Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.9012
Peer-review started: March 16, 2022
First decision: May 30, 2022
Revised: June 13, 2022
Accepted: July 21, 2022
Article in press: July 21, 2022
Published online: September 6, 2022
Processing time: 162 Days and 17.5 Hours
Pancreatic metastases account for only a small proportion of all pancreatic malignancies. Isolated pancreatic metastasis from renal cell cancer (isPM-RCC) is extremely rare and may be difficult to differentiate from more common primary neoplasms. A history of nephrectomy is crucial for the diagnosis.
We report the case of a 64-year-old Asian man who was diagnosed with a mass in the pancreatic head using computed tomography. He had no related symptoms, and his medical history was unremarkable, except for unilateral nephrectomy performed to remove a “benign” tumor 19 years ago. All preoperative imaging findings suggested a diagnosis of pancreatic neuroendocrine tumor. However, ultrasound-guided biopsy revealed features of clear cell renal cell carcinoma (ccRCC). Re-examination of the specimen resected 19 years ago confirmed that he had a ccRCC. The pancreatic mass was resected and pathological examination confirmed isPM-RCC.
Misdiagnosis of isPM-RCC is common because of its rarity and the long interval from resection of the primary tumor and manifestation of the metastasis. The history of the previous surgery may be the only clue.
Core Tip: Pancreatic metastases derived from clear cell renal cell carcinoma are rare and can be maintained for a long time and progress until years later. It is easily confused with hypervascular tumors, such as pancreatic neuroendocrine tumors (pNET). We present a case of a pancreatic mass that had undergone nephrectomy 19 years ago. Due to the lack of medical records, the clinical and imaging findings were misdiagnosed as pNET due to a false medical history. Preoperative biopsy was performed prudently, resulting in a renal-derived tumor, consistent with the findings of the pathological section obtained from the previous surgery hospital. This case highlights the importance of accurate clinical data, especially with a history of surgery, even a long time ago.
- Citation: Liang XK, Li LJ, He YM, Xu ZF. Misdiagnosis of pancreatic metastasis from renal cell carcinoma: A case report. World J Clin Cases 2022; 10(25): 9012-9019
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/9012.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.9012
Renal cell carcinoma (RCC) is an aggressive tumor, and metastasis to the pancreas accounts for only 2%–5% of all metastases[1]. Isolated pancreatic metastasis from RCC (isPM-RCC) is rare and unique[2]. The biological characteristics of the disease are completely different from those of metastases from sarcomas or colorectal carcinoma: the disease-free period can be very long (mean, 10.1 years), the localization within the pancreas is independent of the nephrectomy side, and the overall survival (OS) is optimistic, regardless of the number and size of isPM[3,4]. The long period of slow tumor growth or quiescence often leads to the lesion being mistaken for the more common primary tumor[5]. A history of nephrectomy may be the only indication for an actual diagnosis. We report the case of a patient with isPM-RCC that was initially misdiagnosed as a primary neuroendocrine tumor (pNET). Endoscopic ultrasound-guided needle biopsy helped establish the diagnosis.
A 64-year-old man was referred to our hospital after computed tomography (CT) revealed a pancreatic mass.
There was no associated symptoms.
The patient had a nephrectomy performed for a “benign” tumor 19 years ago at another hospital, without any relevant paper information. The patient had no other relevant personal or family history.
There was no associated personal and family history.
Upon examination, the patient’s temperature was 36.5 °C, heart rate was 87 bpm, respiratory rate was 16 breaths/min, blood pressure was 145/88 mmHg, and oxygen saturation in room air was 99%. The abdomen was soft with no tenderness or palpable masses. An old surgical scar was observed in the left flank region.
Blood glucose levels and liver function test results were normal. Other blood investigation results were as follows: Serum hepatitis B surface antigen, 1794.61 IU/mL (< 0.05 IU/mL); hepatitis B virus deoxyribonucleic acid, 2.23 (< 1.0E + 03); serum alpha-fetoprotein, 3.12 ng/mL (< 20 ng/mL); carcinoembryonic antigen (CEA), 1.45 ng/mL (< 5 ng/mL); carbohydrate antigen (CA) 12–5, 18.79 U/mL (< 35 U/mL); CA 19–9, 16.87 (< 39 U/mL); and squamous cell carcinoma antigen, 0.84 ng/mL (< 1.5 ng/mL).
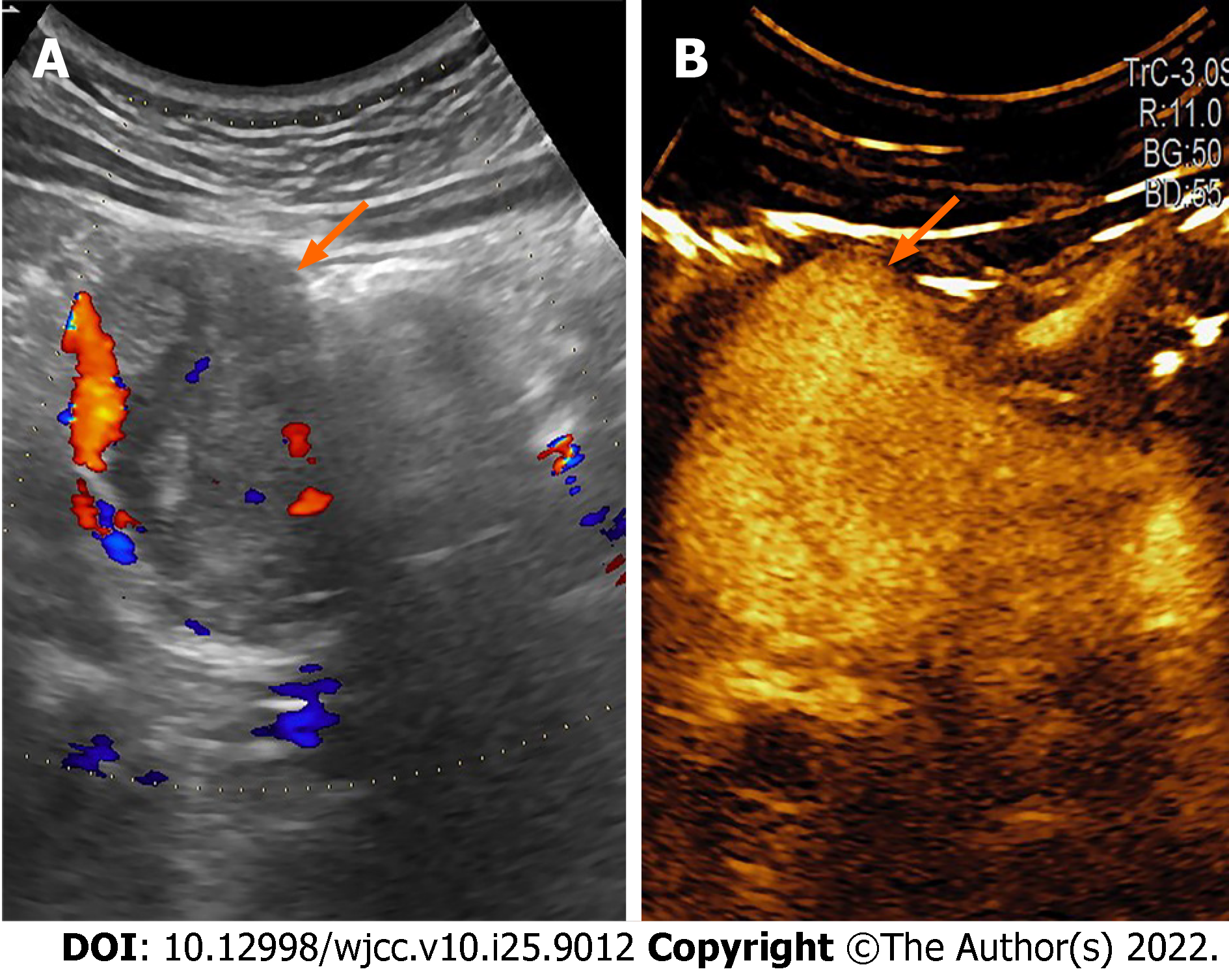
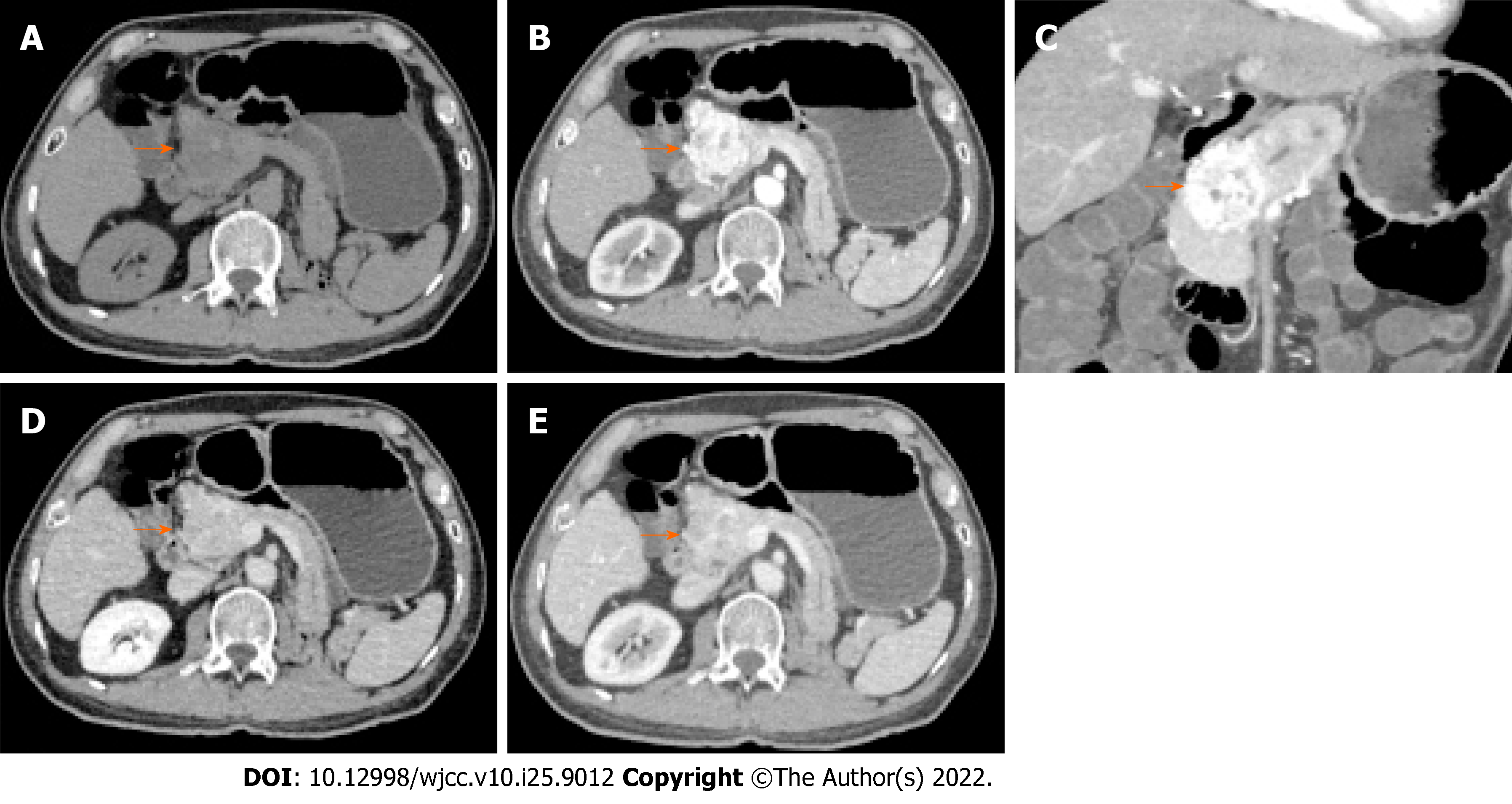
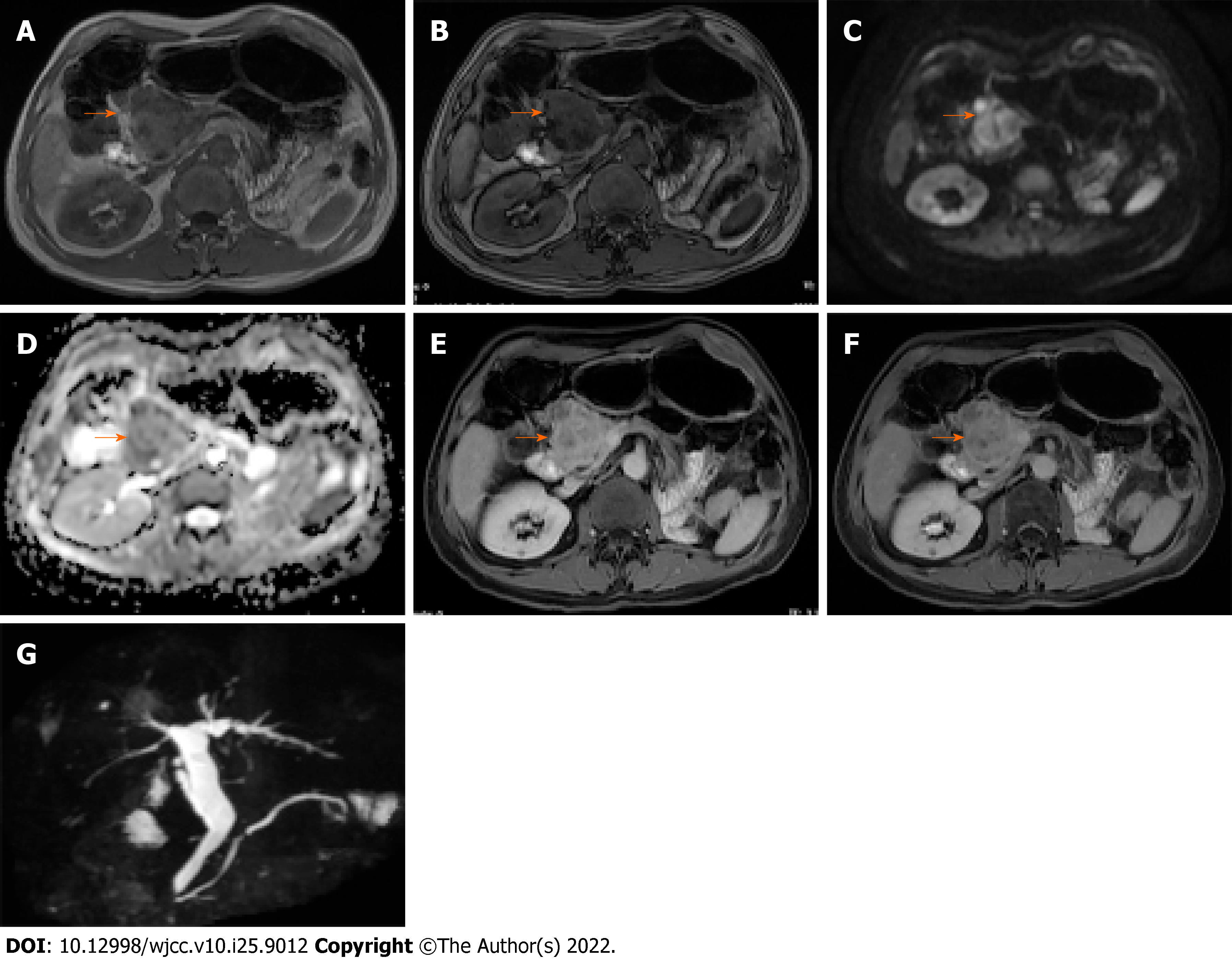
Conventional ultrasonography (Figure 1) revealed a well-defined hypoechoic mass with increased blood flow in the pancreas head. Contrast-enhanced ultrasound (CEUS) showed rapid inhomogeneous enhancement of the mass 17 s after injection of contrast; there was no significant washout in the venous phase. A small area of necrosis was observed at the center of the tumor. The CEUS findings were consistent with a pNET. Contrast-enhanced CT (Figure 2) showed a lobulated low-density mass, with the diameter of 6.1 cm × 5.6 cm × 4.2 cm, extending outward from the pancreatic head, with obvious heterogeneous enhancement in the early arterial phase. There was decreased enhancement in the venous phase with the “fast in fast out” mode, but the intensity was still higher than that of the surrounding pancreatic parenchyma. Patchy, non-enhancing areas were observed at the center of the mass. The size, shape, and enhancement of the remainder of the pancreas were normal. No signs of lymph node metastases were observed. On magnetic resonance imaging (MRI), the lesion was hypointense and moderately hyperintense on T1- and T2-weighted images, respectively (Figure 3). The chemical-shift MRI sequence showed a drop in the signal on out-of-phase images. In contrast, heterogeneous enhancement was observed in the arterial phase, with a thin hyperintense rim; there was washout in the portal and venous phases. MR cholangiogram showed dilatation of the bile duct and main pancreatic duct, suggesting an obstruction. Based on the imaging findings, the provisional diagnosis was a pNET.
After discussion with a multidisciplinary team, and keeping in mind the history of nephrectomy, we performed endoscopic ultrasound-guided needle biopsy. Pathological examination of the biopsy specimen revealed large polygonal cells with a clear cytoplasm arranged in an alveolar pattern. This picture, combined with the immuno-profile, was suggestive of clear cell renal cell carcinoma (ccRCC). Suspecting metastases from a previous renal tumor, we persuaded the relatives of the patient to approach the hospital where the nephrectomy had been performed and to borrow the specimen for re-examination. The material obtained from the kidney resected 19 years ago also showed a histological pattern of clear cell carcinoma.
The final diagnosis of the presented case was isPM-RCC.
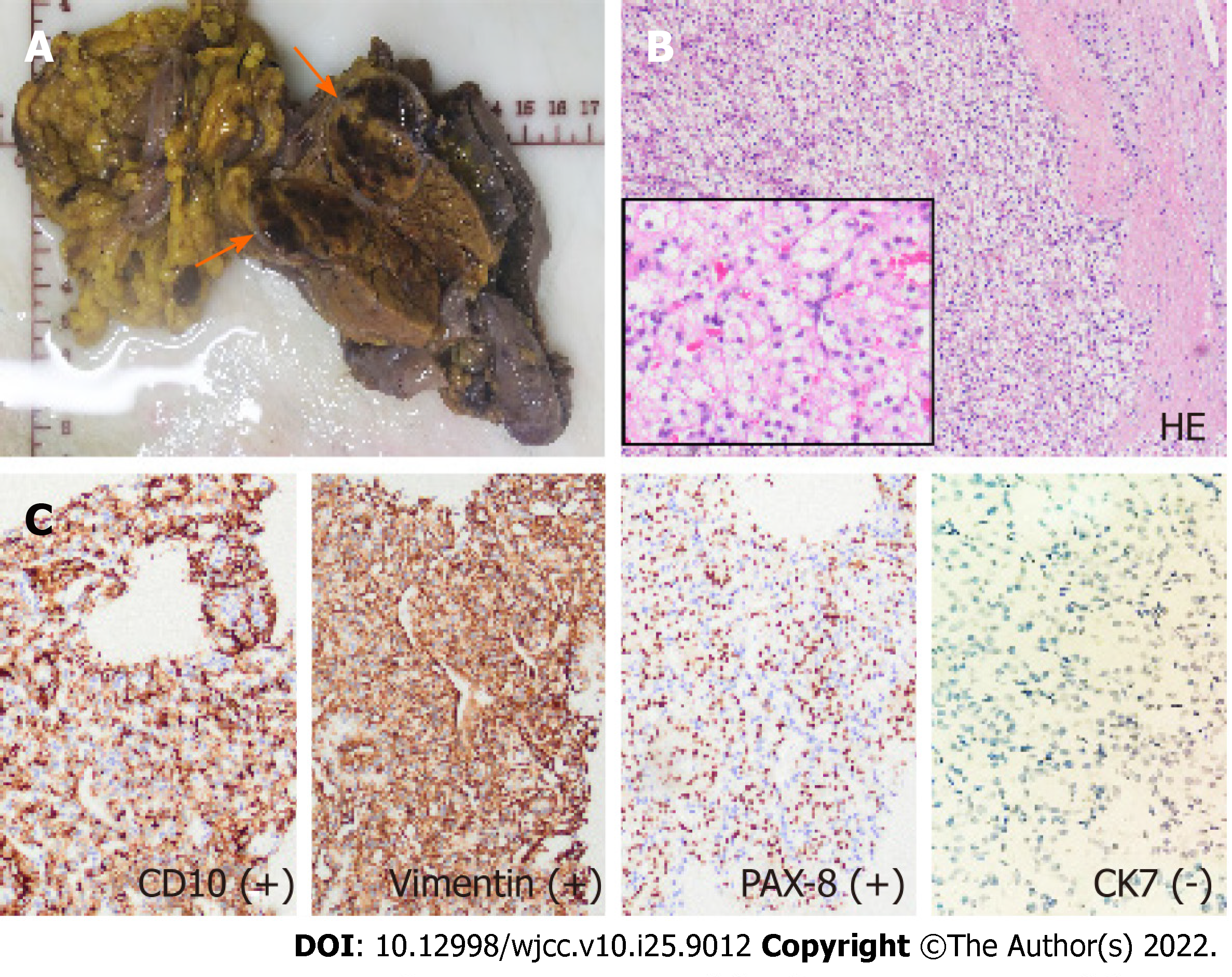
The patient was treated with five courses of apatinib and sunitinib conversion chemotherapy, followed by pancreaticoduodenectomy 6 mo later. During surgery, there was no malignant ascites, omental implants, or metastases to other organs. The border of the tumor was clear and situated close to the junction of the superior mesenteric and portal veins. The pancreatic and common bile ducts were compressed; however, no invasion was observed. Classical surgery was performed. The regional lymph nodes 12A, 12b, and 12p were also dissected. The surgical specimen showed a brownish nodule with a pseudocapsule located in the pancreas head, with some hemorrhagic or necrotic areas in the mass. Examination of hematoxylin and eosin–stained specimens and immunohistochemistry confirmed that it was an isolated metastasis from ccRCC (Figure 4).
Postoperatively, the patient did not experience malabsorption or diarrhea due to decreased exocrine pancreatic function. The patient was discharged on postoperative day 24 and was regularly followed up. To date, there has been no tumor recurrence (2.5 years after surgery).
In 2018 alone, there was over 400000 new cases of RCC worldwide, and over 170000 deaths. Even after surgical resection of RCC, 15%–25% of patients develop metastases to the lungs, bones, liver, or brain[3]. However, in approximately 20% of cases, metastasis grows slowly or remains stable for several years. According to the literature, the interval from nephrectomy to manifestation of pancreatic metastasis may be as long as 33 years[6]. Metachronous metastases occurred in 93% of cases. The prognosis after resection of metastasis is good, with a cumulative 5-year survival rate of 72%[3]. Only approximately 800 cases of isPM-RCC have been reported since 1952[3]. Multiple pancreatic metastases are observed in 38.1% of cases, but the number of metastases (single vs multiple) has no influence on survival[7,8]. The location of metastasis in the pancreas (48%, 22%, and 30% in the head, body, and tail, respectively) does not appear to be related to the side of the affected kidney[9]. In an analysis of previous reports, peripancreatic lymph nodes were found in only 18 of 309 cases (5.8%)[3], while adjacent retroperitoneal structures were not involved. These features suggest that metastases occur via the hematogenous route. Isolated pancreatic metastasis may be an indication of the seed and soil mechanisms. Because of high intratumoral heterogeneity, metastatic tumor cells can develop into clinically manifest metastases in the pancreas but become dormant or undergo apoptosis in other organs[3]. The genetic mechanism responsible for this extreme organotropism remains unknown; however, it probably involves interactions between the tumor microenvironment, immune system, and altered epigenetic states. Turajlic et al[10] showed that primary RCC with high intratumor heterogeneity and low weighted genome instability index progresses slowly, and that metastasizing clones lacking 9p or 14q loss tend to be less aggressive and more likely to establish isolated pancreatic metastasis. Approximately 35% of patients with isPM-RCC have no clinical symptoms, while others present with abdominal pain (20%), gastrointestinal tract bleeding due to duodenal infiltration (20%), obstructive jaundice (9%), weight loss (9%), pancreatitis (3%), or diabetes (3%)[9]. Tumor markers, such as CEA, CA12-5, and CA19-9 are either within normal limits or only slightly elevated, and therefore have no diagnostic value. When isPM-RCC occurs many years after the diagnosis of the primary tumor, it may not be suspected, and without careful elicitation of medical history, the tumor may be mistaken for the more common primary pancreatic tumor.
On imaging, isPM-RCC presented as a well-circumscribed hypervascular mass (Table 1). While these tumors usually show homogeneous enhancement after contrast injection, larger tumors may show rim enhancement, which has been described as a typical feature of isPM-RCC. This imaging sign was present in our patient. Low et al[11] suggested that rim enhancement was due to the tumor parasitizing blood supply from surrounding structures and therefore having greater perfusion at the periphery. Invasion of the common bile duct or main pancreatic duct is rare in isPM-RCC[12]. In our patient, the bile duct was dilated owing to distal compression by the mass, but there was no invasion. These features can help distinguish isPM-RCC from clear cell carcinoma of the pancreas, which is a variant of pancreatic ductal carcinoma. It is usually hypovascular and is more likely to invade adjacent structures[13]. Differentiation of isPM-RCC from pNET, a rare neoplasm that develops from endocrine tissues of the pancreas, can be challenging[5]. Both tumors were hypervascular and did not invade adjacent structures. However, although 20%–45% of isPM-RCCs are multifocal, pNET usually present as a single mass[14,15]. When a tumor is associated with one of the hereditary cancer syndromes (e.g., von Hippel–Lindau disease), pNET is the most probable diagnosis[16]. A drop in signal on chemical-shift MRI sequence, as observed in our patient, favors a diagnosis of isPM-RCC, since 80% of ccRCC metastases contain intracellular lipids similar to the primary tumor[17]. Another imaging feature that could help differentiate these two tumor types is the mean relative percentage washout on CT. Tae et al. found that the relative percentage washout was significantly higher in isPM-RCC than in pNET (29.4% vs 3.2%, P < 0.001) [18]. When the diagnosis is uncertain, EUS-guided biopsy can be useful, with a diagnostic accuracy of 90% [19]. Histologically, metastasis is similar to that in primary RCC. The nests of polygonal cells are associated with a rich vascular network. The Fuhrman nuclear grade tends to be higher in primary renal neoplasms than in pancreatic metastases. Immunohistochemistry showed that the tumor was diffusely positive for PAX8, CD10, CK8/18, vimentin, and PAX-8 and negative for synaptophysin, CgA, CD56, CK7, and CK20[20].
| Symptoms | Number, location, and size | Unenhanced CT | Enhanced CT | Indirect sign | Histology | |
| isPM-RCC | Asymptomatic, non-specific | Solitary/ multiple; No special location; 37.0 ± 21.4 mm | Lower or equal density; Clear boundary; Homogeneous | Hypervascular; Fast in fast out; Homogeneous; Rim enhancement | Rare Infringement of bile duct or main pancreatic duct; No parenchymal atrophy, clear retroperitoneal structure | Similar to the primary RCC, nests of polygonal cells with a rich vascular network. Pseudocapasule. CD10+, PAX8+, c-kit/CD117+, CK7- |
| pNET | Hormone-related symptoms in functional tumor | Solitary; More in tail; Small in Functional tumor (< 20 mm); Large in nonfunctional tumor (> 50 mm) | Lower density; Clear boundary; Heterogeneous; Calcification | Hypervascular; Obvious and continuous; Heterogeneous | Main pancreatic duct dilation; No parenchymal atrophy, clear retroperitoneal structure | Reveals cords, gyriform patterns of tumor cell arrangement, central hemorrhage, CgA+, Synaptophysin+, PAX8- |
| Clear cell carcinoma of the pancreas | Subxiphoid abdominal pain, jaundice, athrepsy | Solitary; More in head; 10 mm to 100 mm | Lower density; Unclear boundary; Heterogeneous; | Hypovascular; Mild in arterial phase but delayed in venous phase | Parenchymal atrophy and pancreatic and bile duct cutoff and dilatation; early metastasis | Virtue of tubular/glandular structures lined by clear cells with varying degrees of nuclear atypia; areas of conventional pancreatic ductal adenocarcinoma are present; CD10-, PAX8- |
Surgical resection remains the most effective treatment for primary RCC and its metastases. The type of surgery mainly depends on whether the metastasis is solitary or diffuse and on the location of the pancreatic lesion. Whipple’s procedure is performed for proximal lesions that are limited to the head or uncinate process of the pancreas, whereas distal pancreatectomy is performed for lesions in the pancreatic body or tail[21]. In a study with long-term follow-up, the cumulative 3-year and 5-year OS rates after pancreatic resection for RCC metastases were 72% and 63%, respectively. However, lymph node involvement and extrapancreatic metastases are associated with poor OS[22,23]. Over the last decade, the medical treatment of metastatic RCC has been revolutionized by the introduction of highly effective targeted therapies with tyrosine kinase inhibitors, mammalian target of rapamycin inhibitors, and monoclonal antibodies, such as angiogenesis and immune checkpoint inhibitors[24,25, 26]. The Memorial Sloan Kettering Cancer Center has suggested that, for risk stratification of cancer, the selection of treatment should be based on the type of malignancy[27]. The first-line treatment for initial patients with favorable or intermediate prognosis is bevacizumab or sunitinib plus interferon a, while those who fail the first-line treatment can receive a high dose of IL-2[27]. Patients with poor prognosis can receive temsirolimus or sunitinib as first-line therapy, respectively. Those who had multitargeted therapy can have everolimus as first-line therapy and tyrosine kinase inhibitors as second-line therapy.
isPM-RCC is extremely rare and usually manifests many years after the resection of the primary tumor. Misdiagnosis is possible without meticulous elicitation of medical history and careful evaluation of the imaging findings. EUS-guided needle biopsy can help establish a diagnosis, and surgical resection can prolong survival.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: De Raffele E, Italy; Moez R, Tunis S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Karimi KM, McFadden DW. Pancreatic resection for metastatic renal cell carcinoma to the pancreas. Am Surg. 2007;73:1158-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Sperti C, Moletta L, Patanè G. Metastatic tumors to the pancreas: The role of surgery. World J Gastrointest Oncol. 2014;6:381-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Sellner F. Observations on Solitary Versus Multiple Isolated Pancreatic Metastases of Renal Cell Carcinoma: Another Indication of a Seed and Soil Mechanism? Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Sellner F. Isolated Pancreatic Metastases of Renal Cell Carcinoma-A Paradigm of a Seed and Soil Mechanism: A Literature Analysis of 1,034 Observations. Front Oncol. 2020;10:709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Ma Y, Yang J, Qin K, Zhou Y, Ying X, Yuan F, Shi M, Jin J, Wang D, Gu J, Cheng D. Resection of pancreatic metastatic renal cell carcinoma: experience and long-term survival outcome from a large center in China. Int J Clin Oncol. 2019;24:686-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Thompson LD, Heffess CS. Renal cell carcinoma to the pancreas in surgical pathology material. Cancer. 2000;89:1076-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Konstantinidis IT, Dursun A, Zheng H, Wargo JA, Thayer SP, Fernandez-del Castillo C, Warshaw AL, Ferrone CR. Metastatic tumors in the pancreas in the modern era. J Am Coll Surg. 2010;211:749-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Volk A, Kersting S, Konopke R, Dobrowolski F, Franzen S, Ockert D, Grutzmann R, Saeger HD, Bergert H. Surgical therapy of intrapancreatic metastasis from renal cell carcinoma. Pancreatology. 2009;9:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Sellner F, Tykalsky N, De Santis M, Pont J, Klimpfinger M. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol. 2006;13:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Turajlic S, Xu H, Litchfield K, Rowan A, Chambers T, Lopez JI, Nicol D, O'Brien T, Larkin J, Horswell S, Stares M, Au L, Jamal-Hanjani M, Challacombe B, Chandra A, Hazell S, Eichler-Jonsson C, Soultati A, Chowdhury S, Rudman S, Lynch J, Fernando A, Stamp G, Nye E, Jabbar F, Spain L, Lall S, Guarch R, Falzon M, Proctor I, Pickering L, Gore M, Watkins TBK, Ward S, Stewart A, DiNatale R, Becerra MF, Reznik E, Hsieh JJ, Richmond TA, Mayhew GF, Hill SM, McNally CD, Jones C, Rosenbaum H, Stanislaw S, Burgess DL, Alexander NR, Swanton C; PEACE; TRACERx Renal Consortium. Tracking Cancer Evolution Reveals Constrained Routes to Metastases: TRACERx Renal. Cell. 2018;173:581-594.e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 672] [Cited by in RCA: 591] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 11. | Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011;31:993-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Shi HY, Zhao XS, Miao F. Metastases to the Pancreas: Computed Tomography Imaging Spectrum and Clinical Features: A Retrospective Study of 18 Patients With 36 Metastases. Medicine (Baltimore). 2015;94:e913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Burk KS, Lo GC, Gee MS, Sahani DV. Imaging and Screening of Pancreatic Cancer. Radiol Clin North Am. 2017;55:1223-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce AA, Di Carlo V. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol. 2008;15:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | van der Pol CB, Lee S, Tsai S, Larocque N, Alayed A, Williams P, Schieda N. Differentiation of pancreatic neuroendocrine tumors from pancreas renal cell carcinoma metastases on CT using qualitative and quantitative features. Abdom Radiol (NY). 2019;44:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Radu EC, Saizu AI, Grigorescu RR, Croitoru AE, Gheorghe C. Metastatic neuroendocrine pancreatic tumor - Case report. J Med Life. 2018;11:57-61. [PubMed] |
| 17. | Koyama H, Maruta T, Kudo T, Mayahara H, Yoshida K. Multiple pancreatic metastases from clear cell renal carcinoma: diagnosis with chemical shift magnetic resonance imaging before surgery. Australas Radiol. 2005;49:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Kang TW, Kim SH, Lee J, Kim AY, Jang KM, Choi D, Kim MJ. Differentiation between pancreatic metastases from renal cell carcinoma and hypervascular neuroendocrine tumour: Use of relative percentage washout value and its clinical implication. Eur J Radiol. 2015;84:2089-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Hasan MK, Hawes RH. EUS-guided FNA of solid pancreas tumors. Gastrointest Endosc Clin N Am. 2012;22:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Cheng SK, Chuah KL. Metastatic Renal Cell Carcinoma to the Pancreas: A Review. Arch Pathol Lab Med. 2016;140:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Kusnierz K, Mrowiec S, Lampe P. Results of surgical management of renal cell carcinoma metastatic to the pancreas. Contemp Oncol (Pozn). 2015;19:54-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Schwarz L, Sauvanet A, Regenet N, Mabrut JY, Gigot JF, Housseau E, Millat B, Ouaissi M, Gayet B, Fuks D, Tuech JJ. Long-term survival after pancreatic resection for renal cell carcinoma metastasis. Ann Surg Oncol. 2014;21:4007-4013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Adler H, Redmond CE, Heneghan HM, Swan N, Maguire D, Traynor O, Hoti E, Geoghegan JG, Conlon KC. Pancreatectomy for metastatic disease: a systematic review. Eur J Surg Oncol. 2014;40:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Lauro S, Onesti EC, Righini R, Carbonetti F, Cremona A, Marchetti P. A Synchronous Pancreatic Metastasis from Renal Clear Cell Carcinoma, with Unusual CT Characteristics, Completely Regressed after Therapy with Sunitinib. Case Rep Med. 2014;2014:473431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Flippot R, Escudier B, Albiges L. Immune Checkpoint Inhibitors: Toward New Paradigms in Renal Cell Carcinoma. Drugs. 2018;78:1443-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Kamli H, Li L, Gobe GC. Limitations to the Therapeutic Potential of Tyrosine Kinase Inhibitors and Alternative Therapies for Kidney Cancer. Ochsner J. 2019;19:138-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Ballarin R, Spaggiari M, Cautero N, De Ruvo N, Montalti R, Longo C, Pecchi A, Giacobazzi P, De Marco G, D'Amico G, Gerunda GE, Di Benedetto F. Pancreatic metastases from renal cell carcinoma: the state of the art. World J Gastroenterol. 2011;17:4747-4756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |