Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.9044
Peer-review started: March 31, 2022
First decision: May 12, 2022
Revised: May 25, 2022
Accepted: July 29, 2022
Article in press: July 29, 2022
Published online: September 6, 2022
Processing time: 147 Days and 20.4 Hours
Kidney transplantation is associated with an increased risk of tumors in the urinary bladder. Among all the pathological types of tumors in the bladder, paraganglioma, which arises from extra-adrenal paraganglia and consists of chromaffin cells, is rare. Paragangliomas might cause severe clinical symptoms due to catecholamine hypersecretion or mass compression. Bladder paragangliomas are rare, especially those appearing after kidney transplantation. Here, we report a case of bladder paraganglioma developing after kidney transplan
A 63-year-old woman received a kidney transplant 12 years ago and took oral immunosuppressants (cyclosporine, mizoribine, and methylprednisolone) for regular post-transplant treatment. The patient felt no discomfort and she came to the hospital for a routine checkup. A mass located in the bladder was incidentally discovered by computed tomography, and she underwent surgical treatment. A 2 cm × 2 cm invasive mass was found in the trigone of the bladder and the mass was removed. The diagnosis of paraganglioma was confirmed by morphology and immunophenotyping. The patient had a good prognosis and is still alive.
Paraganglioma can grow in the bladder, which might cause no clinical symptoms. The diagnosis mainly depends on morphology and immunophenotyping. Surgical resection is an important treatment option for such patients.
Core Tip: Paraganglioma is a rare neuroendocrine carcinoma with a morbidity of 2-8 cases per million. Paraganglioma in the urinary bladder is an infrequent condition, and it is even more rarely reported in kidney transplant recipients. The clinical manifestations of paraganglioma lack specificity. The tumor might cause severe clinical symptoms, such as hypertension and hematuria, or no symptoms, making an accurate preoperative diagnosis difficult. Pathology is essential for diagnosis. Although there is still no standard therapeutic consensus, surgical resection is an important treatment. Here, we report a case of paraganglioma in the bladder of a patient after kidney transplantation.
- Citation: Wang L, Zhang YN, Chen GY. Bladder paraganglioma after kidney transplantation: A case report. World J Clin Cases 2022; 10(25): 9044-9049
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/9044.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.9044
In recent years, with the development and application of new types of immunosuppressants, the survival rate of kidney transplantation has been significantly improved. However, long-term use of immunosuppressants increases the risk of tumors in kidney transplantation recipients. Malignant tumor is one of the common long-term complications after kidney transplantation, with a reported incidence of 7.6%-10.9%[1]. The most common tumors in kidney transplant recipients are Kaposi’s sarcoma, skin cancer, non-Hodgkin’s lymphoma, colorectal cancer, renal cell cancer, and anogenital tract cancer[2]. Although kidney transplantation has been proved to increase the incidence of bladder tumors[3], the incidence is low. A large study showed that the incidence of bladder malignancies was only 1.9% in the population who received kidney transplantation[4]. Paragangliomas were even more rarely found in such situations.
Paragangliomas are nonepithelial tumours originating from neural crest-derived paraganglion cells situated in the region of the autonomic nervous system ganglia and accompanying nerves[5]. It has been reported that the morbidity is only 2-8 per million population[6]. The first case of bladder paraganglioma was reported by Zimmerman et al[7] in 1953, and more cases were then reported. Current data show that paraganglioma arising in the urinary bladder is extremely rare, and accounts for only 0.06% of all bladder cancers[8]. Due to the rarity, the diagnostic and therapeutic methods are still not well established. Here, we report a pathologically confirmed bladder paraganglioma that arose after kidney transplantation, which is a rare clinical entity.
A 63-year-old female patient was hospitalized to the Urology Department of our hospital in October 2016. She was admitted to hospital just for a regular post-transplantation physical examination.
The patient underwent right allograft kidney transplantation 12 years ago at our hospital due to kidney failure caused by chronic nephritis. After transplantation, she had regularly taken cyclosporine (50 mg bid), mizoribine (150 mg qd), and methylprednisolone (5 mg qd). Her physical condition was good after transplantation. Also, she underwent medical examination at a local hospital or our hospital and the last examination occurred 2 years ago. The follow-up protocol included routine blood tests, serum bio
No positive sign.
Routine blood tests and blood coagulation function tests were normal. Blood glucose and lipid levels, transaminase levels, and tumor markers [carcinoembryonic antigen, alpha-fetoprotein, carbohydrate antigen (CA) 12-5, and CA 19-9] were within normal ranges. Blood urea (9.2 mmol/L) and creatinine (132 mmol/L) were slightly elevated.
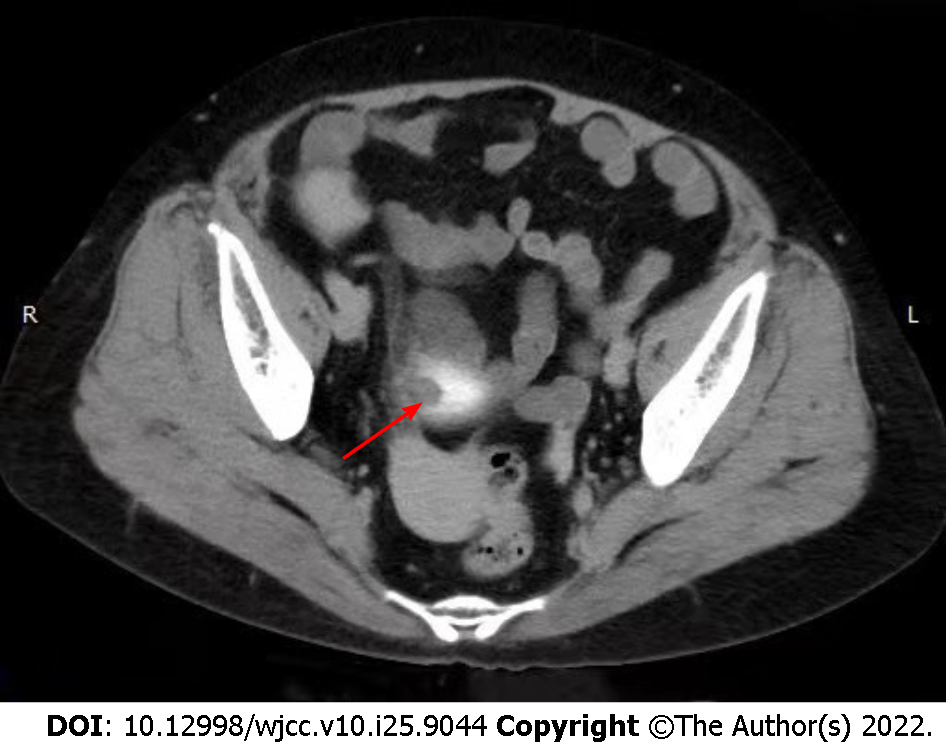
CT revealed a decrease in size of both kidneys. Also, a shadow with heterogeneous soft tissue density was found on the upper bladder wall (Figure 1). The shape of the tissue was irregular with indistinct edges. CT suggested a malignant tumor. No enhanced imaging was performed because non-contrast CT had already indicated a tumor and surgical excision was chosen for treatment.
Paraganglioma.
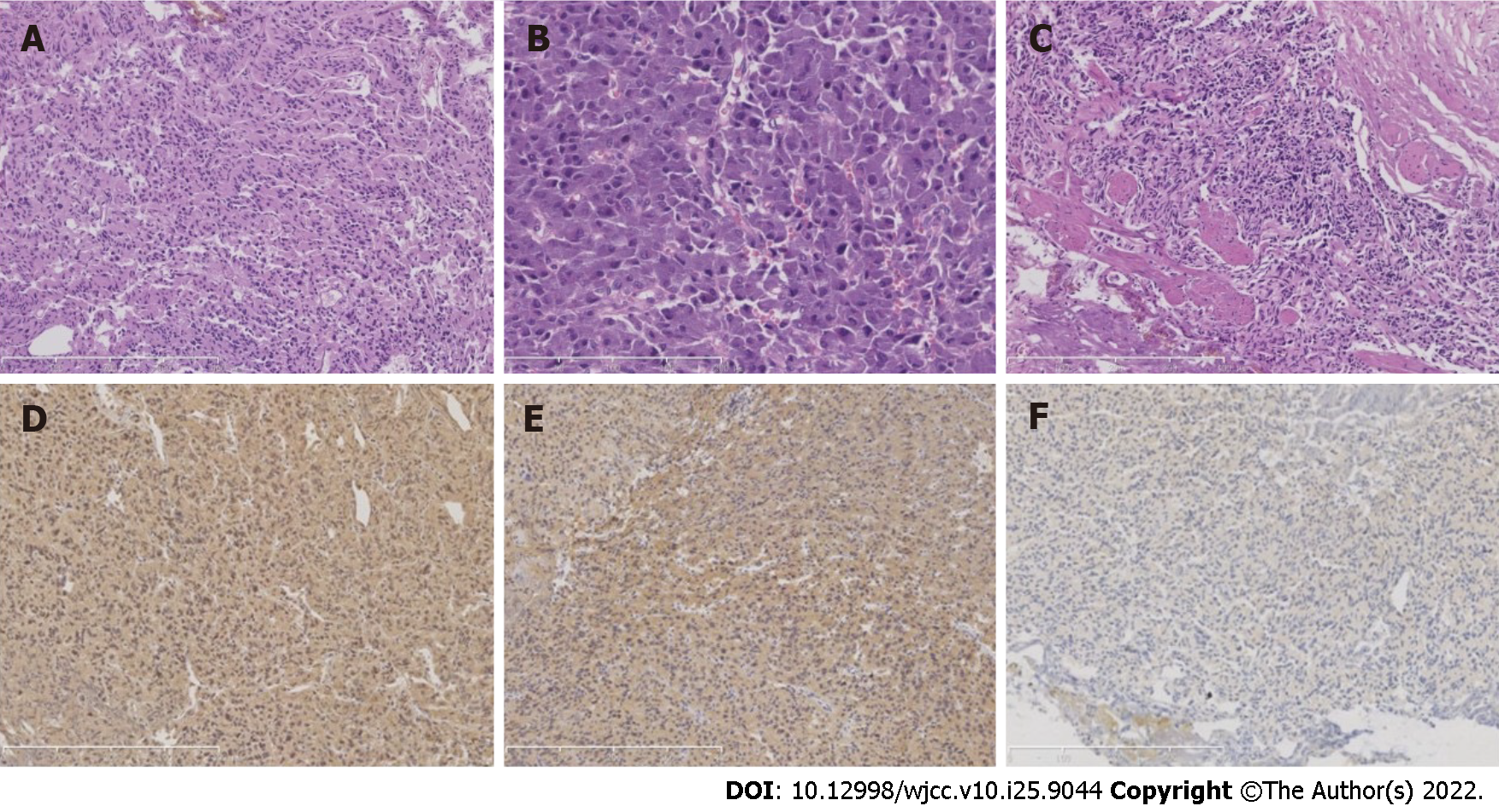
The patient underwent transurethral resection of the bladder tumor after preoperative risk assessment. First, cystoscopy was performed to excise the tumor in the bladder. The invasive mass was observed in the trigone of the bladder under cystoscopy, with a size of 2 cm × 2 cm. It was observed that the tumor had invaded to the muscle layer of the bladder. Then, the tumor was removed with an electric scalpel. The excised neoplasm was sent for pathological examination. The operation was successful. The gross morphology of the excised bladder neoplasm presented irregular gray–white tissue with a diameter of 1.5 cm. Microscopically, the tumor cells were arranged like sheets and nests (Figure 2A). The cytoplasm was eosinophilic to amphophilic after hematoxylin and eosin staining (Figure 2B). The nuclei had a hyperchromatic and irregular shape without mitotic figures. The neoplasm infiltrated the muscle layer (Figure 2C). For further characterization, immunohistochemical staining was performed. The tumor cells were positive for CD56, Syn, CgA, and S-100, and negative for CK, SDHB, and CK7 (Figure 2D-F). Ki-67 staining revealed a proliferative index of approximately 1%. The pathological diagnosis supported paraganglioma.
The patient recovered well after surgery and was followed by telephone. At the 6-year follow-up visit, she reported that her health condition was good without discomfort. The patient is still alive when this case report is written.
Although paragangliomas can arise in a variety of locations, the majority are found in predictable regions, including the middle ear, jugular foramen, carotid body, mediastinum, and para-aortic region[9]. However, paragangliomas arising in the bladder are rare. It is reported that the proportion of bladder paragangliomas among all catecholamine-secreting neoplasms is < 1% and only 0.5% of all bladder tumors[10]. The main clinical manifestations of bladder paraganglioma are tumor mass effects, such as micturition, hematuria, dysuria, and bladder irritation[11]. Paragangliomas secreting cate
Kidney transplantation gives patients with end-stage renal failure a longer life expectancy, with the help of immunosuppression. However, life-long immunosuppression has caused serious complications in this patient group, including tumors. It is now recognized that kidney-transplant recipients have a 2-4-fold increased risk of tumors compared with the age- and gender-matched general population. Immunosuppressive therapy might impair immunosurveillance and cause an inadequate immune response and immunological control of oncogenic viral infections, which might lead to an increased risk of tumorigenesis[13]. In China, statistics show that the total incidence of malignant tumors in post-kidney transplant patients is 2.95%, which is 3-5 times higher than that in the gender- and age-matched general population[14]. Kaposi’s sarcoma and skin cancer are the most common tumors in such patient groups outside China. In China, urinary tract tumors are the most common, followed by digestive system tumors[15]. The top ten most common tumors in post-kidney transplant patients are urothelial carcinoma, hepatocellular carcinoma, renal cell carcinoma, gastrointestinal cancer, lymphoma, breast cancer, lung cancer, cervical cancer, prostate cancer, Kaposi sarcoma, and squamous cell carcinoma of the skin[14]. Paragangliomas are rare post-kidney transplantation tumors. Despite the low morbidity of paragangliomas in the genitourinary system, the bladder is the most common site for paragangliomas, followed by the urethra, pelvis, and ureter[16]. Hence, the possibility of paragangliomas should be suspected for tumors occurring in the bladder. However, paragangliomas of the bladder in post-kidney transplant patients are extremely rare. Until now, we have only found one such case reported[17]. As far as we know, this is the first reported case of post-kidney transplantation paraganglioma of the bladder in the Chinese population.
Paraganglioma is a neuroendocrine tumor originating from neural crest cells. According to the site of occurrence, tumors in the adrenal medulla are called pheochromocytomas, while those outside the adrenal glands are generally referred to as paragangliomas[18]. The diagnosis of paraganglioma mostly relies on the morphological characteristics and immunophenotype, including immunohistochemical staining with neuroendocrine markers[19]. In the 2017 edition of the WHO Classification of Endocrine Organ Tumors[5], the definition of paraganglioma has been updated. This kind of tumor is considered malignant and has the potential to metastasize, and the previous benign classification has been removed. Paraganglioma in this case was invasive in the muscularis mucosae of the trigone of the bladder and was malignant.
Genetic studies have found that mutations of succinate dehydrogenase (SDH) are associated with prognosis. Three types of SDH mutations in bladder paragangliomas have been found: SDHA, SDHB, and SDHD. Bladder paraganglioma with SDHB gene mutation has the highest probability of distant metastasis and malignant change, which means that it might have the worst prognosis[20,21]. No mutations of SDHB protein were found in this case, suggesting a low risk of metastasis. Indeed, no metastasis was found in the later follow-up, and the patient is in good condition currently, which is consistent with the inspection results.
Given that the most common bladder tumor is urothelial carcinoma, this case should be distinguished from it. The diffuse and invasive growth pattern of bladder paraganglioma might be easily misdiagnosed as urothelial carcinoma, especially when the tissue is broken and has been compressed and burned. In addition, urothelial carcinoma may also present with a nest-like growth pattern that is similar to that of paraganglioma. The main differences between the two tumors are the immunophenotypes. CK is negative, neuroendocrine markers are positively expressed in the paraganglioma tumor cells, and S-100 is positively expressed in the supporting cells. Urothelial carcinoma tumor cells express CK7, P63, PAX-8, and GATA-3. The immunophenotypes of this case supported the diagnosis of paraganglioma.
The incidence of bladder paraganglioma after kidney transplantation is rare and few cases have been reported. As far as we know, this is the first reported case in the Chinese population. Paraganglioma in the bladder might cause no clinical symptoms and the diagnosis mainly depends on morphology and immunophenotyping. Surgical resection is an important treatment option for such patients. The exact pathogenesis of paraganglioma of the urinary bladder after kidney transplantation is still unclear because of the low incidence, and long-term use of immunosuppressant might be the cause.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Ghazanfar A, United Kingdom; Gofrit O, Israel; Tieranu CG, Romania S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | Kleine-Döpke D, Oelke M, Schwarz A, Schwager Y, Lehner F, Klempnauer J, Schrem H. Renal cell cancer after kidney transplantation. Langenbecks Arch Surg. 2018;403:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Manickavasagar R, Thuraisingham R. Post renal-transplant malignancy surveillance. Clin Med (Lond). 2020;20:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Yan L, Chen P, Chen EZ, Gu A, Jiang ZY. Risk of bladder cancer in renal transplant recipients: a meta-analysis. Br J Cancer. 2014;110:1871-1877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Besarani D, Cranston D. Urological malignancy after renal transplantation. BJU Int. 2007;100:502-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Asa SL, Mete O, Perry A, Osamura RY. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr Pathol. 2022;33:6-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 282] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 6. | Lin S, Peng L, Huang S, Li Y, Xiao W. Primary pancreatic paraganglioma: a case report and literature review. World J Surg Oncol. 2016;14:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Zimmerman IJ, Biron RE, Macmahon HE. Pheochromocytoma of the urinary bladder. N Engl J Med. 1953;249:25-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 130] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Bhalani SM, Casalino DD, Manvar AM. Paraganglioma of the bladder. J Urol. 2011;186:279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Lee KY, Oh YW, Noh HJ, Lee YJ, Yong HS, Kang EY, Kim KA, Lee NJ. Extraadrenal paragangliomas of the body: imaging features. AJR Am J Roentgenol. 2006;187:492-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Siatelis A, Konstantinidis C, Volanis D, Leontara V, Thoma-Tsagli E, Delakas D. Pheochromocytoma of the urinary bladder: report of 2 cases and review of literature. Minerva Urol Nefrol. 2008;60:137-140. [PubMed] |
| 11. | Martinez Rodriguez RH, Buisan Rueda O, Ibarz L. Bladder cancer: Present and future. Med Clin (Barc). 2017;149:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Williams P, Siref L, Feloney M. Pheochromocytoma of the bladder. JAAPA. 2017;30:23-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Elserwy NA, Lotfy EE, Fouda MA, Mahmoud MI, Donia AF, Mashaly ME, Abbas MH, Abuelmagd MM, Abouelenein RK, Ismail MI, Bakr MA. Postrenal transplant malignancy: Incidence, risk factors, and prognosis. Saudi J Kidney Dis Transpl. 2017;28:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Li L, Sun Q. Renal transplantation in China: ten years of experience at Nanjing Jinling Hospital. Clin Transpl. 2006;71-77. [PubMed] |
| 15. | Zhang J, Ma L, Xie Z, Guo Y, Sun W, Zhang L, Lin J, Xiao J, Zhu Y, Tian Y. Epidemiology of post-transplant malignancy in Chinese renal transplant recipients: a single-center experience and literature review. Med Oncol. 2014;31:32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Beilan JA, Lawton A, Hajdenberg J, Rosser CJ. Pheochromocytoma of the urinary bladder: a systematic review of the contemporary literature. BMC Urol. 2013;13:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Lazareth H, Cohen D, Vasiliu V, Tinel C, Martinez F, Grünfeld JP, Mamzer MF, Legendre C, Sberro-Soussan R. Paraganglioma of the bladder in a kidney transplant recipient: A case report. Mol Clin Oncol. 2017;6:553-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Capatina C, Ntali G, Karavitaki N, Grossman AB. The management of head-and-neck paragangliomas. Endocr Relat Cancer. 2013;20:R291-R305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Cheung VKY, Gill AJ, Chou A. Old, New, and Emerging Immunohistochemical Markers in Pheochromocytoma and Paraganglioma. Endocr Pathol. 2018;29:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Rijken JA, van Hulsteijn LT, Dekkers OM, Niemeijer ND, Leemans CR, Eijkelenkamp K, van der Horst-Schrivers ANA, Kerstens MN, van Berkel A, Timmers HJLM, Kunst HPM, Bisschop PHLT, Dreijerink KMA, van Dooren MF, Hes FJ, Jansen JC, Corssmit EPM, Hensen EF. Increased Mortality in SDHB but Not in SDHD Pathogenic Variant Carriers. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Niemeijer ND, Rijken JA, Eijkelenkamp K, van der Horst-Schrivers ANA, Kerstens MN, Tops CMJ, van Berkel A, Timmers HJLM, Kunst HPM, Leemans CR, Bisschop PH, Dreijerink KMA, van Dooren MF, Bayley JP, Pereira AM, Jansen JC, Hes FJ, Hensen EF, Corssmit EPM. The phenotype of SDHB germline mutation carriers: a nationwide study. Eur J Endocrinol. 2017;177:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |