Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.9064
Peer-review started: April 30, 2022
First decision: May 30, 2022
Revised: June 14, 2022
Accepted: August 1, 2022
Article in press: August 1, 2022
Published online: September 6, 2022
Processing time: 117 Days and 23.8 Hours
Gastrointestinal metastasis of breast cancer is rare, and clinicians may not have previously encountered this disease in clinical practice.
We report a patient with invasive lobular carcinoma of the breast who developed gastrointestinal metastasis two years after modified radical surgery. Mild elevation of carbohydrate antigen 15-3 was observed in the patient at an early stage; however, diagnosis and treatment were delayed due to non-specific clinical manifestations and no identifiable metastasis observed on imaging.
Clinicians should pay attention to gastrointestinal metastasis of breast cancer, especially invasive lobular carcinoma of the breast.
Core Tip: Gastrointestinal metastasis of breast cancer should be vigilant when tumor markers are elevated or when patients have digestive symptoms such as abdominal distention and defecation difficulties of unknown cause. Early detection, diagnosis, and treatment are needed to prevent disease progression and complications.
- Citation: Li LX, Zhang D, Ma F. Gastrointestinal metastasis secondary to invasive lobular carcinoma of the breast: A case report. World J Clin Cases 2022; 10(25): 9064-9070
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/9064.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.9064
Breast cancer is the most common malignant tumor in women worldwide, with the highest incidence at present[1]. Despite treatment advances[2], prognosis for patients with metastatic breast cancer remains poor[3].Breast cancer-related distant metastasis sites commonly involve bone, lung, liver, and brain tissue, with gastrointestinal metastasis from breast cancer being extremely rare. Patients with breast cancer, complicated with gastrointestinal tumors, are often misdiagnosed as having double primary cancers and receive inappropriate treatment. Accurate and timely diagnosis of gastrointestinal metastatic breast cancer is vital. Few reports have involved breast cancer with rare metastatic sites[4]. We report a patient with colon metastasis secondary to invasive lobular carcinoma (ILC) of the breast (Luminal B type), accompanied with detailed clinicopathological features, diagnosis, and treatment.
On November 24, 2018, a 56-year-old female patient, 2 years after breast cancer surgery, presented with persistent elevation of carbohydrate antigen 15-3 (CA15-3) for 1 year, accompanied by abdominal distention and difficulty defecating for one month.
In October 2016, the patient underwent a physical examination that resulted in the identification of a breast mass. Subsequently, after the excluding surgical contraindications, the patient underwent a modified radical mastectomy for right breast cancer with sentinel lymph node dissection at our hospital in December 2016. Postoperative pathology results showed infiltrating lobular carcinoma of the right breast (maximum diameter, 3 cm; sentinel lymph node metastasis, 2/6). Immunohistochemical (IHC) results showed that the expression status of estrogen receptor (ER) and progesterone receptor (PR) were positive at 90% and 5%, respectively. The expression status of human epidermal growth factor receptor 2 (HER2) was negative (1+) and Ki-67 was low (3%). According to American Joint Committee on Cancer 8th edition staging and Chinese Society of Clinical Oncology 2021 edition guidelines, the patient was diagnosed with ILC of the right breast, and postoperative staging was: pT2N1M0, IIB, Luminal B type (HER-2 negative). After surgery, docetaxel combined with cyclophosphamide adjuvant chemotherapy was administered for four cycles, and followed by local radiotherapy and letrozole (2.5 mg qd) as maintenance therapy.
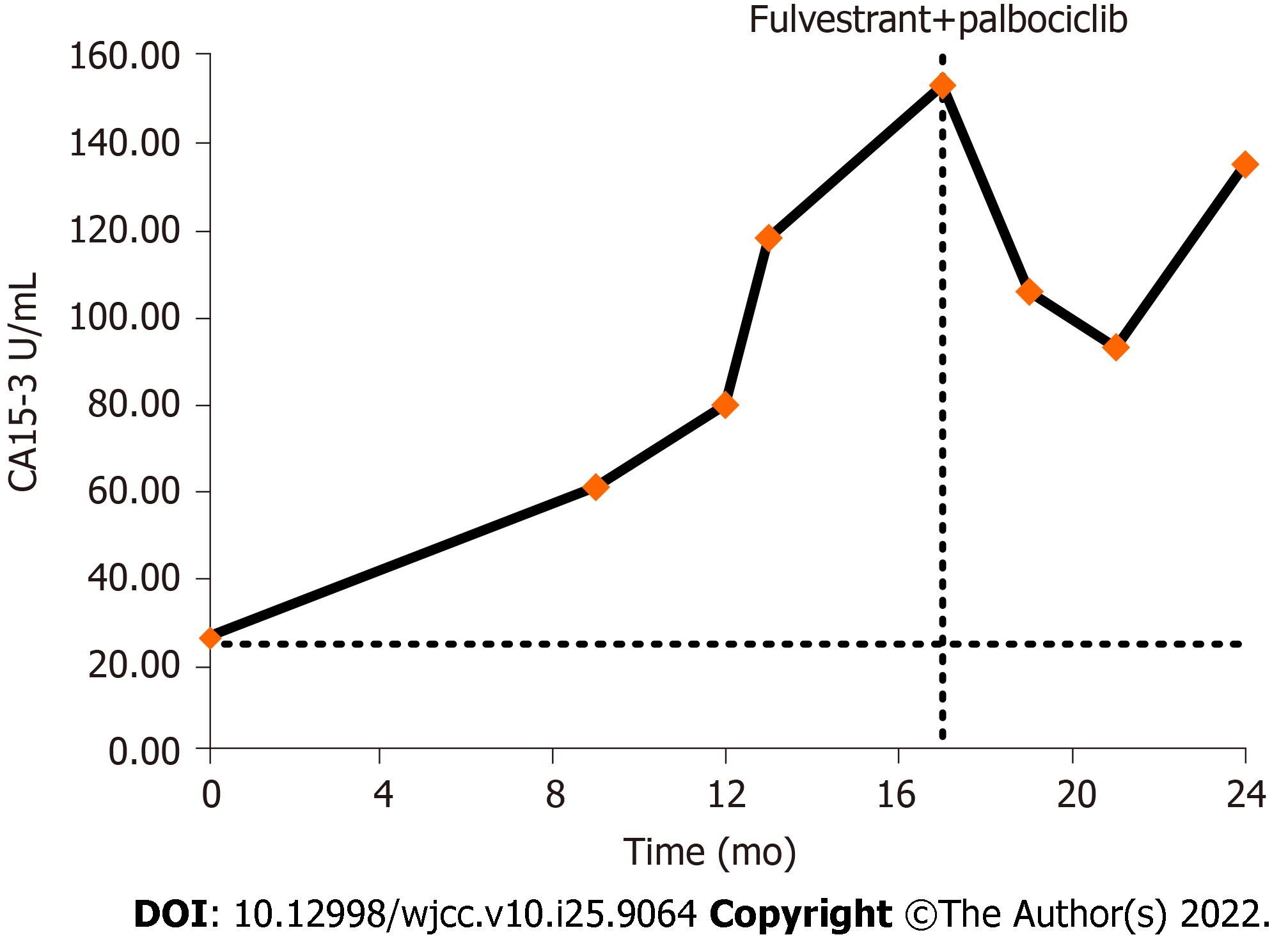
Until July 10, 2017, the CA15-3 Level was 26.44 U/mL (electro-chemiluminescence immunoassay detection, reference range 0.0–25.0 U/mL), but the patient’s CEA and CA125 levels were within normal ranges. Imaging examinations, including chest computed tomography (CT) and abdominal, breast, axillary, and supraclavicular lymph node ultrasonography, showed no abnormalities. In October 2017, the CA15-3 Level was found to be increased again than before. However, positron emission tomography-computed tomography-CT (PET-CT) findings showed no obvious abnormal glucose metabolism foci. Subsequently, the patient continued treatment with Letrozole. During a routine follow-up, levels of CA15-3 continued to rise up to 61.10 U/mL in April 2018 (Figure 1); however, other tumor markers were not shown to be abnormal. Conversely, contrast-enhanced CT neck, chest, and abdomen scans and bone scans showed no obvious abnormalities. While breast cancer-related lesions indicating recurrence and metastasis were not identified on imaging, we could not completely rule this out. Subsequently, endocrine therapy was switched from letrozole to exemestane.
In July 2018, digestive system clinical symptoms such as eating less, fullness of the abdomen, and fatigue appeared, and the patient’s CA15-3 Levels still showed an upward trend. The patient had been reviewed regularly for the previous breast cancer with no obvious abnormality found during follow-up. On November 5, 2018, the patient’s fullness of the abdomen is worse than before. Then, the patient underwent a colonoscopy, which revealed multiple lesions in the colon. Pathologic findings showed a heterogeneous cell mass with a tendency toward poorly differentiated adenocarcinoma, suggesting a source of breast cancer. In addition, IHC analysis showed GATA-3 positive and CK20 and CDX negative results. According to the above results, the patient was diagnosed with gastrointestinal metastasis of breast cancer. On November 24, 2018, the patient came to our hospital again, and the pathological consultation results showed small round tumor cells observed in the ileocecum, transverse colon, and sigmoid colon and in the propria layer of the rectum and focal mucosal muscle, suggesting metastasis secondary to ILC of the breast. IHC analysis showed ER (40%+), PR (5%+), HER-2 (2+, no amplification by FISH), and Ki-67 (20%).
Aside from a > 20-year history of chronic viral hepatitis B, the patient's past medical history was otherwise unremarkable.
Menopause started at age 50 years, and the patient denied any family history of cancer or any relevant genetic history.
Physical examination findings on admission were as follows: Temperature, 36.4 °C; heart rate, 61 beats/min; blood pressure, 114/69 mmHg; and Karnofsky Performance Scale score, 80. No systemic involvement or enlargement of the superficial lymph nodes was observed. A surgical scar was observed on the right chest wall. The abdomen was slightly distended, with no tenderness or rebound pain, and the ribs overlying the liver and spleen were not tender on palpation nor swollen. Mobile dullness was negative, and there was no hyperactivity or weakening of bowel sounds.
Pathology results indicated small round tumor cells in the ileocecum, transverse colon, sigmoid colon, propria layer of the rectum, and the focal mucosal muscle, suggesting metastasis secondary to ILC of the breast. IHC analysis showed ER (40%+), PR (5%+), HER-2 (2+, no amplification by FISH), and Ki-67 (20%). Blood analysis and biochemistry analysis showed no obvious abnormalities. However, both CA15-3 and CA125 Levels were elevated, with values of 118.50 U/mL and 63.55 U/mL, respectively.
Endoscopic examination suggested multiple lesions in the ileocecum, transverse colon, sigmoid colon and rectum. Enhanced CT scans of the neck, chest and abdomen showed no obvious abnormalities.
Right breast cancer invasive lobular carcinoma pT2N1M0 Stage IIB to Stage IV Luminal B (HER-2 negative), ileum cecum, transverse colon, sigmoid colon, and rectum metastases after modified radical resection combined with sentinel lymph node biopsy.
Since December 2018, the patient was administrated an ER modulator combined with a CDK4/6 inhibitor (fulvestrant, 500 mg, intramuscular injection). A subsequent 500 mg was administrated two weeks after initial administration along with palbociclib (125 mg, d1-21, Q4W). According to the RECIST 1.1 evaluation criteria for solid tumors, the patient presented with no evaluable target lesions and declined to undergo repeated colonoscopy for evaluation; therefore, tumor markers were used as efficacy evaluation indicators. In February 2019, the patient’s CA15-3 Level had decreased to 106.00 U/mL and symptoms had significantly improved. In April 2019, the CA15-3 levels had further decreased to 93.24 U/mL, and we advised the patient to continue to attend regular follow-up consultations for active surveillance.
In July 2019, the CA15-3 Level had increased to 135.46 U/mL and the CA125 level was 48.30 U/mL. A small amount of peritoneal effusion was noted on imaging examination. Tumors were assessed for disease progression based on tumor marker elevations and the patient's symptoms, with a progression-free survival of 7 mo. As the patient was subsequently examined at a local hospital, further details could not be obtained through follow-up visits.
Breast cancer often metastasizes to the bones, lungs, liver, and brain. Gastrointestinal metastatic breast cancer has a low incidence and may be misdiagnosed or missed. In a retrospective analysis of approximately 12000 patients with metastatic breast cancer, only 73 patients were reported to have gastrointestinal metastasis[5]. The most common sites in relation to gastrointestinal metastases secondary to breast cancer are the colon or the rectum, followed by the stomach (28%), small intestine (19%), and esophagus (8%). Patients with ILC are reported to be twice as likely to have gastrointestinal metastases than those with invasive ductal carcinoma[5]. In a report involving 206 patients with gastrointestinal metastases of breast cancer, Ambroggi et al[6] found that the sites of gastrointestinal metastases of breast cancer included the stomach (60%), colon (11%), rectum (8%), and oropharynx (1%). Interestingly, ILC of the breast has been reported to differ from invasive ductal carcinoma in distant metastasis[7]. Invasive ductal carcinoma often metastasizes to the lung, liver, and bone, whereas ILC has been reported to metastasize to the gastrointestinal tract, ovaries, peritoneum, and retroperitoneum [8]. It has been reported that 53%–64% of metastatic gastrointestinal breast cancers are ILC, and the common molecular types detected are ER/PR positive and HER-2 negative[5,9].
Non-specific clinical symptoms of gastrointestinal metastasis secondary to breast cancer are similar to those in primary gastrointestinal tumors[10-12]. For example, patients‘ symptoms can manifest as dyspepsia, stomachache, and gastrointestinal obstruction, or present as an asymptomatic abdominal mass. Due to the relatively poor specificity of tumor markers and their susceptibility to interference with other factors, tumor markers alone may not effective as the only basis in which to determine tumor recurrence. Because gastrointestinal metastasis is extremely rare, gastroenteroscopy is not a routinely undertaken postoperatively, which may explain why gastrointestinal metastasis secondary to breast cancer could be easily overlooked.
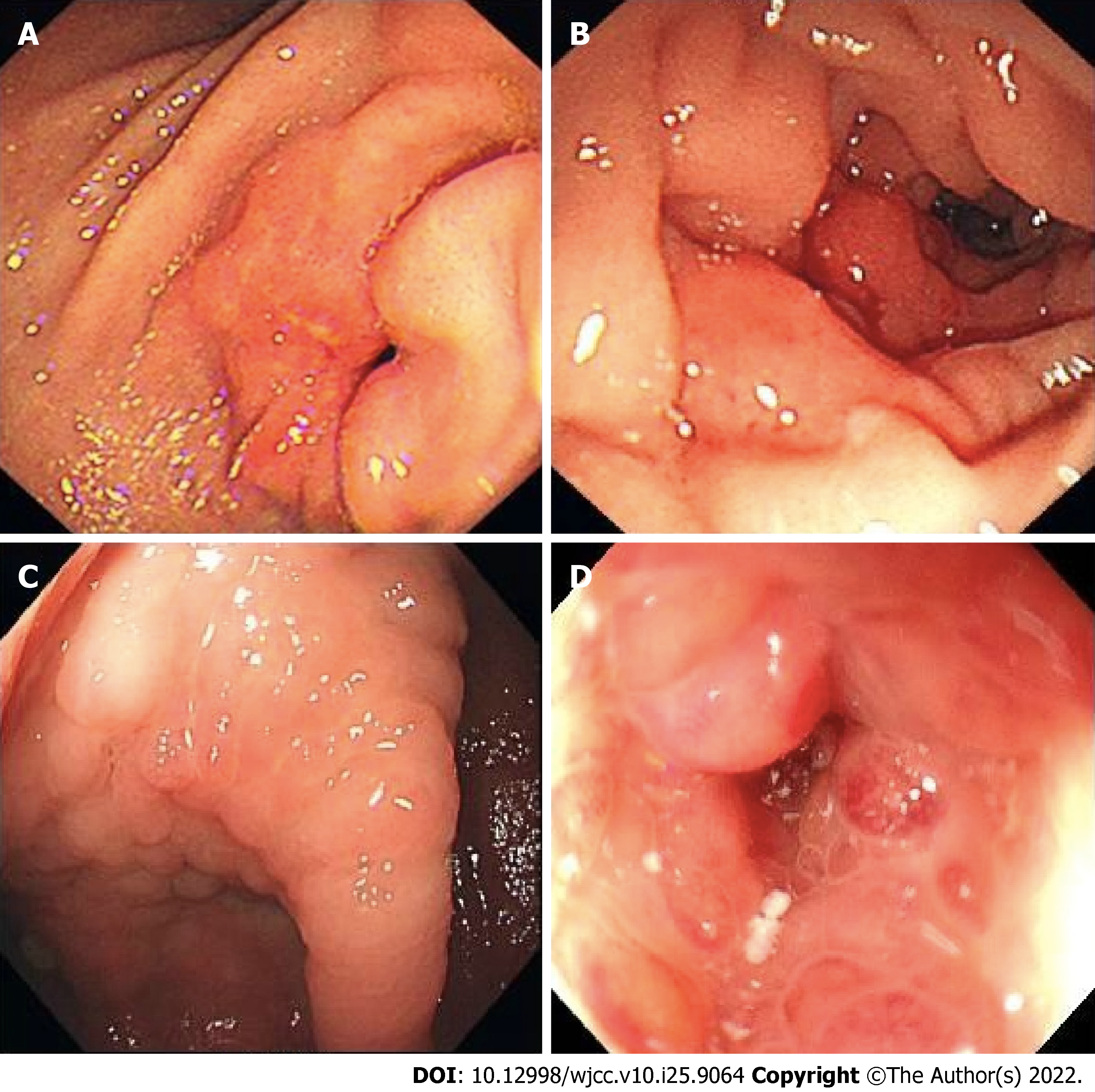
Figure 2 shows the endoscopic images concerning gastric antrum, duodenal, ileocecal, and colon metastases in different patients with breast cancer at our hospital to demonstrate the endoscopic status of gastrointestinal metastatic breast cancer. Notably, endoscopic images of gastrointestinal metastasis secondary to breast cancer lack specificity, and it is necessary to distinguish between primary and secondary tumors. Due to the poor specificity of histomorphology, IHC staining is particularly important in the differential diagnosis. Currently, commonly used differential molecules include CK7, CK20, gross cystic disease fluid protein 15 (GCDFP-15), and GATA-3[13]. CK7+/CK20- is most common in breast tissue, while CK7-/CK20+ is most common in the gastrointestinal tract[10]. GCDFP-15 is a high molecular weight breast cyst fluid protein that is positive in 35%–74% of breast cancers and has strong specificity[14]. GATA-3 has been considered as a specific marker for breast cancer and is expressed in almost all cases of ILC. Additionally, the positive expression rate of GATA-3 in gastrointestinal primary tumors is reported to be < 5%[15].
The median interval between diagnosis of breast cancer and discovery of gastrointestinal metastasis is approximately seven years, ranging from simultaneous diagnosis to a diagnosis made many years later[5,9]. In our patient, the elevation of CA15-3 was elevated < 1 year after breast cancer surgery, which is a typical finding.
The patient had no specific clinical symptoms at the early disease stage of the disease, with only the tumor marker CA15-3 being slightly higher than the normal upper limit, and no tumor metastasis found on imaging examination. CA15-3 is a specific marker of breast cancer, and elevation of CA15-3 often indicates the onset, recurrence, or progression of breast cancer. CA15-3 Level in this patient with ILC of breast increased 10 mo after surgery, which was first considered as a possible recurrence and metastasis secondary to breast cancer. However, neither ultrasound nor CT nor systemic PET-CT could localize signs of tumor recurrence or metastasis. After switching to exemestane endocrine therapy, the level of CA15-3 Level still showed an upward trend. As the disease progressed, the patient began to develop clinical symptoms of the digestive system, and colonoscopy revealed multiple lesions in the colon. Combined with the results of biopsy pathology, and IHC staining results, the negative CK20 and CDX-2 allowed for an exclusion of a primary colorectal tumor, and a positive GATA-3 and ER highly supported breast cancer as the primary source.
In terms of diagnosis and treatment, individual and multidisciplinary approaches should be emphasized. Currently, there is no standardized guidance for the diagnosis and treatment of gastrointestinal metastasis secondary to breast cancer, and chemotherapy and endocrine therapy remain the main treatment methods. patients with hormone receptor-positive breast cancer with only gastrointestinal metastases, endocrine therapy with or without CDK4/6 inhibitors is a more appropriate option. However, for patients with breast cancer and gastrointestinal metastases and metastases to other organs, chemotherapy, compared with endocrine therapy, can rapidly reduce tumor burden. Surgical intervention has been shown to not significantly prolong overall survival[5], and palliative surgery may be considered to improve patients' quality of life when necessary, such as in cases of gastrointestinal obstruction. The IHC results of our patient showed that both ER and PR were positive, and HER2 was not amplified. This patient was intolerant to chemotherapy and her tumor was resistant to the aromatase inhibitor exemestane. According to relevant guidelines, such patients should be prescribed fulvestrant in combination with palbociclib. After treatment, the patient's symptoms were significantly relieved and the CA15-3 Level was significantly lower than previously, indicating that endocrine therapy combined with CDK4/6 inhibitors was effective. The survival benefit of surgical treatment for gastrointestinal metastasis secondary to breast cancer lacks evidence-based support. In this case, the patient did not have an intestinal obstruction, intestinal perforation, or other complications, so surgical treatment was not considered.
This case report provides useful information for clinicians. Although the incidence of postoperative breast cancer metastasis involving the digestive tract is low, the number of patients with breast cancer patients is substantial, and thus the possibility of this complication needs to be considered. Typically, there are no flagrant clinical symptoms in the early stage of gastrointestinal metastasis secondary to breast cancer. If tumor markers increase postoperatively and other causes are excluded, and metastatic lesions cannot be detected using conventional imaging, clinicians should be alerted to the possibility of digestive tract metastasis or metastases at other rare sites should be kept on alert. Moreover, patients with breast cancer and symptoms such as abdominal distention, labored defecation, or other unknown symptoms should be offered a timely gastroenteroscopy to exclude gastrointestinal metastasis. Due to the lack of specificity in endoscopy examinations, diagnosis should be made based on pathology and IHC results. In addition, endocrine therapy in combination with a CDK4/6 inhibitor was found to be effective in managing gastrointestinal metastasis secondary to breast cancer; however, future studies are needed to further validate this finding.
Gastrointestinal metastasis secondary to breast cancer is rare. Due to a lack of specific symptoms in the early stage, diagnosis may be delayed. The possibility of metastatic disease should be considered for patients with a history of breast cancer, digestive tract symptoms, or significant tumor marker abnormalities, and no metastatic lesions detected on imaging. Endoscopic ultrasonography, CT-guided puncture, or surgery could be used for prompt diagnosis, with appropriate treatment methods selected.
The authors thank the patient for her participation and her agreement to publication of this case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He S, China; Nel I, Germany S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64652] [Article Influence: 16163.0] [Reference Citation Analysis (176)] |
| 2. | Miglietta F, Bottosso M, Griguolo G, Dieci MV, Guarneri V. Major advancements in metastatic breast cancer treatment: when expanding options means prolonging survival. ESMO Open. 2022;7:100409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | Yu J, Mu Q, Fung M, Xu X, Zhu L, Ho RJY. Challenges and opportunities in metastatic breast cancer treatments: Nano-drug combinations delivered preferentially to metastatic cells may enhance therapeutic response. Pharmacol Ther. 2022;236:108108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Di Micco R, Santurro L, Gasparri ML, Zuber V, Fiacco E, Gazzetta G, Smart CE, Valentini A, Gentilini OD. Rare sites of breast cancer metastasis: a review. Transl Cancer Res. 2019;8:S518-S552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | McLemore EC, Pockaj BA, Reynolds C, Gray RJ, Hernandez JL, Grant CS, Donohue JH. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol. 2005;12:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Ambroggi M, Stroppa EM, Mordenti P, Biasini C, Zangrandi A, Michieletti E, Belloni E, Cavanna L. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int J Breast Cancer. 2012;2012:439023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | El-Hage A, Ruel C, Afif W, Wissanji H, Hogue JC, Desbiens C, Leblanc G, Poirier É. Metastatic pattern of invasive lobular carcinoma of the breast-Emphasis on gastric metastases. J Surg Oncol. 2016;114:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery. 1993;114:637-641. [PubMed] |
| 9. | Hong J, Kim Y, Cho J, Lim SW, Park SE, Kim HK, Lee H, Cho SY, Kim JY, Ahn JS, Im YH, Park YH. Clinical features and prognosis of breast cancer with gastric metastasis. Oncol Lett. 2019;17:1833-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Saranovic D, Kovac JD, Knezevic S, Susnjar S, Stefanovic AD, Saranovic DS, Artiko V, Obradovic V, Masulovic D, Micev M, Pesko P. Invasive lobular breast cancer presenting an unusual metastatic pattern in the form of peritoneal and rectal metastases: a case report. J Breast Cancer. 2011;14:247-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Zhang LL, Rong XC, Yuan L, Cai LJ, Liu YP. Breast cancer with an initial gastrointestinal presentation: a case report and literature review. Am J Transl Res. 2021;13:13147-13155. [PubMed] |
| 12. | Liu M, Zhang L, Guo L, Lv J, Shi W, Liu B. Intestinal metastasis from breast invasive ductal carcinoma after a long latency: case report and literature review. Onco Targets Ther. 2018;11:8599-8603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol. 2004;121:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Kuncman W, Orzechowska M, Kuncman Ł, Kordek R, Taran K. Intertumoral Heterogeneity of Primary Breast Tumors and Synchronous Axillary Lymph Node Metastases Reflected in IHC-Assessed Expression of Routine and Nonstandard Biomarkers. Front Oncol. 2021;11:660318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Jin X, Tang H, Chen H, Chen G. Case Report: Metastatic Signet-Ring-Cell Carcinoma of the Bladder From Breast Invasive Lobular Carcinoma Detected by Computed Tomography. Front Oncol. 2022;12:835487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |