Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.8922
Peer-review started: April 7, 2022
First decision: June 16, 2022
Revised: June 21, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: September 6, 2022
Processing time: 141 Days and 0.2 Hours
Lymphocytic choriomeningitis virus (LCMV) is a neglected rodent-borne arenavirus associated with transplacental transmission and fetal infection.
To summarize the epidemiological, clinical, and diagnostic features of reported patients with congenital LCMV infection.
A literature search was conducted in PubMed, Medline, Google Scholar, and ResearchGate. The keywords used were ‘congenital lymphocytic choriomeningitis virus,’ and 48 studies were included. In addition, we conducted a relevant search by Reference Citation Analysis (RCA) (https://www.referencecitationanalysis.com).
The results have shown 27 reports of congenital LCMV infection in 86 patients, with 52.73% of them being males. Patients presented with chorioretinitis (83.53%), hydrocephalus (54.12%), and psychomotor retardation or developmental delay (54.12%). Computed tomography and/or magnetic resonance imaging most often demonstrated ventriculomegaly (74.07%), periventricular calcifications (66.67%), and microcephaly (40%). Most mothers of congenitally infected infants were exposed to rodents during pregnancy, predominantly mice, with flu-like symptoms mainly occurring during the first two trimesters of gestation. Mortality in congenitally infected children was 16.47%. The diagnosis of congenital LCMV infection was confirmed serologically in most patients (86.67%).
LCMV is still an insufficiently recognized fetal teratogen that often leads to long-term neurologic sequelae. Clinicians need to be familiar with LCMV and its potential teratogenic effect and as well as to effectively differentiate LCMV from other TORCH (T: Toxoplasma gondii, O: Other pathogens, R: Rubella virus, C: Cytomegalovirus, H: Herpes simplex virus) pathogens.
Core Tip: Lymphocytic choriomeningitis virus (LCMV) is an under-recognized rodent-borne arenavirus associated with transplacental transmission and fetal infection. Patients often present with chorioretinitis, hydrocephalus, and neurologic sequelae. Maternal exposure to rodents during gestation is a risk factor for developing viral infection. The golden standard for diagnosis is the detection of LCMV antibodies in fetal and maternal serum samples. This mini-review systematically summarizes the epidemiological, clinical, and diagnostic features of 86 reported patients with confirmed congenital LCMV infection.
- Citation: Ferenc T, Vujica M, Mrzljak A, Vilibic-Cavlek T. Lymphocytic choriomeningitis virus: An under-recognized congenital teratogen. World J Clin Cases 2022; 10(25): 8922-8931
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/8922.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.8922
Lymphocytic choriomeningitis virus (LCMV) is a neglected rodent-borne arenavirus associated with acquired or congenital human infections. Armstrong and Lillie first described it in 1933 as a cause of aseptic meningitis[1]. In the following years, the house mouse (Mus musculus, M. domesticus) was found to be a natural reservoir host of the virus[2]. Nevertheless, other rodents such as bank vole, yellow-necked mice, pet hamsters, rats, and guinea pigs could also be the origin of infection in humans[3-6]. Most infections occur during autumn and winter, reflecting mice migration and invasion into human habitats during cold periods[7]. LCMV can be transmitted by inhalation or ingestion of infected rodent excreta in direct contact with rodents and their bites[5]. Person-to-person transmission has not been detected; however, there are reports of viral transmission among solid organ transplant recipients and transplacentally infected fetuses[8,9]. Congenital LCMV infection with a fatal outcome 12 d after birth was first reported in England in 1955[10]. Numerous patients diagnosed with congenital LCMV infection have been documented across Europe two decades later. They predominantly presented with hydrocephalus, chorioretinal degeneration, and long-term neurologic abnormalities[11-13]. In the early 1990s, the first case of congenital LCMV infection was reported in the United States[14]. Since then, sporadic cases have been documented every few years. Due to a lack of commercially available tests, the true prevalence of congenital LCMV infection is undetermined. This mini-review analyzed reported cases and systematically summarized the epidemiological, clinical, and diagnostic features of patients with confirmed congenital LCMV infection.
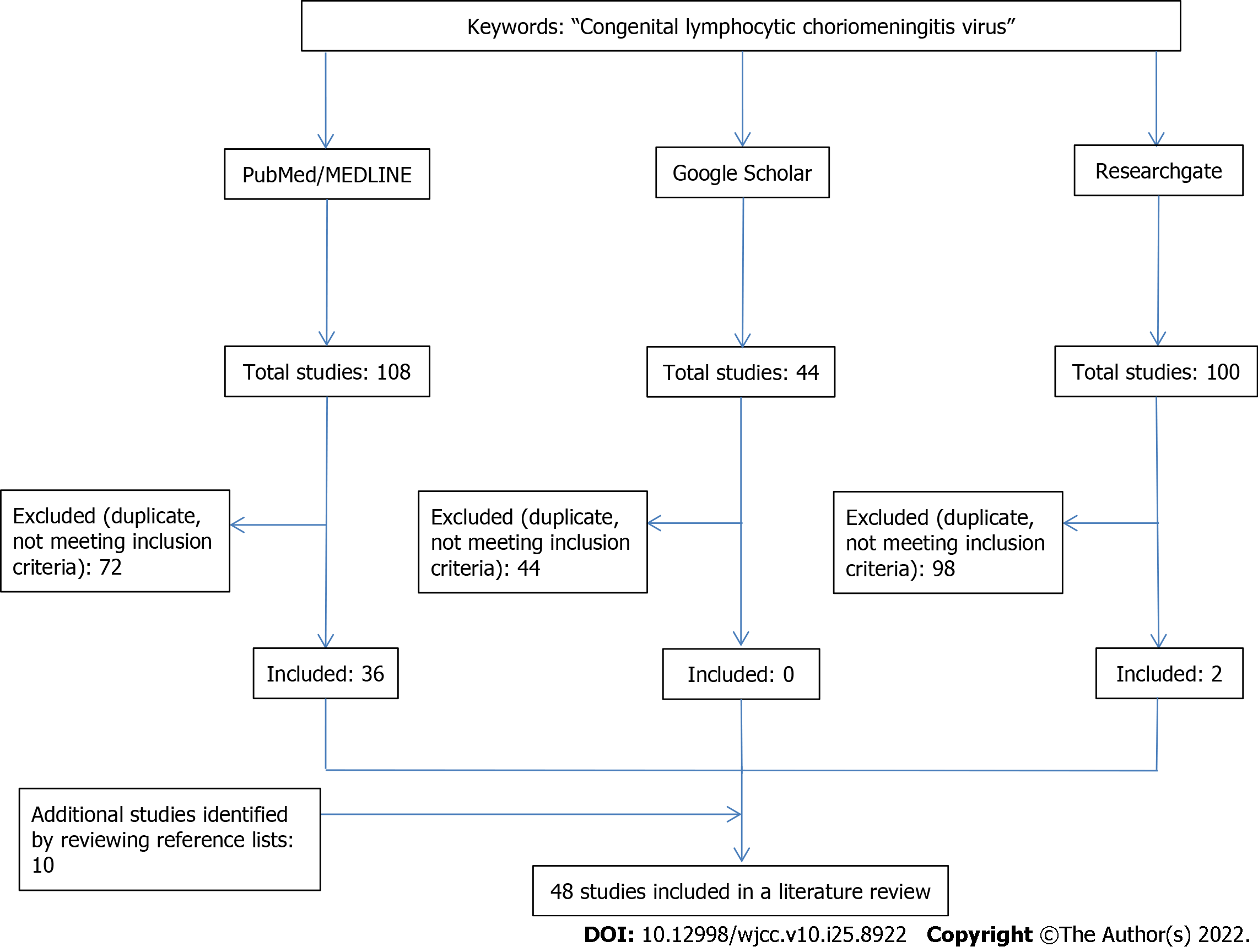
A literature search was conducted in PubMed, Medline, Google Scholar, and ResearchGate with no restrictions placed on the year of publication and study language. The used keywords included: ‘congenital lymphocytic choriomeningitis virus.’ A total of 252 articles were initially found. Studies involving animal models and duplicate papers were excluded. After the list of abstracts was assembled, studies appearing to meet inclusion criteria were reviewed in full. Additional studies were identified by reviewing reference lists of retrieved articles. Finally, 48 studies (original research articles, review articles, and case reports) were included (Figure 1). In addition, we conducted a relevant search by Reference Citation Analysis (RCA) (https://www.referencecitationanalysis.com).
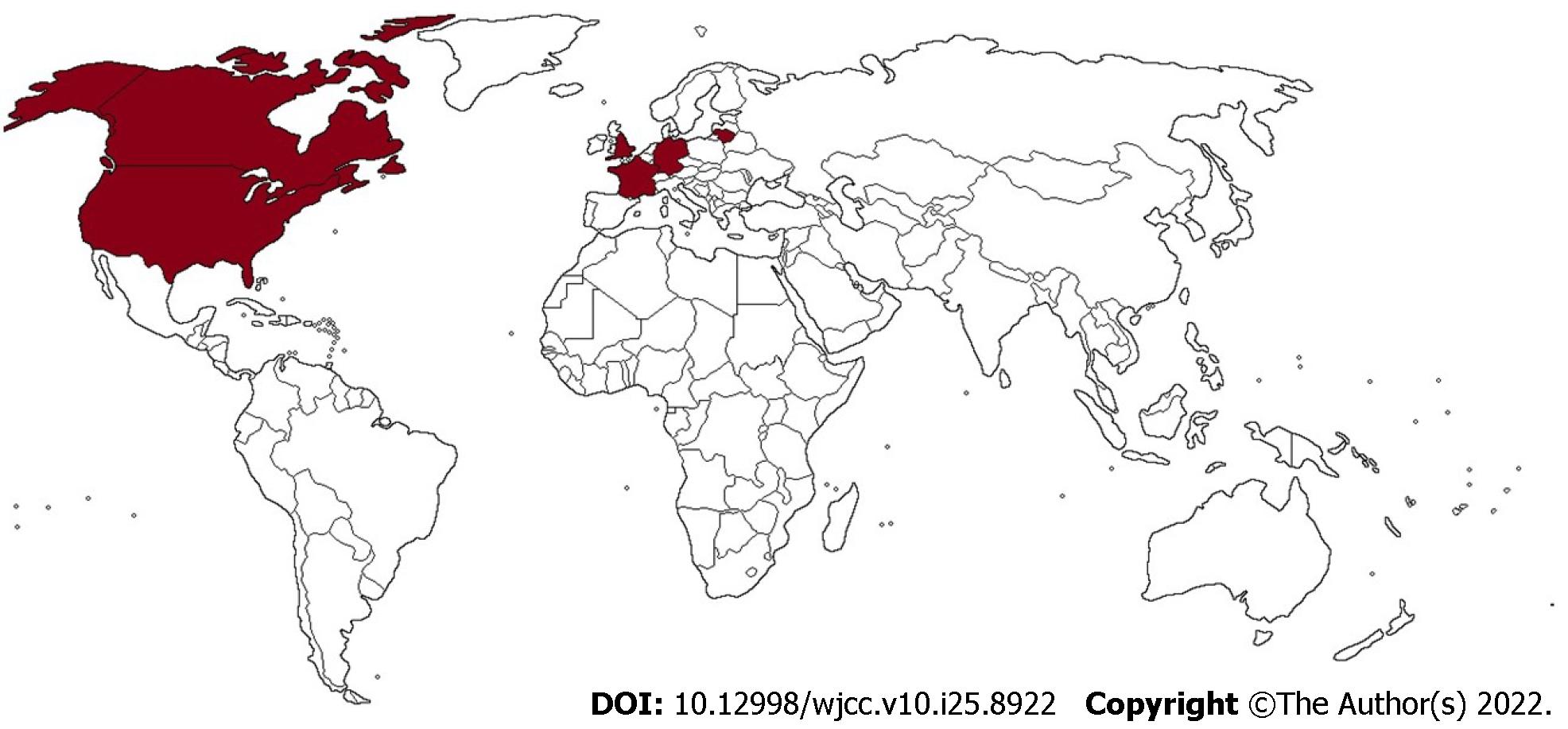
Until January 30, 2022, there have been 27 reports of congenital LCMV infection in 86 patients, mainly in the United States (Table 1 and Figure 2). In 70% of cases, pregnancies were full term (≥ 37 wk). The median birth weight of infected infants was 3080 g (interquartile range [IQR] 2550-3600 g), and 52.73% of them were males. Patients presented with chorioretinitis (83.53%), hydrocephalus (54.12%), psychomotor retardation or developmental delay (54.12%), microcephaly (38.82%), spastic quadriplegia (36.47%), epilepsy or epilepsy-like symptoms (35.29%), and optic nerve atrophy (21.18%). Visual and hearing impairment was documented in 18 patients. The median time to diagnosis was 2 mo after birth (IQR 8-270 d). Computed tomography (CT) and magnetic resonance imaging (MRI) scans most often displayed ventriculomegaly (74.07%), periventricular calcifications (66.67%), microcephaly (40%), gyral malformations (36.67%), and cerebral atrophy (22.22%). Serology was the mainstay for diagnosing congenital LCMV infection in 73 patients, whereas reverse transcription-polymerase chain reaction (RT-PCR) was used in only 2 patients. The indirect immunofluorescence assay (IFA) and enzyme-linked immunoassay (ELISA) were used almost equally (44% and 42.67%, respectively). Mortality in congenitally infected children was 16.47%, with four terminated pregnancies and one intrauterine death. The median age of infants at the time of decease was 19 d (IQR 8-90 d). Epidemiological and clinical features of maternal LCMV infections are presented in Table 2. A total of 43 mothers were serologically tested, and IFA was predominantly used compared to ELISA (69.77%, 23.26%, respectively). Serology tests were not performed in two mothers, and one mother was negative for LCMV infection, whereas serology data were not available in 38 cases.
| Country (year) | Cases, n | Clinical characteristics | CT/MRI imaging | Ref. |
| England (1955) | 1 | Fever, epilepsy-like symptoms, opisthotonus, intracranial hemorrhage, skin lesions, hydrocephalus | ND | Komrower et al[10], 1955 |
| Germany (1974, 1999) | 8 (2- twins) | Hydrocephalus, chorioretinitis, myopia, hyperbilirubinemia, developmental delay, heart failure, psychomotor retardation, epilepsy-like symptoms, dystrophia, microcephaly, visual impairment, intracranial calcifications | ND | Ackermann et al[11], 1974; Enders et al[37], 1999 |
| Lithuania (1976, 1981) | 22 | Hydrocephalus, spastic quadriplegia, epilepsy-like symptoms, chorioretinitis, optic nerve atrophy, psychomotor retardation, blepharoptosis, microphthalmy, cataract, cerebral palsy, microcephaly | ND | Sheinbergas[12], 1976; Sheinbergas et al[38], 1981 |
| France (1978, 2000, 2009, 2017) | 6 (2- twins)1 | Hydrocephalus, chorioretinitis, fetal hydrops, hepatosplenomegaly, cardiomegaly, microcephaly, abnormal gyration, cerebral atrophy, periventricular calcifications, ascites, anemia, thrombocythemia | MRI: normal | Chastel et al[13], 1978; |
| United States (1993, 1995-1997, 2000, 2002, 2003, 2006, 2007, 2013, 2014, 2017, 2018, 2021) | 47 (3- twins)2 | Microcephaly, optic nerve atrophy, nystagmus, chorioretinitis, developmental delay, hydrocephalus, microphthalmia, visual impairment, psychomotor retardation, periventricular calcifications, intracranial calcifications, nystagmus, exotropia, dolichocephaly, cortical thumb, skin lesions, spastic quadriplegia, epilepsy-like symptoms, esotropia, heart abnormality (single ventricle with pulmonary atresia), glaucoma, conjunctivitis, clinodactyly, hearing impairment, progressive supranuclear palsy, epilepsy, ataxia, spastic diplegia, fetal hydrops, ascites, cataract, retinal coloboma | CT: subependymal calcifications, cerebral atrophy, ventriculomegaly, ependymal calcifications, calcification of the lens, periventricular calcifications, shizencephaly, microcephaly, colpocephaly, encephalomalacia, gyral malformations, cerebellar dysgenesis, periventricular cysts, basal ganglia, and parenchymal calcifications MRI: ventriculomegaly and ventricular dysmorphology, cerebral atrophy, gyral malformations, corpus callosum atrophy, brain stem and cerebellum displacement, ‘migration disorder’, prominent cortical sulci, cortical lesions, cerebellar dysgenesis, agenesis of the corpus callosum colpocephaly, agenesis of the septum pellucidum, periventricular calcifications, microcephaly, encephalomalacia, porencephaly, periventricular cysts, fetal hydrops, ascites, intracranial hemorrhage, subependymal calcifications, porencephalic cysts | Larsen et al[14], 1993; Barton et al[41], 1993; Barton[42], 1995; Barton et al[43], 1996; Wright et al[17], 1997; Bechtel et al[44], 1997; Mets et al[24], 2000; Barton et al[45], 2002; Greenhow et al[25], 2003; Schulte et al[15], 2006; Yu et al[35], 2006; Bonthius et al[19], 2007; El Feghaly et al[27], 2013; Anderson et al[20], 2014; Bou Ghannam et al[46], 2017; Kinori et al[22], 2018; Ansari et al[47], 2021 |
| Canada, 2015 | 2 | Microcephaly, abnormal gyration, hydrocephalus, pontocerebellar hypoplasia | ND | Fallet-Bianco et al[48], 2015 |
| Characteristic | Prevalence |
| Rodent exposure | 71.11% |
| Type of rodent | |
| Mice | 44.44% |
| Hamster | 8.89% |
| Rat | 4.44% |
| Mice + hamster | 8.89% |
| Mice + rat | 4.44% |
| Flu-like illness | 60.71% |
| First symptoms (trimester) | |
| First | 27.27% |
| Second | 33.33% |
| Third | 6.06% |
| No symptoms | 33.33% |
Seroprevalence studies conducted in the general population have shown that up to 15% of individuals are LCMV seropositive. In rodents, LCMV antibodies have been detected in 2.90% to 66% of mice and 0.40% to 25% of rats[9]. However, the true prevalence of congenital LCMV infection is still unknown. Congenital LCMV infection is associated with transplacental transmission of the virus to the fetal central nervous system during maternal viremia[15]. Sheinbergas et al[12] conducted a serologic study in 833 healthy newborns, 110 infants under the age of 2 with various neurologic symptoms, and 40 infants under the age of 1 with hydrocephalus. Among the patients’ selected groups, the prevalence of LCMV antibodies was 0.8%, 2.7%, and 30%, respectively. A recent study by Enninga and Theiler[16] used human placental explants infected with LCMV to model viral infection and observe differences in the innate immune response during the first and the third trimester of pregnancy. Viral replication was detected in the first trimester, whereas it was absent in the third trimester placentae, which was in accordance with the findings of a more robust immune response of human placental tissue to LCMV infection in the third trimester compared to the first trimester. These findings may explain a decrease in transplacental transmission of the virus and subsequent less severe congenital manifestations in the later stages of gestation. LCMV demonstrates a strong neurotropism, especially for neuroblasts. Infection of mitotically active neuroblasts in the periventricular region of the human fetal brain can explain findings of periventricular calcifications during CT/MRI examinations[9]. Viral replication in ependymal cells and periventricular germinal matrix results in inflammation and cell necrosis, leading to necrotizing ependymitis, aqueductal obstruction, and development of hydrocephalus and intracranial lesions[15,17]. Gyral malformations in congenitally infected children can be explained by LCMV disruption of neuronal migrations[9]. Brain tissue analysis of deceased neonates unveiled lymphocytic infiltration, encephalomalacia, glial proliferation, and perivascular edema[18]. Other histologically examined tissues revealed lymphocytic myocarditis and extramedullary hematopoiesis[18].
According to the analyzed results, 70% of pregnancies were full term, and the median birth weight of infected infants was 3080 g. A study by Wright et al[17] reviewed reported cases of congenital LCMV infection up to that time. Most infants were the product of term gestation, and their median birth weight was 3520 g. In another study, 14 of 20 infected newborns had birth weight appropriate for gestational age[19]. These data suggest that congenitally acquired LCMV infection does not cause significant intrauterine growth restriction. This review’s descriptions of clinical manifestations were available for 85 congenitally infected children. Most of them presented with neurologic manifestations: chorioretinitis, hydrocephalus, psychomotor retardation or developmental delay, microcephaly, spastic quadriplegia, epilepsy or epilepsy-like symptoms, and optic nerve atrophy. These findings were expected since LCMV infection transmitted in utero damages the brain and retina in 87.50% of cases[20]. Besides the above-mentioned, ocular findings also included visual impairment (12 patients), nystagmus (5), esotropia (3), microphthalmia (2), exotropia (2), cataract (2), blepharoptosis (1), glaucoma (1), conjunctivitis (1), and retinal coloboma (1). Previous studies have shown that chorioretinitis is the most common manifestation of congenital LCMV infection in 88%-100% of patients[17-19,21]. Based on 34 eye examinations in 17 reported United States cases, generalized chorioretinal scars in the periphery (71%) and macular chorioretinal scars (29%) were the most prevalent findings, followed by optic nerve atrophy and nystagmus (24%). Hearing loss is seldom associated with congenital LCMV infection[7,20,22], and to date, it has been documented in only 6 patients (7.06%). In the review by Cohen et al[23], a similar incidence was noted (7.40% of cases), while the hearing deficits were often bilateral. In a study by Bonthius et al[19], the auditory sensation was preserved in 15 of 18 evaluated children. A low number of detected hearing deficits in infected infants may be due to under-diagnosis; therefore, a baseline auditory assessment in these patients is recommended[20]. Among other rare features of congenital LCMV infection, 3 patients presented with fetal hydrops, 3 with skin lesions, 2 with splenomegaly or hepatosplenomegaly, and 1 with heart abnormality (single ventricle with pulmonary atresia), and 1 with limb dysplasia (clinodactyly)[17,20,24,25].
Imaging techniques such as CT and/or MRI have been used to assess structural intracranial anomalies in patients with congenital LCMV infection. The most common findings were periventricular calcifications, ventriculomegaly, microcephaly, and gyral malformations. CT scans have also displayed parenchymal, ependymal, or subependymal calcifications (7 patients in total), encephalomalacia (3), cerebellar hypoplasia (2), shizencephaly (1), and colpocephaly (1). MRI demonstrated cerebellar dysgenesis (6), colpocephaly (3), encephalomalacia (2), agenesis of the septum pellucidum or corpus callosum (2), migration disorders (1), and porencephaly (1). In a study from 2007, Bonthius et al[19] reported similar findings on a sample size of 20 patients. By the time of birth, many of newborns with congenital LCMV infection no longer harbor the virus; therefore, in these cases, serological testing is the mainstay for the diagnosis[9]. However, transplacentally transferred maternal immunoglobulin G (IgG) antibodies may interfere with serology results, and for this reason, it is advised to include both IgM and IgG titers on both infant and maternal serum samples[9]. IFA and ELISA were used almost equally in the reported cases, while RT-PCR detected LCMV in 2 infected infants. The usual gene target for RT-PCR was LCMV nucleoprotein[26]. Information regarding outcomes was available in 85 children. There were 14 deaths in documented cases, including four terminated pregnancies and one intrauterine death. In total, mortality in congenitally infected children was 16.47%. This data differed from the previously reported mortality rate of 35%[17]. A possible explanation is a larger number of confirmed cases and better recognition due to the greater availability of different diagnostic methods. Long-term neurologic sequelae after congenital LCMV infection are common and may be severe in 66-67% of patients[27,28]. In this review, some form of developmental delay or psychomotor retardation was present in 63.38%, epilepsy or epilepsy-like symptoms in 35.21%, and spastic quadriplegia in 33.80% of children.
In a study by Bonthius et al[19], 12 of 20 women who gave birth to congenitally infected children with LCMV were exposed to mice during pregnancy, and the same number of mothers developed flu-like illness during gestation. A study by Vilibic-Cavlek et al[29] showed that the significant predictors for LCMV seropositivity were the presence of rodents in the house or yard or cleaning their nests. The risk of LCMV infection in individuals who reported such information was three times higher[29]. Data regarding rodent exposure, development of flu-like illness, and the period (trimester) of first symptoms were available in 45, 46, and 33 cases, respectively. This review showed that 71.11% of mothers reported exposure to rodents, 44.44% mice. The flu-like illness developed in 60.71% of women. According to the available studies, transplacental LCMV infection primarily occurs during the first and second trimesters[5]. In addition, acquired maternal LCMV infection during the first trimester has been associated with an increased risk of spontaneous abortion[9,18,27]. There is a limited number of studies about the prevalence of LCMV in pregnant women. Riera et al[30] found that 1.6% of Argentinian mothers have been seropositive to LCMV, but the absence of LCMV antibodies in the newborn excluded infection during pregnancy. A French study found no positive serology in 155 maternal serum samples[31]. Similar results were obtained in the recent Croatian study, where 3.9% of pregnant women have been seropositive to LCMV but with no detection of IgM antibodies[29].
Due to similar clinical symptoms, the major pathogens of expanded TORCH (T: Toxoplasma gondii, O: Other pathogens, R: Rubella virus, C: Cytomegalovirus [CMV], H: Herpes simplex virus [HSV]) acronym (parvovirus B-19, varicella-zoster virus [VZV], and Treponema pallidum) should be included in the differential diagnosis of congenital LCMV infection[18,32,33]. Congenital toxoplasmosis and congenital LCMV infection may significantly overlap in clinical presentation since both can cause microcephaly or macrocephaly, intracranial calcifications, and chorioretinitis[18,33]. However, congenital toxoplasmosis usually manifests with diffuse intracranial calcifications in contrast to congenital LCMV infection, which has been mostly associated with periventricular calcifications[18]. Parvovirus B-19 is a known cause of fetal hydrops. However, there have been several cases of fetal hydrops in infants with congenital LCMV infection, which must be taken into consideration in the differential diagnosis. Clinical manifestations of congenital varicella syndrome include chorioretinitis, optic nerve atrophy, microcephaly, hydrocephalus, limb hypoplasia, congenital cataract, microphthalmia, and Horner syndrome. The four latter features are rare in congenitally infected infants with LCMV[32,33]. Congenital rubella syndrome is associated with heart abnormalities (atrial and ventricular septal defects, patent ductus arteriosus), cataracts, and hearing loss, which are uncommon manifestations of LCMV. Generalized salt-and-pepper retinopathy, also a manifestation of congenital rubella syndrome, has never been documented in LCMV-infected infants[18,24,34]. Congenital CMV infection can be particularly difficult to differentiate from LCMV infection since its main ocular finding is chorioretinitis, which can also be combined with microcephaly or macrocephaly and intracranial calcifications[18,24,32-34]. However, fetal CMV infection is also associated with hepatosplenomegaly, hearing impairment, and skin lesions, which was a rarity in reported cases of congenital LCMV infection[18,33,34]. There may be some overlap between congenital HSV and LCMV ocular manifestations, yet acute retinal necrosis syndrome and scarring after HSV infection are quite distinctive from LCMV[24,34]. Characteristic signs of congenital syphilis include skin lesions, lymphadenopathy, hepatosplenomegaly, salt-and-pepper retinopathy, and bone abnormalities. All of these are infrequent or non-existent in congenital LCMV infection[18,24,33,34]. Reports have demonstrated systemic and ocular similarities between congenital LCMV infection and Aicardi syndrome, an X-linked chromosomal disorder fatal for males, occurring only in females[22,35]. The clinical features distinctive of Aicardi syndrome are hemivertebrae or fused vertebrae and agenesis of the corpus callosum[17,22,35]. However, Yu et al[35] have found agenesis of the corpus callosum in an infant boy who was congenitally infected with LCMV. Therefore, in patients suspected of having Aicardi syndrome, besides genetic testing, it is advisable to perform serologic analysis for LCMV antibodies[22,35]. In terms of genetic disorders, congenital LCMV infection must not be mistaken for Aicardi-Goutières syndrome, a completely distinct from similarly named Aicardi syndrome. The syndrome has four known genotypes, and it is distinguished from congenital LCMV infection by progressive clinical course, worsening of acute neurological episodes, high levels of interferon alpha in cerebrospinal fluid, and intracranial calcifications mainly located in basal ganglia[36].
Effective antiviral therapy for congenital LCMV infection has yet to be developed. Ribavirin was the first to demonstrate inhibitory activity against LCMV in vitro, however, clinical trials have not confirmed its efficacy and is limited to off-label use only, particularly due to possible teratogenic effects[5]. During the past decade, favipiravir has emerged as a promising antiviral agent with low cytotoxicity and robust in vitro activity against arenaviruses but with no clinical trials to determine the anti-LCMV effect to this date[7]. Most recent in vitro studies also showed that umifenovir and human monoclonal antibodies may be possible therapeutic options against LCMV[9].
This literature mini-review has some limitations regarding certain unavailability of previously discussed data, and potential conclusions were drawn from analysis of small size samples. Further studies with a larger number of participants are needed to better understand congenital LCMV infection.
In summary, LCMV is a rodent-borne arenavirus that should be recognized as an emerging fetal teratogen and included in the TORCH acronym. There have been 86 patients with congenital LCMV infection reported to date, mainly presenting with neurologic symptoms and long-term developmental disorders. Maternal exposure to rodents during pregnancy is a risk factor for developing LCMV infection and consequent transplacental transmission of the virus. The mainstay of diagnosis is the detection of LCMV antibodies in fetal and maternal serum samples. Specific epidemiological, clinical, and radiological findings differentiate LCMV from other congenital pathogens. Primary prevention of congenital LCMV infection is crucial, with a need for improvement in public education about reducing rodent household migrations and avoiding unnecessary contact with infected rodents and their excreta. Furthermore, clinicians should also become more familiar with this pathogen and its importance in congenital infections. In cases of unresolved fetal hydrocephalus and/or chorioretinitis, the diagnosis of congenital LCMV infection should always be suspected.
Lymphocytic choriomeningitis virus (LCMV) is a rodent-borne arenavirus that can be transmitted transplacentally and cause congenital infection.
Data on LCMV infection are scarce.
To summarize the epidemiological, clinical, and diagnostic features of reported patients with congenital LCMV infection.
A literature search was conducted in PubMed, Medline, Google Scholar, and Researchgate using ‘congenital lymphocytic choriomeningitis virus’ keywords.
In this mini-review, 48 studies (original research articles, review articles, and case reports) describing 86 children with congenital LCMV infection from 1955 to 2021 were included. Patients were from England (the first reported case), United States, Germany, Lithuania, France, and Canada. The main clinical presentations were chorioretinitis (83.53%), hydrocephalus (54.12%), and psychomotor retardation or developmental delay (54.12%). The most common findings on computed tomography/magnetic resonance imaging scans were ventriculomegaly (74.07%) and periventricular calcifications (66.67%). Congenitally infected children showed a mortality rate of 16.47%, with four terminated pregnancies and one intrauterine death.
Children with congenital LCMV infection mainly presented with neurologic symptoms and long-term developmental disorders. LCMV should be considered in the differential diagnosis in cases of unresolved fetal hydrocephalus and/or chorioretinitis.
Further studies on congenital LCMV infections are needed to determine the prevalence and clinical significance of this neglected viral pathogen.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bieńkowski C, Poland; Routray S, India S-Editor: Wang LL L-Editor: Filipodia A P-Editor: Wang LL
| 1. | Armstrong C, Lillie RD. Experimental lymphocytic choriomeningitis of monkeys and mice produced by a virus encountered in studies of the 1933 St. Louis encephalitis epidemic. Public Health Rep. 1934;49:1019-1027. [DOI] [Full Text] |
| 2. | Traub E. A FILTERABLE VIRUS RECOVERED FROM WHITE MICE. Science. 1935;81:298-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 79] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Tagliapietra V, Rosà R, Hauffe HC, Laakkonen J, Voutilainen L, Vapalahti O, Vaheri A, Henttonen H, Rizzoli A. Spatial and temporal dynamics of lymphocytic choriomeningitis virus in wild rodents, northern Italy. Emerg Infect Dis. 2009;15:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Barton LL, Mets MB. Lymphocytic choriomeningitis virus: pediatric pathogen and fetal teratogen. Pediatr Infect Dis J. 1999;18:540-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Jamieson DJ, Kourtis AP, Bell M, Rasmussen SA. Lymphocytic choriomeningitis virus: an emerging obstetric pathogen? Am J Obstet Gynecol. 2006;194:1532-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Barton LL, Hyndman NJ. Lymphocytic choriomeningitis virus: reemerging central nervous system pathogen. Pediatrics. 2000;105:E35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Bonthius DJ. Lymphocytic choriomeningitis virus: an underrecognized cause of neurologic disease in the fetus, child, and adult. Semin Pediatr Neurol. 2012;19:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Mrzljak A, Novak R, Pandak N, Tabain I, Franusic L, Barbic L, Bogdanic M, Savic V, Mikulic D, Pavicic-Saric J, Stevanovic V, Vilibic-Cavlek T. Emerging and neglected zoonoses in transplant population. World J Transplant. 2020;10:47-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 9. | Vilibic-Cavlek T, Savic V, Ferenc T, Mrzljak A, Barbic L, Bogdanic M, Stevanovic V, Tabain I, Ferencak I, Zidovec-Lepej S. Lymphocytic Choriomeningitis-Emerging Trends of a Neglected Virus: A Narrative Review. Trop Med Infect Dis. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Komrower GM, Williams BL, Stones PB. Lymphocytic choriomeningitis in the newborn; probable transplacental infection. Lancet. 1955;268:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Ackermann R, Körver G, Turss R, Wönne R, Hochgesand P. [Prenatal infection with the virus of lymphocytic choriomeningitis: report of two cases (author's transl)]. Dtsch Med Wochenschr. 1974;99:629-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Sheinbergas MM. Hydrocephalus due to prenatal infection with the lymphocytic choriomeningitis virus. Infection. 1976;4:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Chastel C, Bosshard S, Le Goff F, Quillien MC, Gilly R, Aymard M. Transplacental infection by lymphocytic choriomeningitis virus. Results of a retrospective serological study in France (author's transl). Nouv Presse Med. 1978;7:1089-1092. [PubMed] |
| 14. | Larsen PD, Chartrand SA, Tomashek KM, Hauser LG, Ksiazek TG. Hydrocephalus complicating lymphocytic choriomeningitis virus infection. Pediatr Infect Dis J. 1993;12:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Schulte DJ, Comer JA, Erickson BR, Rollin PE, Nichol ST, Ksiazek TG, Lehman D. Congenital lymphocytic choriomeningitis virus: an underdiagnosed cause of neonatal hydrocephalus. Pediatr Infect Dis J. 2006;25:560-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Enninga EAL, Theiler RN. Lymphocytic Choriomeningitis Virus Infection Demonstrates Higher Replicative Capacity and Decreased Antiviral Response in the First-Trimester Placenta. J Immunol Res. 2019;2019:7375217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Wright R, Johnson D, Neumann M, Ksiazek TG, Rollin P, Keech RV, Bonthius DJ, Hitchon P, Grose CF, Bell WE, Bale JF Jr. Congenital lymphocytic choriomeningitis virus syndrome: a disease that mimics congenital toxoplasmosis or Cytomegalovirus infection. Pediatrics. 1997;100:E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Barton LL, Mets MB. Congenital lymphocytic choriomeningitis virus infection: decade of rediscovery. Clin Infect Dis. 2001;33:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Bonthius DJ, Wright R, Tseng B, Barton L, Marco E, Karacay B, Larsen PD. Congenital lymphocytic choriomeningitis virus infection: spectrum of disease. Ann Neurol. 2007;62:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Anderson JL, Levy PT, Leonard KB, Smyser CD, Tychsen L, Cole FS. Congenital lymphocytic choriomeningitis virus: when to consider the diagnosis. J Child Neurol. 2014;29:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Mets MB. Childhood blindness and visual loss: an assessment at two institutions including a ‘new’ cause. Trans Am Ophthalmol Soc. 1999;97:653-696. [PubMed] |
| 22. | Kinori M, Schwartzstein H, Zeid JL, Kurup SP, Mets MB. Congenital lymphocytic choriomeningitis virus-an underdiagnosed fetal teratogen. J AAPOS. 2018;22:79-81.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Cohen BE, Durstenfeld A, Roehm PC. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 24. | Mets MB, Barton LL, Khan AS, Ksiazek TG. Lymphocytic choriomeningitis virus: an underdiagnosed cause of congenital chorioretinitis. Am J Ophthalmol. 2000;130:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Greenhow TL, Weintrub PS. Your diagnosis, please. Neonate with hydrocephalus. Pediatr Infect Dis J. 2003;22:1099, 1111-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Rawlinson WD, Hall B, Jones CA, Jeffery HE, Arbuckle SM, Graf N, Howard J, Morris JM. Viruses and other infections in stillbirth: what is the evidence and what should we be doing? Pathology. 2008;40:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | El Feghaly RE, Hunstad DA. A Newborn With Hydrops, Hydrocephalus, and Ophthalmologic Abnormalities. J Pediatric Infect Dis Soc. 2013;2:391-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Delaine M, Weingertner AS, Nougairede A, Lepiller Q, Fafi-Kremer S, Favre R, Charrel R. Microcephaly Caused by Lymphocytic Choriomeningitis Virus. Emerg Infect Dis. 2017;23:1548-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Vilibic-Cavlek T, Oreski T, Korva M, Kolaric B, Stevanovic V, Zidovec-Lepej S, Tabain I, Jelicic P, Miklausic-Pavic B, Savic V, Barbic L, Avsic-Zupanc T. Prevalence and Risk Factors for Lymphocytic Choriomeningitis Virus Infection in Continental Croatian Regions. Trop Med Infect Dis. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Riera L, Castillo E, Del Carmen Saavedra M, Priotto J, Sottosanti J, Polop J, Ambrosio AM. Serological study of the lymphochoriomeningitis virus (LCMV) in an inner city of Argentina. J Med Virol. 2005;76:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Hannachi N, Freymuth F, Luton D, Herlicoviez M, Oury JF, Boukadida J, Lebon P. [Lymphocytic choriomeningitis virus and fetal anomalies]. Pathol Biol (Paris). 2011;59:e85-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Mets MB, Chhabra MS. Eye manifestations of intrauterine infections and their impact on childhood blindness. Surv Ophthalmol. 2008;53:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Bale JF Jr. Congenital infections. Neurol Clin. 2002;20:1039-1060, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Mets MB. Eye manifestations of intrauterine infections. Ophthalmol Clin North Am. 2001;14:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Yu JT, Culican SM, Tychsen L. Aicardi-like chorioretinitis and maldevelopment of the corpus callosum in congenital lymphocytic choriomeningitis virus. J AAPOS. 2006;10:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Farmer M, Sébire G. Genetic mimics of congenital lymphocytic choriomeningitis virus encephalitis. Ann Neurol. 2008;64:353-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Enders G, Varho-Göbel M, Löhler J, Terletskaia-Ladwig E, Eggers M. Congenital lymphocytic choriomeningitis virus infection: an underdiagnosed disease. Pediatr Infect Dis J. 1999;18:652-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Sheinbergas MM, Lewis VJ, Thacker WL, Verikiene VV. Serological diagnosis in children infected prenatally with lymphocytic choriomeningitis virus. Infect Immun. 1981;31:837-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Meritet JF, Krivine A, Lewin F, Poissonnier MH, Poizat R, Loget P, Rozenberg F, Lebon P. A case of congenital lymphocytic choriomeningitis virus (LCMV) infection revealed by hydrops fetalis. Prenat Diagn. 2009;29:626-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Brézin AP, Thulliez P, Cisneros B, Mets MB, Saron MF. Lymphocytic choriomeningitis virus chorioretinitis mimicking ocular toxoplasmosis in two otherwise normal children. Am J Ophthalmol. 2000;130:245-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Barton LL, Budd SC, Morfitt WS, Peters CJ, Ksiazek TG, Schindler RF, Yoshino MT. Congenital lymphocytic choriomeningitis virus infection in twins. Pediatr Infect Dis J. 1993;12:942-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Barton LL, Peters CJ, Ksiazek TG. Lymphocytic choriomeningitis virus: an unrecognized teratogenic pathogen. Emerg Infect Dis. 1995;1:152-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Barton LL, Peters CJ, Seaver LH, Chartrand SA. Congenital lymphocytic choriomeningitis virus infection. Arch Pediatr Adolesc Med. 1996;150:440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Bechtel RT, Haught KA, Mets MB. Lymphocytic choriomeningitis virus: a new addition to the TORCH evaluation. Arch Ophthalmol. 1997;115:680-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Barton LL, Mets MB, Beauchamp CL. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am J Obstet Gynecol. 2002;187:1715-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Bou Ghannam A, Chang T, Jantausch BA, Vézina G, Miller M. Congenital Lymphocytic Choriomeningitis Virus in a Member of a Twin Pregnancy. J Pediatr Neurol. 2017;15:076-079. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 47. | Ansari N, Demmler-Harrison G, Coats DK, Paysse EA. Severe congenital chorioretinitis caused by congenital lymphocytic choriomeningitis virus infection. Am J Ophthalmol Case Rep. 2021;22:101094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 48. | Fallet-Bianco C. Congenital Lymphocytic Choriomeningitis Virus: A Neuropathological Study. Can J Neurol Sci. 2015;42:S4-S4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |