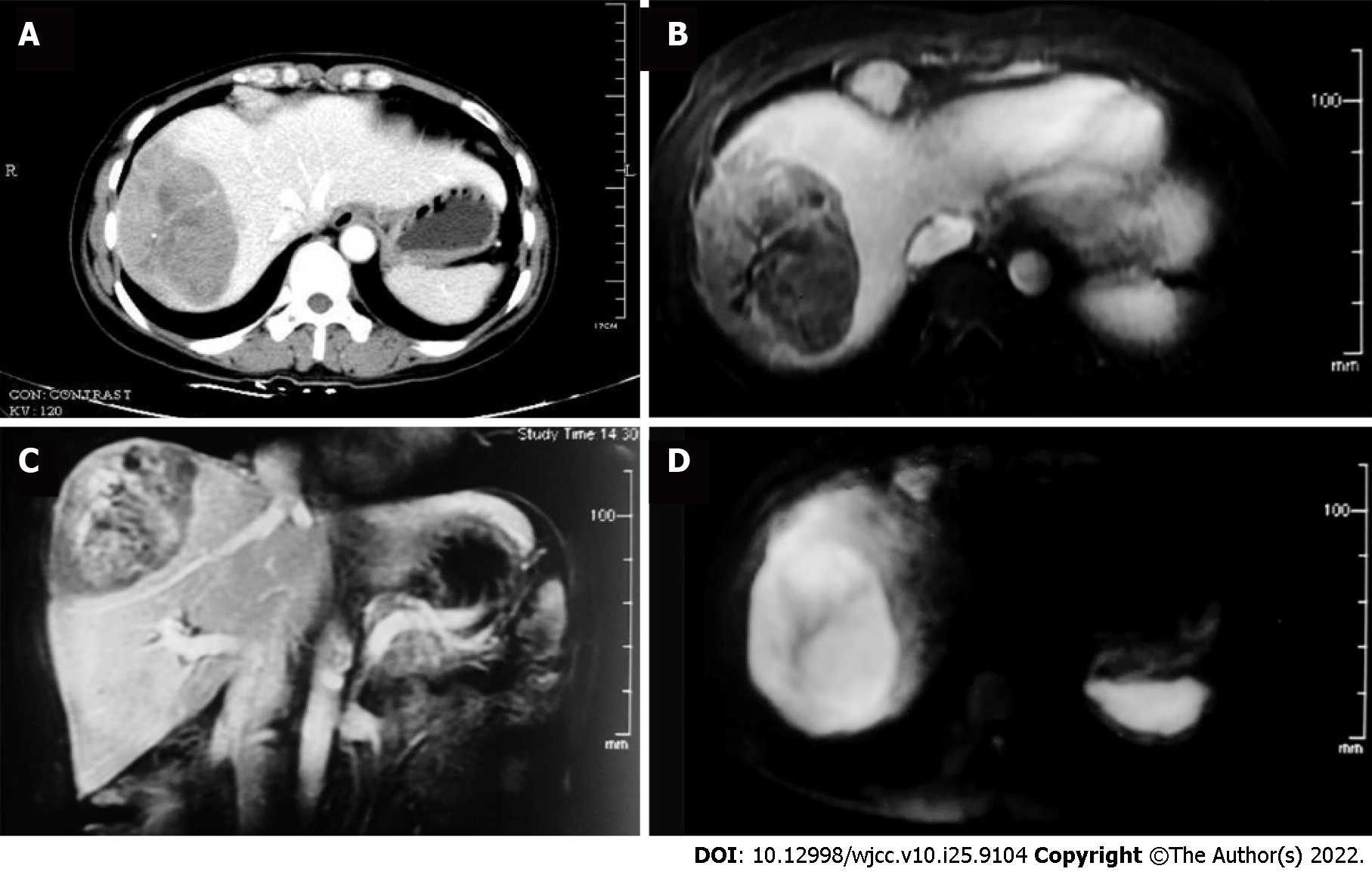
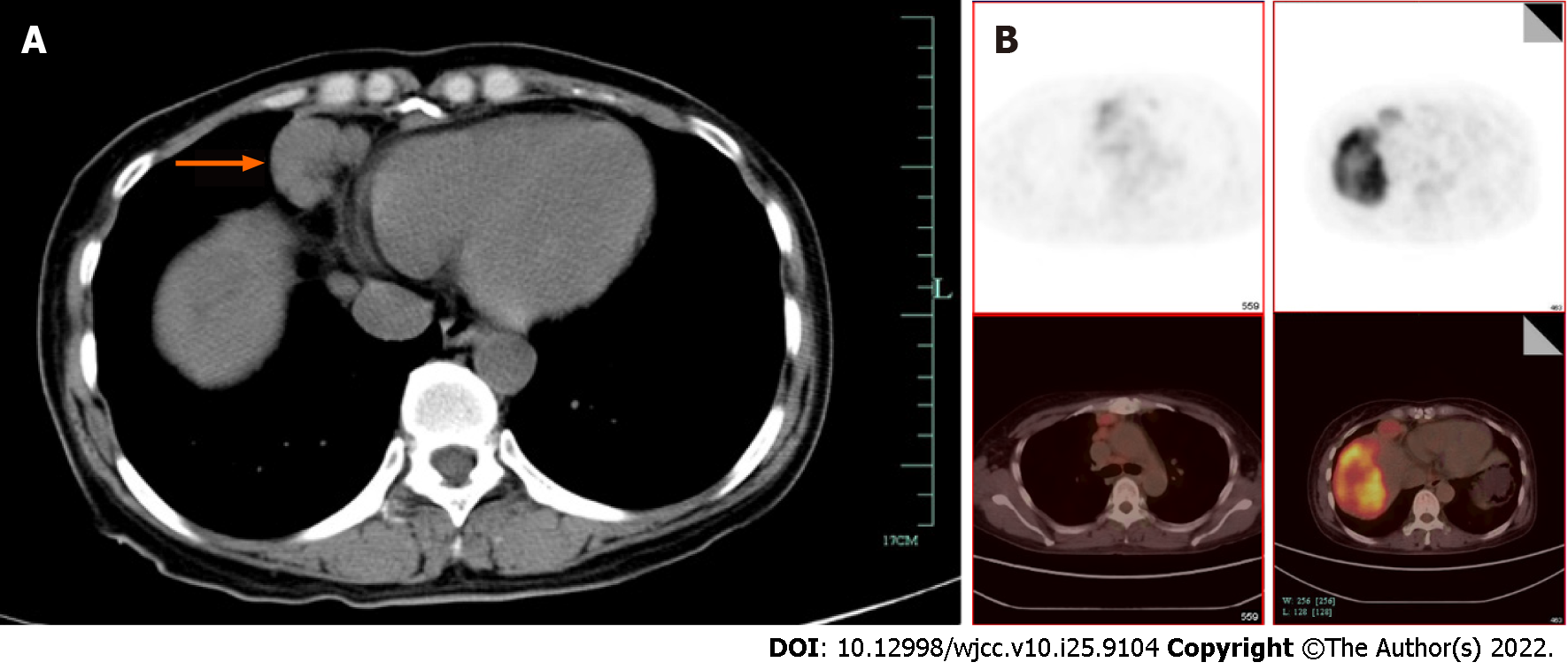
The patient was referred to an oncologic surgeon. Contrast abdominal computed tomography (CT), and subsequent magnetic resonance imaging showed a 9.2 cm × 5.9 cm solitary mass in hepatic segments VII and VIII, which was unevenly enhanced during the arterial phase and washed out during the venous phase (Figure 1). Additionally, pulmonary CT showed multiple enlarged merging mediastinal and cervical lymph nodes (Figure 2A). The leading diagnosis based on imaging was malignant liver carcinoma, possibly hepatocellular carcinoma or intrahepatic cholangiocarcinoma, with extensive lymph node metastasis. To exclude possible distant metastasis, positron emission tomography/ computed tomography was conducted, which showed uneven fluorodeoxyglucose (FDG) uptake (maximum SUV 6.7) in the liver mass, elevated FDG uptake (maximum SUV 1.9) in multiple mediastinal and cervical lymph nodes (Figure 2B and C), and no suspicious FDG elevation in the lung, brain, or pelvic cavity.
A core needle biopsy was suggested to guide systemic treatment. Unexpectedly, the pathological finding for the biopsy suggested an epithelial neoplasm with a tubuloglandular structure. The epithelial cells immunohistochemically stained positive for cytokeratin (CK) 7 and CK19 but negative for glypican-3, p53, and hepatocyte-specific antigen, implying a biliary origin. The ki-67 index was approximately 5%. The morphology and molecular pathology were compatible with adenofibroma with a malignant transformation. The patient underwent a cervical lymph node excisional biopsy to exclude distant lymph node metastasis. Three swollen lymph nodes were resected, which were negative for cancerous components.
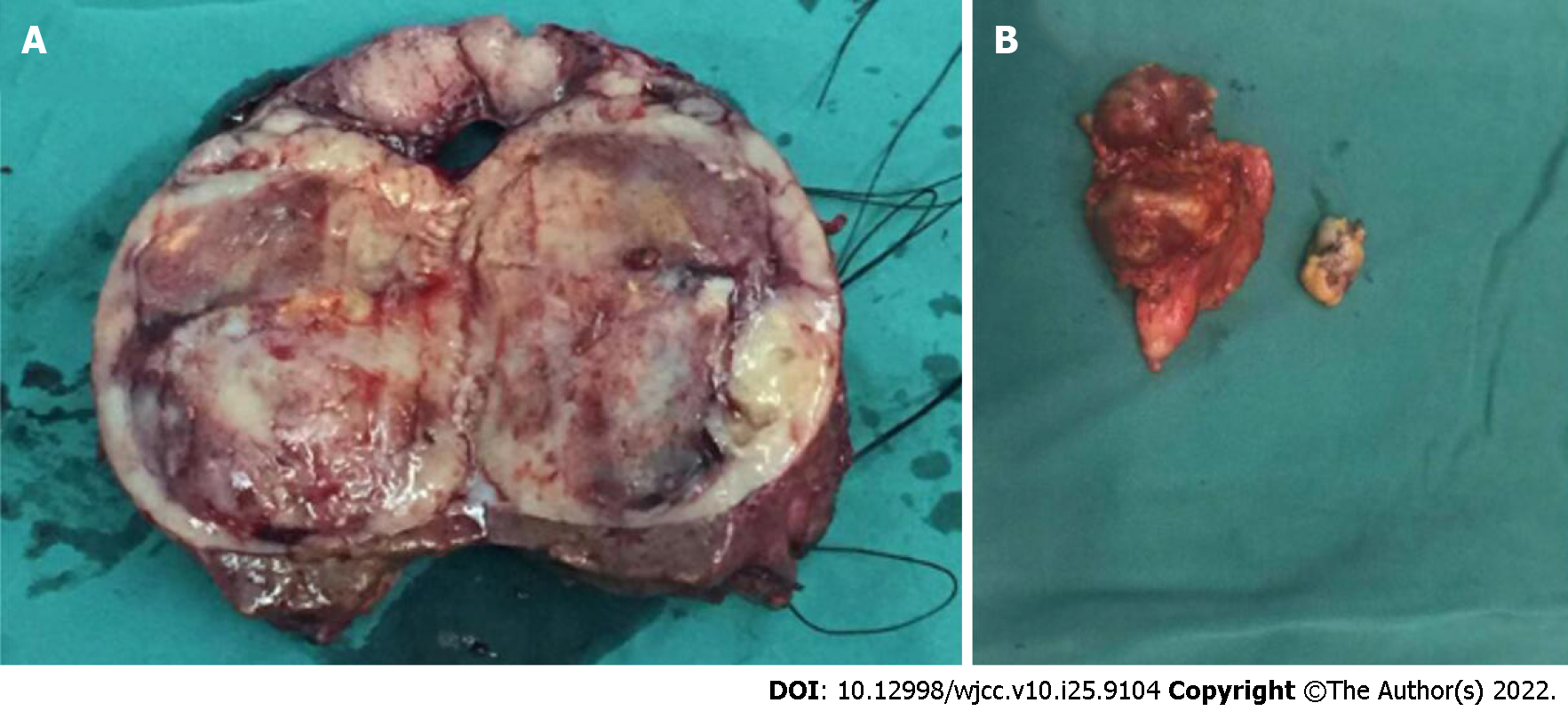
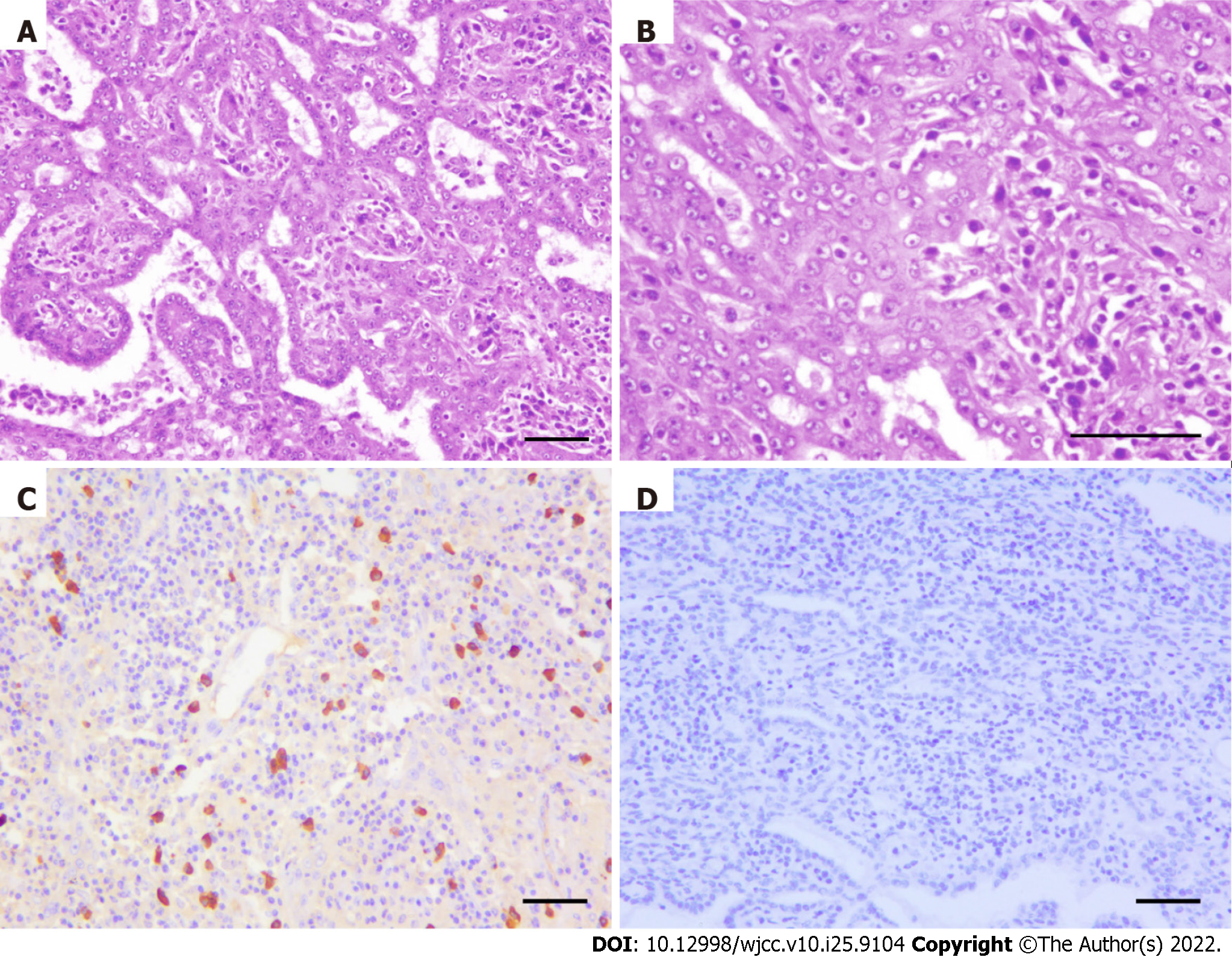
Our team decided to perform surgery on this patient based on the current knowledge of biliary adenofibroma with malignant transformation. Complete local resection of the hepatic lesion and resection of multiple merged lymph nodes in the anterior-inferior mediastinum were performed (the macroscopic view is presented in Figure 3). In detail, under general anesthesia, open atypical hepatectomy was done by Cavitron Ultrasonic Surgical Aspirator to remove the tumor with a margin of at least 1cm. The mediastinum lymph nodes were dissected through the trans-esophageal hiatus approach. The surgery lasted 3.5 h and estimated blood lost was 200 mL. The patient was well-recovered and discharged 11 days after surgery (Table 1). Subsequent pathological findings of the hepatic lesion were consistent with those of needle biopsy. The resected tissue was composed of epithelial cells with irregular tubular shapes, exhibiting cytological atypia and collagenous stroma with plasmocytic infiltration (Figure 4A and B). All margins were negative. Again, no neoplastic composition was found in the resected lymph nodes, whereas lymphatic hyperplasia with massive plasmocytic infiltration was observed.
| Time (day) | Event |
| 1 | Outpatient evaluation |
| 4 | Hospitalization |
| 6 | Found liver mass by CT |
| 11 | PET-CT |
| 13 | Core needle biopsy of the liver mass |
| 15 | Cervical lymph node biopsy |
| 25 | Surgery |
| 36 | Discharge |
Immunohistochemical staining was positive for CD3, CD20, CD10, CD21, KappaK, Lambda, and IgG4 (Figure 4C) but negative for HHV8 (Figure 4D). For the differential diagnosis of immune-mediated diseases such as IgG4-related lymphadenopathy, the patient’s serum IgG4, IgE, and IL-6 levels were tested, all within normal ranges.
The patient did not develop recurrence of the hepatic lesion during the 12 mo postoperative follow-up (assessed by local CT); no fever, epigastric pain, or other discomfort was present.
BAF is a rare primary hepatic tumor first described in 1993 by Tsui et al[1]. It is pathologically characterized by dilated ductular structures lined with bland cuboidal epithelial cells and an abundant fibroblastic stroma. BAFs are generally regarded as benign lesions, but they should be considered premalignant neoplasms because of their potential for malignant transformation[2,6]. The literature has limited reports of BAFs with malignant features, with only 13 cases to date (Table 2). Based on a recent review[7], the malignant transformation was found in 37% (7/19) of BAF cases that underwent resection. Three cases were reported as cholangiocarcinoma arising from BAFs, 2 cases as adenocarcinomas, and 1 unspecified carcinoma, while the others contained some microscopic malignant features such as dysplastic changes and microinvasion. BAF has been reported to arise from liver cirrhosis secondary to chronic hepatitis C virus infection[7]. Immunohistochemistry (IHC) revealed that the epithelial component of the BAF was positive for CK7 and CK19. Of the 5 cases, 3 were positive for p53 on IHC staining. The Ki-67 index ranged from 2% to 50% in 6 cases with available data. Wide local resection is recommended as the primary treatment for BAFs with malignant transformation, and follow-up imaging is needed for potential recurrence[2]. Only 2 of the reported patients developed recurrence after surgery, presenting as local hepatic recurrence, and 1 with pulmonary metastasis[8,9], whereas other cases, with follow-up data from 1 mo to 9 years, had no postoperative recurrence. Therefore, despite the heterogeneity of the pathological features in these cases, BAFs with malignant transformation generally have a good prognosis after complete resection.
| No. | Ref. | Age | Sex | Tumor location | Size (cm) | Treatment | Ki67, % | p53 | Outcome | Additional information |
| 1 | Akin et al[8], 2002 | 25 | M | Right hepatic lobe | 20 | Right hepatectomy | NA | NA | Hepatic recurrence and pulmonary metastasis after 3 yr | |
| 2 | Kai et al[12], 2012 | 40 | M | Right hepatic lobe | 7 | Right hepatectomy | 5-10 | NA | No recurrence at 8-mo follow-up | |
| 3 | Nguyen et al[13], 2012 | 53 | F | Segment IVb | 6.5 | Resection of segments III/IV | NA | NA | No recurrence after 12 mo | |
| 4 | Tsutsui et al[5], 2014 | 69 | F | Segment VI | 3.5 | Partial hepatectomy | 10-15 | Focally positive | No recurrence at 4-yr follow-up | Dysplastic changes |
| 5 | Thompson et al[4], 2016 | 71 | M | Left hepatic lobe | 14.5 | Left hepatectomy | NA | NA | No recurrence of liver tumor for 9 yr | Moderatlely-differentiated adenocarcinoma |
| 6 | Thompson et al[4], 2016 | 71 | M | Caudate lobe | 6.3 | Codate lobectomy | NA | NA | No recurrence at 1-mo follow-up | Well-differentiated adenocarcinoma |
| 7 | Godambe et al[6], 2016 | 71 | F | Segments II, III and IVa | 5.7 | Left hepatectomy | 50 | Positive | NA | Microinvasive carcinoma |
| 8 | Thai et al[14], 2016 | 77 | M | Segment II | 4 | Left hepatectomy | NA | NA | NA | Cholangiocarcinoma arising from BAF |
| 9 | Kaminsky et al[2], 2017 | 37 | F | Segment V | 4.5 | Partial hepatectomy | 50 | Negative | No recurrence at 4-mo follow-up | Cholangiocarcinoma arising from BAF |
| 10 | Chua et al[7], 2018 | 66 | F | Segment IVb | 6 | Wedge resection | 2 | Positive | No recurrence at 4-mo follow-up | Cholangiocarcinoma arising from BAF |
| 11 | Sturm et al[15], 2019 | 63 | F | Segment IVa | 6.3 | Left hepatectomy | 20-30 | Focally positive | No recurrence at 24-mo follow-up | |
| 12 | Alshbib et al[9], 2022 | 63 | M | Segments IVb and V | 15.5 | Resection of segments IVb and V | 25 | Weak | Hepatic recurrence 3 mo | |
| 13 | Current report | 51 | F | Segments VII and VIII | 7.5 | Partial hepatectomy | 5 | Negative | No recurrence at 12-mo follow-up | |
In the current case, the multiple enlarged lymph nodes were first misinterpreted as metastases arising from the hepatic neoplasm, leading to an initial diagnosis of advanced liver malignancy and, correspondingly, a plan for systemic treatment such as chemotherapy or targeted therapy. For advanced disease with high suspicion of hepatocellular carcinoma, pathology is not mandatory prior to systemic treatment. In this case, the imaging studies did not suggest a definite diagnosis, therefore, a core needle biopsy was conducted after prudent evaluation. Finally, the pathological diagnosis was BAF with malignant transformation, thus providing a rationale for complete resection of the neoplasm.
Lymphadenopathy showed no evidence of malignancy. It was first suspected to be IgG4-related or multicentric Castleman’s disease due to its histology and positive IHC staining for IgG4[10,11]. However, the patient’s serum IgG4, IgE, and IL-6 levels were within the normal range; therefore, we did not reach a definite diagnosis for her lymphadenopathy. The patient’s fever was non-specific, which could be interpreted as an infection or neoplastic fever, apart from immune-mediated diseases. Notably, the fever did not recur, and CRP and erythrocyte sedimentation rates were normal after surgery.
The limitation of this case is that, despite exclusive diagnosis for IgG4-related or multicentric Castleman’s disease, we eventually did not reach a diagnosis for the patient’s lymphadenopathy. Besides, we did not conduct molecular analysis such as MSI state and PD-L1 expression of the BAF sample. Additional molecular analysis may assist follow-up treatment of this disease in case of recurrence.
In summary, BAFs with malignant transformation are clinically unusual and could be misinterpreted as liver carcinoma based on imaging features, especially when comorbid with other suspicious signs of malignancy, such as lymphadenopathy. Our case serves as an example of a rare manifestation of BAF, a biopsy of suspicious lesions is vital for diagnosis. According to previous reports, BAFs with malignant transformation have the potential to develop into intrahepatic cholangiocarcinoma or adenocarcinoma, curative resection should be considered an essential primary treatment, which generally leads to a favorable outcome.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed SK, Iraq; Trébol J, Spain S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Tsui WM, Loo KT, Chow LT, Tse CC. Biliary adenofibroma. A heretofore unrecognized benign biliary tumor of the liver. Am J Surg Pathol. 1993;17:186-192. [PubMed] |
| 2. | Kaminsky P, Preiss J, Sasatomi E, Gerber DA. Biliary adenofibroma: A rare hepatic lesion with malignant features. Hepatology. 2017;65:380-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Arnason T, Borger DR, Corless C, Hagen C, Iafrate AJ, Makhlouf H, Misdraji J, Sapp H, Tsui WM, Wanless IR, Zuluaga Toro T, Lauwers GY. Biliary Adenofibroma of Liver: Morphology, Tumor Genetics, and Outcomes in 6 Cases. Am J Surg Pathol. 2017;41:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Thompson SM, Zendejas-Mummert B, Hartgers ML, Venkatesh SK, Smyrk TC, Mahipal A, Smoot RL. Malignant transformation of biliary adenofibroma: a rare biliary cystic tumor. J Gastrointest Oncol. 2016;7:E107-E112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Tsutsui A, Bando Y, Sato Y, Miyake H, Sawada-Kitamura S, Shibata H, Kakuda Y, Harada K, Sasaki M, Nakanuma Y. Biliary adenofibroma with ominous features of imminent malignant changes. Clin J Gastroenterol. 2014;7:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Godambe A, Brunt EM, Fulling KH, Reza Kermanshahi T. Biliary Adenofibroma with Invasive Carcinoma: Case Report and Review of the Literature. Case Rep Pathol. 2016;2016:8068513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Chua D, Chiow AKH, Ang TL, Wang LM. Malignant Transformation Arising Within Unusual and Rare Hepatic Lesions: Fibropolycystic Disease Form of Ductal Plate Malformation and Biliary Adenofibroma. Int J Surg Pathol. 2018;26:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Akin O, Coskun M. Biliary adenofibroma with malignant transformation and pulmonary metastases: CT findings. AJR Am J Roentgenol. 2002;179:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Alshbib A, Grzyb K, Syversveen T, Reims HM, Lassen K, Yaqub S. Biliary Adenofibroma: A Rare Liver Tumor with Transition to Invasive Carcinoma. Case Rep Surg. 2022;2022:5280884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Zhang X, Zhang P, Peng L, Fei Y, Zhang W, Feng R. Clinical characteristics of a concurrent condition of IgG4-RD and Castleman's disease. Clin Rheumatol. 2018;37:3387-3395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1871] [Article Influence: 143.9] [Reference Citation Analysis (83)] |
| 12. | Kai K, Yakabe T, Kohya N, Miyoshi A, Iwane S, Mizuta T, Miyazaki K, Tokunaga O. A case of unclassified multicystic biliary tumor with biliary adenofibroma features. Pathol Int. 2012;62:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Nguyen NT, Harring TR, Holley L, Goss JA, O'Mahony CA. Biliary adenofibroma with carcinoma in situ: a rare case report. Case Reports Hepatol. 2012;2012:793963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Thai E, Dalla Valle R, Evaristi F, Silini EM. A case of biliary adenofibroma with malignant transformation. Pathol Res Pract. 2016;212:468-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Sturm AK, Welsch T, Meissner C, Aust DE, Baretton G. A case of biliary adenofibroma of the liver with malignant transformation: a morphomolecular case report and review of the literature. Surg Case Rep. 2019;5:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |