Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.8906
Peer-review started: January 25, 2022
First decision: May 9, 2022
Revised: May 25, 2022
Accepted: July 22, 2022
Article in press: July 22, 2022
Published online: September 6, 2022
Processing time: 213 Days and 0.2 Hours
Early quantitative assessment of liver fat content is essential for patients with fatty liver disease. Mounting evidence has shown that magnetic resonance (MR) technique has high accuracy in the quantitative analysis of fatty liver, and is suitable for monitoring the therapeutic effect on fatty liver. However, many packaging methods and postprocessing functions have puzzled radiologists in clinical applications. Therefore, selecting a quantitative MR imaging technique for patients with fatty liver disease remains challenging.
To provide information for the proper selection of commonly used quantitative MR techniques to quantify fatty liver.
We completed a systematic literature review of quantitative MR techniques for detecting fatty liver, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol. Studies were retrieved from PubMed, Embase, and Cochrane Library databases, and their quality was assessed using the Quality Assessment of Diagnostic Studies criteria. The Reference Citation Analysis database (https://www.referencecitationanalysis.com) was used to analyze citation of articles which were included in this review.
Forty studies were included for spectroscopy, two-point Dixon imaging, and multiple-point Dixon imaging comparing liver biopsy to other imaging methods. The advantages and disadvantages of each of the three techniques and their clinical diagnostic performances were analyzed.
The proton density fat fraction derived from multiple-point Dixon imaging is a noninvasive method for accurate quantitative measurement of hepatic fat content in the diagnosis and monitoring of fatty liver progression.
Core Tip: This study focused on properly selecting commonly used quantitative magnetic resonance (MR) techniques to quantify fatty liver disease. We completed a systematic literature review of quantitative MR techniques for detecting fatty liver, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol. Three techniques including spectroscopy, two-point Dixon imaging, and multiple-point Dixon imaging, were compared. We found that proton density fat fraction derived from multiple-point Dixon imaging is a noninvasive method for accurate quantitative measurement of hepatic fat content. It can be used to diagnose fatty liver disease and monitor disease progression as well as treatment effects.
- Citation: Li YW, Jiao Y, Chen N, Gao Q, Chen YK, Zhang YF, Wen QP, Zhang ZM. How to select the quantitative magnetic resonance technique for subjects with fatty liver: A systematic review. World J Clin Cases 2022; 10(25): 8906-8921
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/8906.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.8906
Fatty liver refers to the excessive accumulation of triglycerides within the cytoplasm of hepatocytes. Increased fat deposition in hepatocytes can cause hepatocyte injury, inflammation, fibrosis, and eventually cirrhosis, with a high risk of liver failure and hepatocellular carcinoma[1]. Therefore, early quantitative assessment of hepatic fat content is essential for patients with fatty liver disease.
Liver biopsy is the gold standard for assessing hepatic fat content[2]. This may increase the chances of sampling error commonly encountered in livers with inhomogeneous fat distribution, because only a small fraction of the entire liver is sampled. Additionally, it can cause complications, such as bleeding, infection, and death. More importantly, this operation cannot be repeated and is not conducive for longitudinal monitoring of disease progression[3].
Ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) are commonly used for noninvasive examination of fatty liver. Ultrasound is easy to perform. Quantitative measurements were performed using the attenuation and backscatter coefficients. However, its accuracy in staging fatty liver is low because the images are blurred by hepatic parenchymal structures and ultrasound beam is attenuated significantly by the fatty liver[4], especially in obese patients[5]. Additionally, ultrasound is highly operator-dependent and has low reproducibility[4]. CT evaluation of fatty liver is based on the absolute CT value of liver parenchyma or relative attenuation difference between liver parenchyma and spleen[6,7]. When the threshold was 42 Hounsfield units, the sensitivity and specificity for grade 2–3 fatty liver were 73% and 100%, respectively[8]. The energy spectrum of fat is similar to that of the liver parenchyma in dual-energy CT examination. Therefore, its accuracy in diagnosing fatty liver is lower than that of conventional CT[6]. Moreover, CT exposes patients to radiation and is thus not advisable for repeated use. Various MR techniques have been developed for the quantitative assessment of signal fat fraction (SFF) and/or proton density fat fraction (PDFF). SFF is defined as the signal from fat divided by the combined signal from fat and water. This is measured using fat-suppressed techniques or chemical shift–encoded imaging (CSI) and MR spectroscopy (MRS) techniques[9]. This measurement is biased by one or more confounding factors. Once all confounding factors have been addressed, SFF is equivalent to PDFF[10]. PDFF, which can be measured with MRS or CSI, reflects the true fat content in tissue and thus, has become a reliable, accurate, and standardized MR-based biomarker for tissue fat accumulation. Mounting evidence has shown that MR has high accuracy in quantitatively analyzing fatty liver and can be repeated without radiation exposure[11-14]. However, many packaging methods and postprocessing functions have puzzled radiologists in clinical applications. This study compiled widespread data on MR techniques. This study aimed to provide information for properly selecting quantitative MR techniques to visualize the fatty liver.
A systematic review of the literature was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[15]. Literature from 1984 to 2021 was searched in PubMed, Embase, and Cochrane Library. The Reference Citation Analysis database (https://www.referencecitationanalysis.com) was used to analyze citation of articles which were included in this review. Combined MeSH and free text were used as retrieval strategies (Supplementary Table 1). Only the studies published in English were included. To ensure literature saturation, we scrutinized the reference lists of the included studies. The inclusion criteria were as follows: (1) Studies limited to human participants; (2) Studies related to the principles of MR techniques or systemic review and meta-analysis for measuring hepatic fat content; and (3) Studies involving comparisons of MR techniques with other methods (liver biopsy, ultrasound, or CT) to measure hepatic fat content. Studies conducted on animals, those without full text, review papers, conference proceedings, and case reports were excluded. The studies were independently screened by two authors, and study selection was decided by consensus.
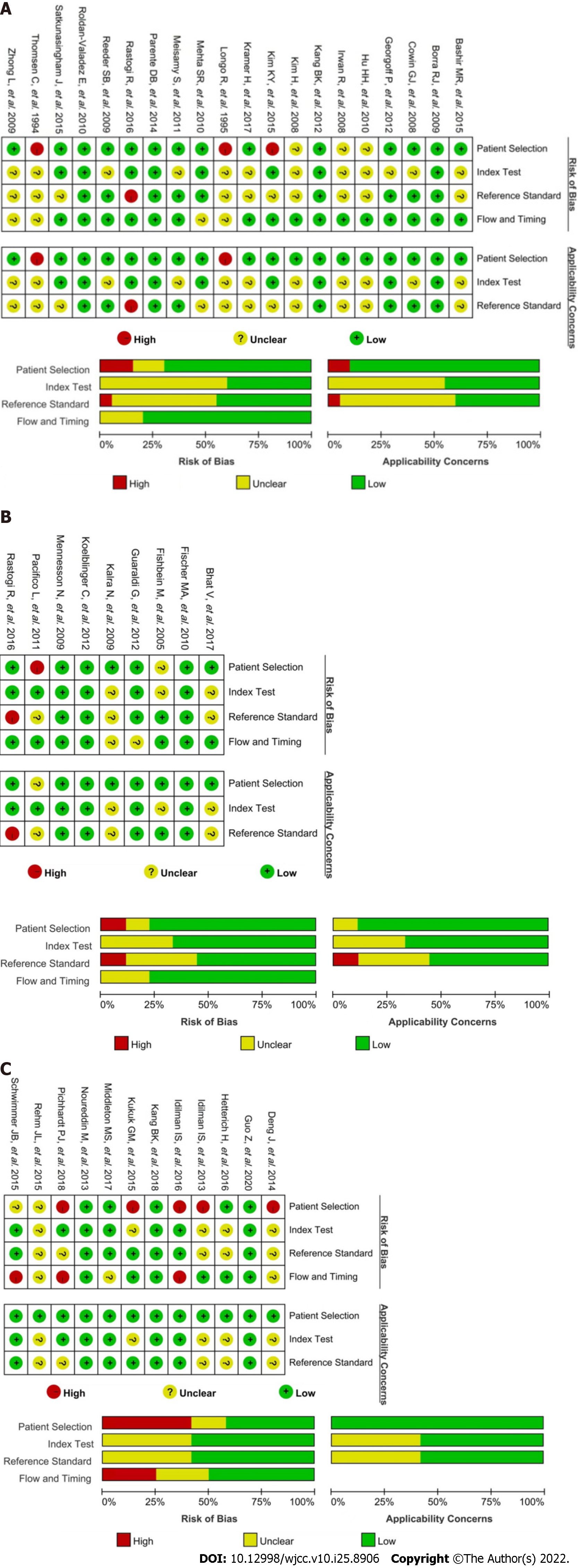
Two authors used the Quality Assessment of Diagnostic Studies (QUADAS)-2 criteria in RevMan 5.4 for judging the risk of bias independently. Each study was given a low, high, or unclear risk of bias (Supplementary material) following QUADAS-2 guidance in the four domains. The signaling question 2 in the first domain was replaced by “Was the study design prospective or retrospective” because a retrospective study had a relatively higher risk of bias[16]. Any disagreements were resolved by a third author.
The following data were extracted: First author, publication year, study design, number of patients, mean age, studied etiology, data on MR techniques such as field strength and scan sequences, comparison, interval between MR methods and comparison, and study outcomes. If a study reported multiple MR methods, the data from the main modality was extracted.
The principles, main technical factors, advantages, and disadvantages of each method were summarized and evaluated. The results of the studies with an overall low and moderate risk of bias were used to analyze the diagnostic performance of one of the three methods.
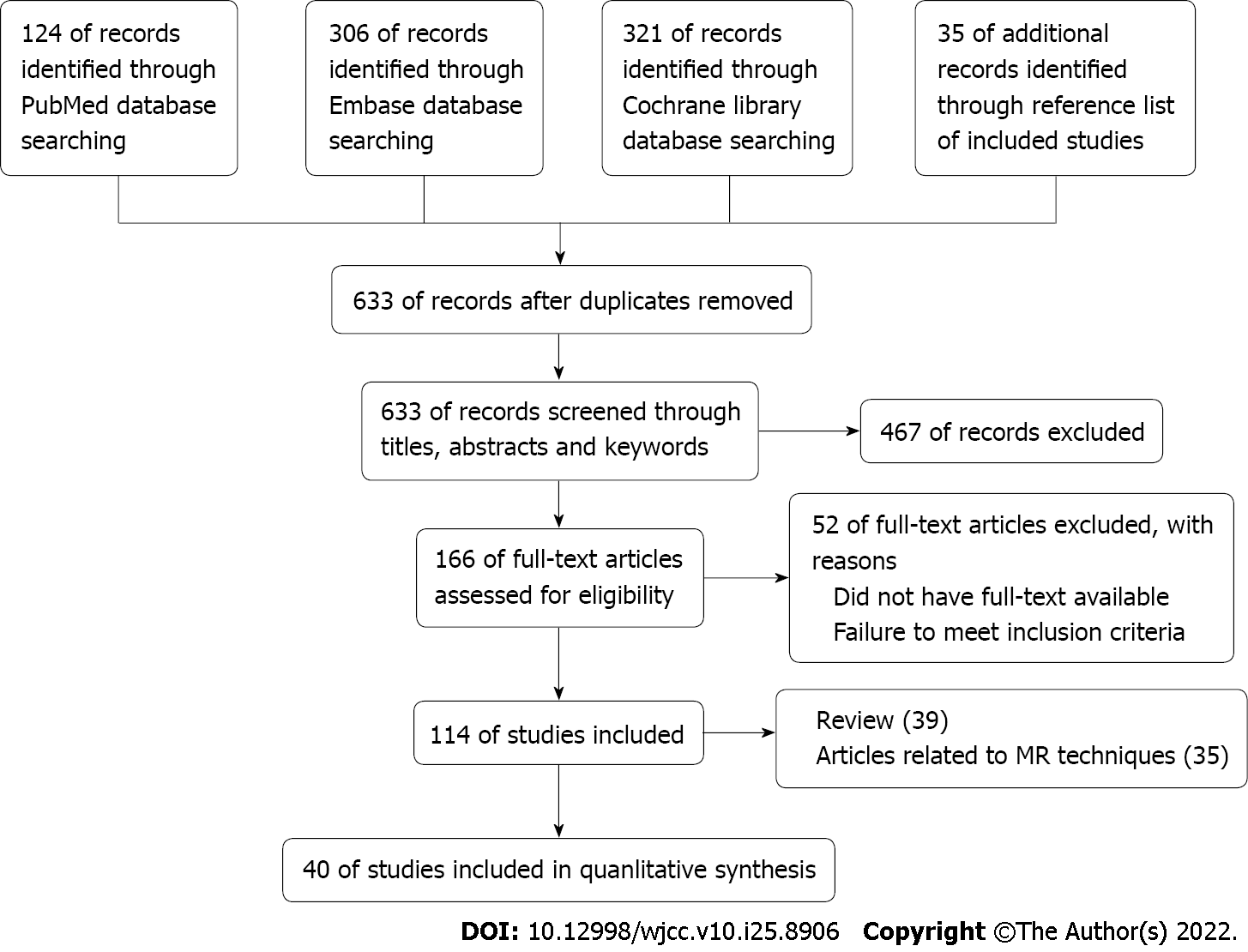
Electronic search identified 633 studies. Of these, 467 studies were excluded after reviewing the titles, abstracts, and keywords of each study. Another 52 studies were excluded after reading full-text articles. Of the 114 included studies, 35 were related to MR techniques, and 39 were reviews and meta-analyses. Consequently, 40 studies were used for further analysis, including 20, 9, and 12 studies for spectroscopy two-point Dixon imaging, and multiple-point Dixon imaging compared with other methods, respectively (Figure 1). Detailed data extraction for each study is shown in Tables 1-3.
| Ref. | Year | Study design | Age (year) | N | Etiology | Field strength sequence | Comparison | Interval | Results |
| Thomsen et al[22] | 1994 | 48 | 14 | Fatty liver | 1.5 T. STEAM (TE = 34 ms) | Liver biopsy | r = 0.897; P < 0.001 | ||
| Longo et al[26] | 1995 | 45 | 29 | Diffuse steatosis | 1.5 T. PRESS (TE = 50–200 ms) | Liver biopsy | r = 0.70 | ||
| Cowin et al[30] | 2008 | 42 | 12 | Steatosis | 1.5 T. PRESS (TE = 30 ms) | Liver biopsy | 6 wk | r = 0.928; P < 0.0001 | |
| Irwan et al[63] | 2008 | Prospective | 47 | 10 | Healthy volunteers | 1.5 T. PRESS (TE = 30 ms) | Dual-echo imaging | One measurement session | r = 0.927. In the range 1%–10%, the MRI-determined the liver fat contents (corrected algorithm) are systematically higher, on average 4% (range: 2.1%–6.1%) than those obtained with MRS |
| Kim et al[64] | 2008 | Prospective | 15.9 ± 5.3 | 28 | Lean and obese | 1.5 TPRESS (TE = 20 ms) | Two-Point Dixon | r = 0.954; P < 0.001 | |
| Borra et al[65] | 2009 | Prospective | 62.8 ± 8.3 | 33 | Type 2 diabetes | 1.5 T. PRESS (TE = 25 ms) | IP/OP (Dixon) | r = 0.959–0.962; P < 0.001 | |
| Reeder et al[66] | 2009 | Prospective | 49.0 ± 12 | 31 | Suspected steatosis and unrelated reasons | 1.5 T. PRESS (TE = 25 ms) | IDEAL | r = 0.83 ± 0.05; P < 0.001. Intercept (1.76 ± 0.76%; P = 0.03) | |
| Zhong et al[31] | 2009 | 50 ± 12 | 36 | Fatty liver | 3.0 T. PRESS (TE = 144 ms) | 16-row multislice CT | r = –0.461; P = 0.005 | ||
| Hu et al[67] | 2010 | 16 | 3.0 T. PRESS (TE = 23 ms) | IDEAL | Slope = 0.90, intercept = 1.07%; r2 = 0.95, P < 0.001 | ||||
| Roldan-Valadez et al[68] | 2010 | 35 | 18 | Steatosis | 3.0 T | Liver biopsy | r = 0.876; P ≤ 0.001 | ||
| Mehta et al[32] | 2010 | 39.9 | 50 | Steatosis | 1.5 T. PRESS (TE = 135 ms) | Ultrasound | BMI > 30, sensitivity 96%; BMI ≤ 30, sensitivity 64% | ||
| Meisamy et al[23] | 2011 | Prospective | 40 | 55 | 1.5 T. STEAM (TE = 10, 20, 30, 40, and 50 ms) | IDEAL | r2 = 0.99 | ||
| Georgoff et al[69] | 2012 | Prospective | 50.6 | 52 | Steatosis | 3.0 T. PRESS (TE = 50 ms) | Liver biopsy | 15 ± 9 d | Diagnostic accuracy was (AUC: 0.95; 95%CI: 0.89–1.0) |
| Kang et al[18] | 2012 | Prospective | 54 | 56 | Steatosis | 1.5 T. STEAM (TE = 20, 30, 40, 50, and 60 ms) | Liver biopsy | 1–28 d | r = 0.95 |
| Parente et al[70] | 2014 | Prospective | 54 ± 9 | 73 | Nonalcoholic fatty liver disease | 3.0 T. PRESS (TE = 40 ms) | Liver biopsy | r = 0.767; P < 0.001 | |
| Bashir et al[71] | 2015 | Prospective | 55 ± 13.8 | 217 | Various hepatic diseases | 1.5 T. STEAM (TE = 12 ms) | Two-point Dixon | r = 0.61; P < 0.001 | |
| Kim et al[57] | 2015 | 52.8 ± 14 | 42 | Various hepatic diseases | 3.0 T. STEAM (TE = 12, 24, 36, 48, and 72 ms) | In- and opposed-phase echo pairs | r = 0.97 | ||
| Satkunasingham et al[72] | 2015 | Retrospective | 57.8 (12–83) | 156 | Various hepatic diseases | 3.0 T. STEAM (TE = 12, 24, 36, 48, and 72 ms) | MRI-PDFF | r = 0.977; P < 0.001 | |
| Rastogi et al[73] | 2016 | Retrospective | 32.5 | 73 | Steatosis | 3.0 T. STEAM (TE = 15, 20, 25, 30, and 35 ms) | Biopsy and surgery | ≤ 20 d | MRS correlated well with the histopathology results (r = 0.882). An accuracy of 96% and sensitivity of 94% |
| Kramer et al[6] | 2017 | Prospective | 57 ± 5 | 50 | Various hepatic diseases | 1.5 T. STEAM (TE = 10, 20, 30, 40, and 50 ms) | PDFF | r2 = 0.992; slope, 0.974; intercept, –0.943 |
| Ref. | Year | Study design | Age (year) | N | Etiology | Field strength sequence | Comparison | Interval | Results |
| Fishbein et al[35] | 2005 | 47 ± 10 | 38 | Various hepatic diseases | 1.5 T. IP/OP (Dixon) | Biopsy | 2 wk | r = 0.773, P < 0.001; Macrovesicular steatosis: r = 0.920, mixed steatosis: r = 0.605, P = 0.05 | |
| Kalra et al[74] | 2009 | Prospective | 41 ± 9.2 | 10 | Nonalcoholic fatty liver disease | 1.5 T. IP/OP (Dixon) | Biopsy | Provides data on fat infiltration without information of hepatic fibrosis | |
| Mennesson et al[41] | 2009 | Prospective | 52.5 | 40 | Various hepatic diseases | 1.5 T. IP/OP (Dixon) | Biopsy | Same day | r = 0.852; P < 0.0001 |
| Fischer et al[37] | 2010 | Prospective | 66 ± 12 | 23 | Various hepatic diseases | 1.5 T IP/OP (Dixon) | Biopsy and surgery | ≤ 10 d | r = 0.92; P < 0.0001 |
| Pacifico et al[75] | 2011 | Case–control | 7-16 | 25 | Nonalcoholicfatty liver disease | 1.5 T. Two-point Dixon | Biopsy | 1–7 d | r = 0.883; P < 0.0001 |
| Guaraldi et al[76] | 2012 | Observational pilot | 16 | 1.5 T. IP/OP (Dixon) | Biopsy | r = 0.88; P < 0.0001 | |||
| Koelblinger et al[77] | 2012 | Prospective | 60.5 | 35 | Various hepatic diseases | 3.0 T. IP/OP (Dixon) | Biopsy | Uncorrected: r = 0.67, P < 0.001. Spleen correction: r = 0.85, P < 0.001 | |
| Rastogi et al[73] | 2016 | Retrospective | 32.5 | 73 | Steatosis | 3.0 T. IP/OP (Dixon) | Biopsy and surgery | ≤ 20 d | Dual-echo MRI correlated well with the histopathology results (r = 0.871). An accuracy of 95% and sensitivity of 97% |
| Bhat et al[78] | 2017 | Prospective | 46 | 30 | Steatosis | 1.5 T. Two-point DIXON | Biopsy | 1 wk | Good correlation between the MR estimation of liver fat and histological grading. 90% of patients had a fat content of less than 10%. The maximal fat content of 28% was observed in one patient |
| Ref. | Year | Study design | Age (year) | N | Etiology | Field strength sequence | Comparison | Interval | Results |
| Noureddin et al[79] | 2013 | Randomized | 50 | Nonalcoholic fatty liver disease | MRI-PDFF | MRS | 0 and 24 wk | r2 = 0.98; P < 0.0001 | |
| Idilman et al[28] | 2013 | Prospective | 44.7 ± 13.1 | 70 | Nonalcoholic fatty liver disease | 1.5 T. IDEAL-IQ | Biopsy | 14.5 d (0–259) | r = 0.820; The correlation of PDFF in mild hepatic steatosis was found to be better than that of moderate or severe steatosis (r = 0.835 and r = 0.402, respectively; P = 0.003) |
| Deng et al[51] | 2014 | Prospective | 3–16 | 10 | Nonalcoholic fatty liver disease | 1.5 T. Multi-point Dixon | Biopsy | r = 0.90; P = 0.0004 | |
| Kukuk et al[59] | 2015 | 51.7 ± 15.2 | 59 | Liver disorders | 3.0 T. Six echo-mDixon | Biopsy | ≤ 6 wk | r = 0.967, P < 0.001. Slightly a higher hepatic fat contents than q Histo (mean difference 2.1% for 6E-mDixon and 1.9% for MRS) | |
| Rehm et al[52] | 2015 | Prospective | 13.3 ± 2 (11–22) | 132 | Healthy females | 3.0 T. Multi-echo Dixon | STEAM (TE = 10, 15, 20, 25, and 30 ms) | r = 0.96 | |
| Schwimmer et al[80] | 2015 | Prospective | 14 | 174 | No steatosis and nonalcoholic fatty liver disease | 3.0 T. Multi-echo Dixon | Biopsy | 57 ± 51 d | r = 0.725; P < 0.01 |
| Idilman et al[55] | 2016 | Retrospective | 41.7 ± 14.6 | 19 | Nonalcoholic fatty liver disease | 1.5 T. DEAL-IQ | Biopsy | r = 0.743; P < 0.001 | |
| Hetterich et al[39] | 2016 | Prospective | 57.2 ± 9.4 | 215 | 3.0 T. STEAM (TE = 12, 24, 36, 48, and 72 ms) | Multi-echo Dixon | r = 0.96; P = 0.001 | ||
| Middleton et al[81] | 2017 | Randomized | 51 ± 11 | 113 | Nonalcoholic steatohepatitis | 1.5 T or 3.0 T. Six echo-mDixon | Biopsy | 51 d | r = 0.80; P < 0.001 |
| Kang et al[46] | 2018 | Prospective | 47.3 ± 14.9 | 29 | NAFLD (34). Alcoholic liver disease (13). Liver cirrhosis (9) | 3.0 T. mDIXON-Quant sequence | Biopsy | Same day | r = 0.809; P < 0.001 |
| Pickhardt et al[82] | 2018 | Retrospective | 54 ± 12 | 221 | 1.5 T or 3.0 T. MRI-PDFF | CT | 0–158 mo | r = 0.88 (≤ 1 mo) substantially worsened with increasing time | |
| Guo et al[83] | 2020 | PProspective | 52.6 (22–83) | 400 | Healthy adults and older adults | 3.0 T mDixon-Quant sequence | CT | Same day | r = 0.79; P < 0.001 |
The outcomes of risk of bias assessment in the 40 studies are summarized in Figure 2. The overall low risk of bias in the 1H-MRS, two-point Dixon imaging, and multiple-point Dixon imaging groups was 50%, 55.5%, and 33.3%, respectively. Qualitative rather than quantitative synthesis was used in this study because of the high bias of included studies.
1H-MRS-principle: 1H-MRS measures the chemical composition of tissues. A signal from a region of interest (ROI) is Fourier transformed into an MR spectrum that displays various metabolites with unique frequencies. Triglycerides are composed of three fatty acid chains connected to a glycerol backbone; hence, at least six peaks can be resolved in the MRS spectrum. The water proton yields a single peak whose position in the spectrum may vary slightly depending on the temperature[17]. Liver SFF can be calculated as follows: Afat/(Afat+ Awater) × 100%, where Afat is the summation of lipid peak areas and Awater is the area under the water peak[18]. After T1 and T2 relaxation effects are corrected, spectroscopy-derived PDFF can be corrected[19,20].
Main technical factors-single-voxel technique vs multi-voxel technique: 1H-MRS spectra can be obtained using single-voxel or multiple-voxel techniques. The single-voxel technique for sampling a voxel of interest with a high signal-to-noise ratio (SNR) is commonly applied in hepatic MRS measurements[19].
Main technical factors-PRESS vs stimulated-echo acquisition mode: The most commonly used techniques for 1H-MRS are point-resolved spectroscopy (PRESS) and stimulated-echo acquisition mode (STEAM). PRESS is a spin-echo technique with a longer minimal echo time (TE) that allows for better visualization of metabolites with long T2 relaxation times. However, STEAM applies a 90°–90°–90° pulse and provides a shorter TE that is suitable for metabolites with short T2 relaxation times. PRESS has a higher SNR and is relatively insensitive to patient motion compared to STEAM, whereas STEAM is less affected by J-coupling and is generally preferred[21].
Main technical factors-correcting T1 and T2 effects: T1 and T2 values affect fat content measurement. In general, T1 relaxation times cause no trouble because the TR of MRS is much longer than the longest T1 of fat[22,23]. However, different T2 relaxation times may be problematic[24]. Both PRESS and STEAM sequences have a TE delay, causing spin-spin relaxation and decrease the signal[25]. Multiple spectroscopic acquisitions with different TEs are required to correct T2 values. If the spectra are acquired at a single TE, the sequence must use minimal TE to reduce T2 effects. Therefore, STEAM with shorter TE is recommended.
Main technical factors-ROI: The ROI was placed at the center of the right hepatic lobe to avoid vascular structures, bile ducts, and the liver edge[19].
Main technical factors-advantages: 1H-MRS is an alternative to liver biopsy. It can accurately quantify fat content with high intra- or inter-individual reproducibility[26] and is not affected by hepatic iron deposition, inflammation, and fibrosis[18].
1H-MRS is a noninvasive method for assessing hepatic lipid composition. Higher indices of hepatic fatty acid saturation and lower indices of unsaturation were observed in patients with obesity-related metabolic disease[27].
Main technical factors-disadvantages: 1H-MRS requires technical expertise for its acquisition and analysis[28]. 1H-MRS introduced sampling errors, especially in the liver with nonhomogeneous fat distribution, because fat is measured in the ROI rather than in the entire liver[29].
Hepatic fat showed multiple peaks on MR spectroscopy. The main lipid peak is at approximately 0.9–2.75 ppm and two unsolved lipid resonances at 4.2 and 5.3 ppm overlapping with the water peak, leading to quantification errors[19].
Diagnostic performance-1H-MRS vs liver biopsy: Five studies with an overall low risk of bias were used to evaluate the diagnostic performance of 1H-MRS[22,26,30-32]. These studies showed that 1H-MRS strongly correlated with the degree of hepatic steatosis on liver biopsy (r = 0.767–0.959). The sensitivity and specificity for 1H-MRS diagnosis of hepatic fat content of 5% or more were 94.4% and 89.5%, respectively[18].
Diagnostic performance-1H-MRS vs other imaging methods: 1H-MRS is considered the gold standard for other imaging methods to quantify hepatic fat content. One study[32] demonstrated that ultrasound detected liver fat in 82% of cases, measurable by 1H-MRS. Zhong et al[31] compared CT with 1H-MRS to quantitatively assess hepatic fat content and found that 1H-MRS correlated with the CT liver/spleen ratio (r = –0.461).
Principle: Two-point Dixon technique produces in-phase (IP) and out-of-phase (OP) images using two acquisitions[33,34]. The signal intensity (SI) on IP images is the sum of water and fat signals within a voxel, whereas that on OP images is the difference between water and fat signals. Thus, SFF can be calculated using the following formula: SFF = [(SIIP – SIOP]/2SIIP] × 100, where SIIP is the SI in a voxel on the IP image and SIOP is the SI on the OP image[35].
Main technical factors-SE vs gradient-recalled echo: Gradient-recalled echo (GRE) is routinely used for hepatic fat estimation. Since the GRE sequence is susceptible to the motion and paramagnetic effects of iron, Dixon used the SE sequence instead of the GRE sequence for CSI. A three-point Dixon method, which introduced a third echo to correct phase errors, was required to overcome long scan time and sensitivity to magnetic field inhomogeneities[34,36].
Main technical factors-2D vs 3D: IP and OP images are typically obtained in 2D acquisitions with multiple breath-holds for nonvolumetric quantification of hepatic fat. The 3D GRE sequence provided volumetric coverage of the liver but increased the post-processing time[37].
Main technical factors-ROI: ROIs were drawn at anatomically matched locations on the hepatic parenchyma on paired sequences, using a co-registration tool to exclude vessels, bile ducts, motion artifacts, and partial volume effects. Two of the 12 circular ROIs were placed in the right liver and two in the left liver above, below, and at the level of the porta hepatis[38].
Main technical factors-advantages: This technique could be used with all types of MR scanners (0.5T-3T). Both IP and OP images were acquired in the same breath-hold, and all imaging parameters, except TE, were similar. Therefore, the SI differences between the two images were based only on parallel, opposing water, and fat protons. The quality of the images was not affected by phase-related effects owing to amplitude imaging without phase information[33].
Main technical factors-disadvantages: The IP and OP images contained T1, T2, and T2*. These estimate the hepatic fat content inaccurately, especially for the liver with a fat content lower than 5%[38,39]. Because the liver SFF is within the dynamic range of 0%–50%[18], when the hepatic fat content was > 50%, the dominant constituent in a voxel is ambiguous on IP and OP images. This require phase-sensitive processing or a dual flip angle (20°and 70°) for removal[40].
The SFF derived from the IP and OP images assumes that water and fat have a single resonance frequency. In fact, this was not true for fat. Therefore, SFF based on IP and OP images is intrinsically incorrect[33].
Diagnostic performance: The sensitivity and specificity of SFF for diagnosing hepatic fat content > 20% are 96% and 93%, respectively[41]. However, a sensitivity of 89% and specificity of 82% were observed for an SFF of 1.8%[37].
Principle: Multiple-point Dixon acquires data from more than three echoes and provides images with both the magnitude and phase information of the echoes. This method addresses many confounding factors and yields accurate PDFF measurements. Simultaneously, transverse relaxation rate maps for measuring the iron content are also obtained[33,34,40]. The whole-liver PDFF was measured by averaging PDFF values from multiple regions in different parts of the liver. However, an optimal ROI-based sampling strategy has not yet been established[42].
Main technical factors-correcting T1 and T2* effects: In general, a long TR or a low flip angle in spoiled GRE acquisitions is used to minimize T1 bias. Echoes were acquired at three or more nominally OP and IP TEs to minimize T2* interference, especially the IDEAL-IQ sequence using a 6-echo 3-point Dixon method[28,33].
Main technical factors-noise and eddy: The noise bias originated from the skewed noise distribution in areas with a low signal during the magnitude operation. It significantly affects low-fat regions and makes the diagnosis of mild steatosis difficult. Noise bias can be mitigated using a hybrid complex/magnitude reconstruction[43].
Eddy currents were generated during rapid gradient switches at multiple TEs, which led to a phase shift and adversely affected the complex-based PDFF. This can be addressed by acquiring additional calibration data[44].
Main technical factors-fat spectral complexity: The fat spectrum consisted of multiple peaks that interfered with each other, as well as water, and made the PDFF incorrect. Multiple spectral models are required to address these multifrequency effects[45].
Main technical factors-ROI: It is recommended to select one to three ROIs per Couinaud segment, with the first ROI in each segment as centrally as possible and the remaining two on the same slice. ROI placement on the source images must avoid vessels, artifacts, and the edge of the liver.
Main technical factors-advantages: Multipoint Dixon imaging can be completed within a single breath-hold[46]. MRI-PDFF calculation used both the phase and magnitude data of the MR signal to measure the fat concentration in the range of 0%–100%[47]. The field strength and imaging manufacturer had negligible effects on the measurements[48].
Main technical factors-disadvantages: The accuracy of MRI-PDFF measurements is affected by fibrosis and severe steatosis[49]. The correlation between liver biopsy findings and MRI-PDFF was weaker in patients with moderate or severe hepatic steatosis than in those with milder forms[50].
Diagnostic performance: The sensitivity and specificity of MRI-PDFF were 83% and 89% for LS ≥ G2, and 79% and 89% for LS = G3, respectively[12]. An excellent correlation (r = 0.96–0.984)[28,51,52] with 1H-MRS has also been shown and confirmed by a previous meta-analysis (r = 0.96)[48].
MR techniques have emerged as reliable tools for the noninvasive estimation of hepatic fat content. This systematic review compared three common MR techniques: 1H-MRS, two-point Dixon imaging, and multiple-point Dixon imaging. These techniques have the same basic physical principles based on the chemical shift between the main peak of fat and that of water[33].
Before 2012, many studies used 1H-MRS and two-point Dixon imaging to measure hepatic fat content. The liver SFF calculated from 1H-MRS was not affected by iron deposition, fibrosis, or coexisting pathology, and provided accurate quantification of liver fat[19,53], especially MRS-PDFF[20]. Therefore, 1H-MRS is commonly used as a reference for other imaging techniques to measure hepatic fat content. However, expensive and complex post-processing procedures as well as only providing accurate data of liver fat content from small parenchymal regions, especially single-voxel 1H-MRS, hampered its widespread clinical application. Moreover, 1H-MRS is not available at every institution. Chemical shift MR imaging can visualize the regional distribution of intrahepatic lipids. The IP and OP images derived from the two-point Dixon technique are simple approaches. This technique requires several data sets with different TEs to calculate the fat content, which contains T1 and T2* effects; therefore, it evaluates the hepatic fat content inaccurately, especially for livers with less than 5% fat[39]. Springer[54] used additional individual time-consuming T1 and T2* measurements to correct the measured intrahepatic lipids; however, most measurements are not applicable in time-restricted examination protocols. This method also measures liver fat concentration within the dynamic range of 0%–50%[18].
Aside from measuring the liver fat concentration in the range of 0%–100%, the PDFF derived from multiple-point Dixon imaging mitigates confounding factors, such as T1, T2*, lipid spectral complex, noise, and eddy current, and has been successfully applied to quantify liver fat. It has been extensively used for detecting and grading hepatic steatosis, especially for differentiating moderate/severe steatosis from mild/no hepatic steatosis[55] accurately. This is due to its good correlation with histopathology and 1H-MRS measurements[12,56] as well as the shorter acquisition time compared with MRS[3,10,12,28,57]. Concurrent with PDFF acquisition, an R2* (1/T2*) map might also be formed, which could measure the iron concentration in the liver[58]. Additionally, PDFF was independent of field strength, scanner platform, and specific scanning parameters. However, this method yields a slightly higher hepatic fat content than liver histology[59] and the accuracy of measurements could be affected by fibrosis and severe steatosis. It lacked the power to detect the changes in non-alcoholic fatty liver disease (NAFLD) such as inflammation or fibrosis[13,47].
Recent studies have also shown that MR elastography and T1-T2 mapping can be useful in detecting hepatic inflammatory and fibrotic changes[13,60,61]. Therefore, the multiparametric MRI protocol may be helpful in liver tissue characterization and in risk stratification and therapeutic management of patients with NAFLD.
How do we choose each technique in daily practice? For epidemiological studies, MR and CT are unsuitable because of the expensive and time-consuming nature of MR and radiation damage from CT. Here, ultrasound is preferred. For clinical studies, especially follow-up or assessment of treatment efficacy, two-point Dixon and multiple-point Dixon imaging are preferred because of their subjective and robust characteristics. However, CT can be selected for shorter follow-up in primary or secondary care where there is no MR machine. MRS is the most accurate noninvasive technique and is a good standard in research studies, although its accuracy depends on expertise and the result is difficult to explain. Multiparametric MRI protocols, including MR elastography and T1-T2 mapping may be useful for stratification and therapeutic management of patients with NAFLD.
This study has several limitations. First, this review may have potential publication bias because gray literature and non-English language literature were not retrieved. Second, the overall moderate and high risks of bias in the 1H-MRS, two-point Dixon imaging, and multiple-point Dixon imaging groups were 50%, 45.5%, and 66.7%, respectively. Therefore, qualitative methods other than quantitative synthesis were used. The diagnostic accuracy of each method requires further investigation through a meta-analysis. Third, less commonly used methods for quantitative analysis of hepatic fat content were not included in this review. These include fat-selective imaging with spectral-spatial excitation, which requires a homogenous static magnetic field for optimal spectral-spatial excitation and is relatively sensitive to breathing artifacts[54,62].
PDFF derived from multiple-point Dixon imaging is a noninvasive method that provides an accurate, quantitative measurement of hepatic fat content. It can be used clinically to diagnose fatty liver and follow-up the progression of the disease and treatment effect.
Fatty liver can cause hepatocyte injury, inflammation, fibrosis, and eventually cirrhosis, with a high risk for liver failure and hepatocellular carcinoma. Early quantitative assessment of liver fat content is essential for patients with fatty liver disease.
Mounting evidence has shown that magnetic resonance (MR) technique has high accuracy in the quantitative analysis of fatty liver disease. However, many packaging methods and postprocessing functions have puzzled radiologists in clinical applications. Hence, selecting quantitative MR imaging (MRI) for patients with fatty liver disease is challenging.
To provide information for the proper selection of commonly used quantitative MR techniques to quantify fatty liver.
A systematic review of the literature from 1983 to May 2021 using PubMed, Embase, and Cochrane Library was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
A total of 114 articles were included, including 35 articles on MR techniques for measurement of hepatic fat content, 39 articles on reviews and meta-analysis, and 40 studies for further qualitative analysis. Because the overall moderate and high risk of bias in the 40 studies was approximately 50.0%, qualitative synthesis other than quantitative synthesis was used in this systematic review. The principle, main technical factors, advantages, and disadvantages of 1H-MR spectroscopy, two-point Dixon imaging, and multiple-point Dixon imaging, as well as their clinical diagnostic performance were summarized and analyzed.
Proton density fat fraction (PDFF) derived from multiple-point Dixon imaging is a noninvasive method that provides an accurate, quantitative measurement of hepatic fat content.
The accuracy of the PDFF derived from multiple-point Dixon imaging can be affected by fibrosis and severe steatosis. Therefore, the multiparametric MRI protocol might be helpful in liver tissue characterization and thereby in the risk stratification and therapeutic management of patients with non-alcoholic fatty liver disease.
We would like to express our gratitude to Dr. Wei Wang and Zhi-Chao Zhou for their great advice and careful modifications on the manuscript. Dr. Wei Wang is from the Research Institute for Translational Medicine on Molecular Function and Artificial Intelligence Imaging & Department of Radiology, The First People’s Hospital of Foshan, and Zhi-Chao Zhou is the Director of Consulting Department, Health Science Library of Peking University.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Athyros VG, Greece; Tarantino G, Italy; Teixeira KN, Brazil S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4949] [Article Influence: 707.0] [Reference Citation Analysis (9)] |
| 2. | Boyd A, Cain O, Chauhan A, Webb GJ. Medical liver biopsy: background, indications, procedure and histopathology. Frontline Gastroenterol. 2020;11:40-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Jayakumar S, Middleton MS, Lawitz EJ, Mantry PS, Caldwell SH, Arnold H, Mae Diehl A, Ghalib R, Elkhashab M, Abdelmalek MF, Kowdley KV, Stephen Djedjos C, Xu R, Han L, Mani Subramanian G, Myers RP, Goodman ZD, Afdhal NH, Charlton MR, Sirlin CB, Loomba R. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J Hepatol. 2019;70:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 4. | Ozturk A, Grajo JR, Gee MS, Benjamin A, Zubajlo RE, Thomenius KE, Anthony BW, Samir AE, Dhyani M. Quantitative Hepatic Fat Quantification in Non-alcoholic Fatty Liver Disease Using Ultrasound-Based Techniques: A Review of Literature and Their Diagnostic Performance. Ultrasound Med Biol. 2018;44:2461-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Starekova J, Reeder SB. Liver fat quantification: where do we stand? Abdom Radiol (NY). 2020;45:3386-3399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Kramer H, Pickhardt PJ, Kliewer MA, Hernando D, Chen GH, Zagzebski JA, Reeder SB. Accuracy of Liver Fat Quantification With Advanced CT, MRI, and Ultrasound Techniques: Prospective Comparison With MR Spectroscopy. AJR Am J Roentgenol. 2017;208:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 7. | Hahn L, Reeder SB, Muñoz del Rio A, Pickhardt PJ. Longitudinal Changes in Liver Fat Content in Asymptomatic Adults: Hepatic Attenuation on Unenhanced CT as an Imaging Biomarker for Steatosis. AJR Am J Roentgenol. 2015;205:1167-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Li Q, Dhyani M, Grajo JR, Sirlin C, Samir AE. Current status of imaging in nonalcoholic fatty liver disease. World J Hepatol. 2018;10:530-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (3)] |
| 9. | Bray TJ, Chouhan MD, Punwani S, Bainbridge A, Hall-Craggs MA. Fat fraction mapping using magnetic resonance imaging: insight into pathophysiology. Br J Radiol. 2018;91:20170344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 371] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 11. | Zhang QH, Zhao Y, Tian SF, Xie LH, Chen LH, Chen AL, Wang N, Song QW, Zhang HN, Xie LZ, Shen ZW, Liu AL. Hepatic fat quantification of magnetic resonance imaging whole-liver segmentation for assessing the severity of nonalcoholic fatty liver disease: comparison with a region of interest sampling method. Quant Imaging Med Surg. 2021;11:2933-2942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 12. | Qu Y, Li M, Hamilton G, Zhang YN, Song B. Diagnostic accuracy of hepatic proton density fat fraction measured by magnetic resonance imaging for the evaluation of liver steatosis with histology as reference standard: a meta-analysis. Eur Radiol. 2019;29:5180-5189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Erden A, Kuru Öz D, Peker E, Kul M, Özalp Ateş FS, Erden İ, İdilman R. MRI quantification techniques in fatty liver: the diagnostic performance of hepatic T1, T2, and stiffness measurements in relation to the proton density fat fraction. Diagn Interv Radiol. 2021;27:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Zheng D, Guo Z, Schroder PM, Zheng Z, Lu Y, Gu J, He X. Accuracy of MR Imaging and MR Spectroscopy for Detection and Quantification of Hepatic Steatosis in Living Liver Donors: A Meta-Analysis. Radiology. 2017;282:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15886] [Article Influence: 1588.6] [Reference Citation Analysis (1)] |
| 16. | De Visschere PJL, Standaert C, Fütterer JJ, Villeirs GM, Panebianco V, Walz J, Maurer T, Hadaschik BA, Lecouvet FE, Giannarini G, Fanti S. A Systematic Review on the Role of Imaging in Early Recurrent Prostate Cancer. Eur Urol Oncol. 2019;2:47-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 17. | Peterson P, Trinh L, Månsson S. Quantitative 1 H MRI and MRS of fatty acid composition. Magn Reson Med. 2021;85:49-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Kang BK, Yu ES, Lee SS, Lee Y, Kim N, Sirlin CB, Cho EY, Yeom SK, Byun JH, Park SH, Lee MG. Hepatic fat quantification: a prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Invest Radiol. 2012;47:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Pasanta D, Htun KT, Pan J, Tungjai M, Kaewjaeng S, Kim H, Kaewkhao J, Kothan S. Magnetic Resonance Spectroscopy of Hepatic Fat from Fundamental to Clinical Applications. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Runge JH, Smits LP, Verheij J, Depla A, Kuiken SD, Baak BC, Nederveen AJ, Beuers U, Stoker J. MR Spectroscopy-derived Proton Density Fat Fraction Is Superior to Controlled Attenuation Parameter for Detecting and Grading Hepatic Steatosis. Radiology. 2018;286:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Hamilton G, Middleton MS, Bydder M, Yokoo T, Schwimmer JB, Kono Y, Patton HM, Lavine JE, Sirlin CB. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging. 2009;30:145-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 271] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, Brittain JH, Reeder SB. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 317] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 24. | Dieckmeyer M, Ruschke S, Cordes C, Yap SP, Kooijman H, Hauner H, Rummeny EJ, Bauer JS, Baum T, Karampinos DC. The need for T₂ correction on MRS-based vertebral bone marrow fat quantification: implications for bone marrow fat fraction age dependence. NMR Biomed. 2015;28:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Gajdošík M, Chmelík M, Just-Kukurová I, Bogner W, Valkovič L, Trattnig S, Krššák M. In vivo relaxation behavior of liver compounds at 7 Tesla, measured by single-voxel proton MR spectroscopy. J Magn Reson Imaging. 2014;40:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Longo R, Pollesello P, Ricci C, Masutti F, Kvam BJ, Bercich L, Crocè LS, Grigolato P, Paoletti S, de Bernard B. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 298] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Willis SA, Bawden SJ, Malaikah S, Sargeant JA, Stensel DJ, Aithal GP, King JA. The role of hepatic lipid composition in obesity-related metabolic disease. Liver Int. 2021;41:2819-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Idilman IS, Aniktar H, Idilman R, Kabacam G, Savas B, Elhan A, Celik A, Bahar K, Karcaaltincaba M. Hepatic steatosis: quantification by proton density fat fraction with MR imaging vs liver biopsy. Radiology. 2013;267:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 308] [Article Influence: 25.7] [Reference Citation Analysis (1)] |
| 29. | Haus JM, Solomon TP, Kelly KR, Fealy CE, Kullman EL, Scelsi AR, Lu L, Pagadala MR, McCullough AJ, Flask CA, Kirwan JP. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2013;98:E1181-E1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Cowin GJ, Jonsson JR, Bauer JD, Ash S, Ali A, Osland EJ, Purdie DM, Clouston AD, Powell EE, Galloway GJ. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging. 2008;28:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Zhong L, Chen JJ, Chen J, Li L, Lin ZQ, Wang WJ, Xu JR. Nonalcoholic fatty liver disease: quantitative assessment of liver fat content by computed tomography, magnetic resonance imaging and proton magnetic resonance spectroscopy. J Dig Dis. 2009;10:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Mehta SR, Thomas EL, Patel N, Crofton ME, McCarthy J, Eliahoo J, Morin SX, Fitzpatrick J, Durighel G, Goldstone AP, Johnston DG, Bell JD, Taylor-Robinson SD. Proton magnetic resonance spectroscopy and ultrasound for hepatic fat quantification. Hepatol Res. 2010;40:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Hong CW, Fazeli Dehkordy S, Hooker JC, Hamilton G, Sirlin CB. Fat Quantification in the Abdomen. Top Magn Reson Imaging. 2017;26:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | van Vucht N, Santiago R, Lottmann B, Pressney I, Harder D, Sheikh A, Saifuddin A. The Dixon technique for MRI of the bone marrow. Skeletal Radiol. 2019;48:1861-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Fishbein M, Castro F, Cheruku S, Jain S, Webb B, Gleason T, Stevens WR. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005;39:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Singh D, Das CJ, Baruah MP. Imaging of non alcoholic fatty liver disease: A road less travelled. Indian J Endocrinol Metab. 2013;17:990-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Fischer MA, Nanz D, Reiner CS, Montani M, Breitenstein S, Leschka S, Alkadhi H, Stolzmann P, Marincek B, Scheffel H. Diagnostic performance and accuracy of 3-D spoiled gradient-dual-echo MRI with water- and fat-signal separation in liver-fat quantification: comparison to liver biopsy. Invest Radiol. 2010;45:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Bahl M, Qayyum A, Westphalen AC, Noworolski SM, Chu PW, Ferrell L, Tien PC, Bass NM, Merriman RB. Liver steatosis: investigation of opposed-phase T1-weighted liver MR signal intensity loss and visceral fat measurement as biomarkers. Radiology. 2008;249:160-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Hetterich H, Bayerl C, Peters A, Heier M, Linkohr B, Meisinger C, Auweter S, Kannengießer SA, Kramer H, Ertl-Wagner B, Bamberg F. Feasibility of a three-step magnetic resonance imaging approach for the assessment of hepatic steatosis in an asymptomatic study population. Eur Radiol. 2016;26:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Eskreis-Winkler S, Corrias G, Monti S, Zheng J, Capanu M, Krebs S, Fung M, Reeder S, Mannelli L. IDEAL-IQ in an oncologic population: meeting the challenge of concomitant liver fat and liver iron. Cancer Imaging. 2018;18:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Mennesson N, Dumortier J, Hervieu V, Milot L, Guillaud O, Scoazec JY, Pilleul F. Liver steatosis quantification using magnetic resonance imaging: a prospective comparative study with liver biopsy. J Comput Assist Tomogr. 2009;33:672-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Vu KN, Gilbert G, Chalut M, Chagnon M, Chartrand G, Tang A. MRI-determined liver proton density fat fraction, with MRS validation: Comparison of regions of interest sampling methods in patients with type 2 diabetes. J Magn Reson Imaging. 2016;43:1090-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 401] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 44. | Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J Magn Reson Imaging. 2011;34:729-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 45. | Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, Middleton MS. In vivo characterization of the liver fat ¹H MR spectrum. NMR Biomed. 2011;24:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 430] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 46. | Kang BK, Kim M, Song SY, Jun DW, Jang K. Feasibility of modified Dixon MRI techniques for hepatic fat quantification in hepatic disorders: validation with MRS and histology. Br J Radiol. 2018;91:20170378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology. 2018;68:763-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 350] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 48. | Yokoo T, Serai SD, Pirasteh A, Bashir MR, Hamilton G, Hernando D, Hu HH, Hetterich H, Kühn JP, Kukuk GM, Loomba R, Middleton MS, Obuchowski NA, Song JS, Tang A, Wu X, Reeder SB, Sirlin CB; RSNA-QIBA PDFF Biomarker Committee. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology. 2018;286:486-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 49. | Lee SJ, Kim SU. Noninvasive monitoring of hepatic steatosis: controlled attenuation parameter and magnetic resonance imaging-proton density fat fraction in patients with nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Lv S, Jiang S, Liu S, Dong Q, Xin Y, Xuan S. Noninvasive Quantitative Detection Methods of Liver Fat Content in Nonalcoholic Fatty Liver Disease. J Clin Transl Hepatol. 2018;6:217-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Deng J, Fishbein MH, Rigsby CK, Zhang G, Schoeneman SE, Donaldson JS. Quantitative MRI for hepatic fat fraction and T2* measurement in pediatric patients with non-alcoholic fatty liver disease. Pediatr Radiol. 2014;44:1379-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Rehm JL, Wolfgram PM, Hernando D, Eickhoff JC, Allen DB, Reeder SB. Proton density fat-fraction is an accurate biomarker of hepatic steatosis in adolescent girls and young women. Eur Radiol. 2015;25:2921-2930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Thiagarajan P, Bawden SJ, Aithal GP. Metabolic Imaging in Non-Alcoholic Fatty Liver Disease: Applications of Magnetic Resonance Spectroscopy. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Springer F, Machann J, Schwenzer NF, Ballweg V, Würslin C, Schneider JH, Fritsche A, Claussen CD, Schick F. Quantitative assessment of intrahepatic lipids using fat-selective imaging with spectral-spatial excitation and in-/opposed-phase gradient echo imaging techniques within a study population of extremely obese patients: feasibility on a short, wide-bore MR scanner. Invest Radiol. 2010;45:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Idilman IS, Keskin O, Celik A, Savas B, Elhan AH, Idilman R, Karcaaltincaba M. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS vs liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2016;57:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 56. | Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 422] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 57. | Kim KY, Song JS, Kannengiesser S, Han YM. Hepatic fat quantification using the proton density fat fraction (PDFF): utility of free-drawn-PDFF with a large coverage area. Radiol Med. 2015;120:1083-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Henninger B, Plaikner M, Zoller H, Viveiros A, Kannengiesser S, Jaschke W, Kremser C. Performance of different Dixon-based methods for MR liver iron assessment in comparison to a biopsy-validated R2* relaxometry method. Eur Radiol. 2021;31:2252-2262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Kukuk GM, Hittatiya K, Sprinkart AM, Eggers H, Gieseke J, Block W, Moeller P, Willinek WA, Spengler U, Trebicka J, Fischer HP, Schild HH, Träber F. Comparison between modified Dixon MRI techniques, MR spectroscopic relaxometry, and different histologic quantification methods in the assessment of hepatic steatosis. Eur Radiol. 2015;25:2869-2879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 60. | Pavlides M, Banerjee R, Tunnicliffe EM, Kelly C, Collier J, Wang LM, Fleming KA, Cobbold JF, Robson MD, Neubauer S, Barnes E. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int. 2017;37:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 61. | Besutti G, Valenti L, Ligabue G, Bassi MC, Pattacini P, Guaraldi G, Giorgi Rossi P. Accuracy of imaging methods for steatohepatitis diagnosis in non-alcoholic fatty liver disease patients: A systematic review. Liver Int. 2019;39:1521-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Machann J, Thamer C, Schnoedt B, Stefan N, Haring HU, Claussen CD, Fritsche A, Schick F. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med. 2006;55:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 63. | Irwan R, Edens MA, Sijens PE. Assessment of the variations in fat content in normal liver using a fast MR imaging method in comparison with results obtained by spectroscopic imaging. Eur Radiol. 2008;18:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Kim H, Taksali SE, Dufour S, Befroy D, Goodman TR, Petersen KF, Shulman GI, Caprio S, Constable RT. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point dixon and three-point IDEAL. Magn Reson Med. 2008;59:521-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 65. | Borra RJ, Salo S, Dean K, Lautamäki R, Nuutila P, Komu M, Parkkola R. Nonalcoholic fatty liver disease: rapid evaluation of liver fat content with in-phase and out-of-phase MR imaging. Radiology. 2009;250:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 66. | Reeder SB, Robson PM, Yu H, Shimakawa A, Hines CD, McKenzie CA, Brittain JH. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging. 2009;29:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 67. | Hu HH, Kim HW, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring). 2010;18:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 68. | Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Ríos C, Méndez-Sánchez N. In vivo 3T spectroscopic quantification of liver fat content in nonalcoholic fatty liver disease: Correlation with biochemical method and morphometry. J Hepatol. 2010;53:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Georgoff P, Thomasson D, Louie A, Fleischman E, Dutcher L, Mani H, Kottilil S, Morse C, Dodd L, Kleiner D, Hadigan C. Hydrogen-1 MR spectroscopy for measurement and diagnosis of hepatic steatosis. AJR Am J Roentgenol. 2012;199:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Parente DB, Rodrigues RS, Paiva FF, Oliveira Neto JA, Machado-Silva L, Lanzoni V, Campos CF, Eiras-Araujo AL, do Brasil PE, Garteiser P, Gomes MB, de Mello Perez R. Is MR spectroscopy really the best MR-based method for the evaluation of fatty liver in diabetic patients in clinical practice? PLoS One. 2014;9:e112574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Bashir MR, Zhong X, Nickel MD, Fananapazir G, Kannengiesser SA, Kiefer B, Dale BM. Quantification of hepatic steatosis with a multistep adaptive fitting MRI approach: prospective validation against MR spectroscopy. AJR Am J Roentgenol. 2015;204:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 72. | Satkunasingham J, Besa C, Bane O, Shah A, de Oliveira A, Gilson WD, Kannengiesser S, Taouli B. Liver fat quantification: Comparison of dual-echo and triple-echo chemical shift MRI to MR spectroscopy. Eur J Radiol. 2015;84:1452-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Rastogi R, Gupta S, Garg B, Vohra S, Wadhawan M, Rastogi H. Comparative accuracy of CT, dual-echo MRI and MR spectroscopy for preoperative liver fat quantification in living related liver donors. Indian J Radiol Imaging. 2016;26:5-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Kalra N, Duseja A, Das A, Dhiman RK, Virmani V, Chawla Y, Singh P, Khandelwal N. Chemical shift magnetic resonance imaging is helpful in detecting hepatic steatosis but not fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). Ann Hepatol. 2009;8:21-25. [PubMed] |
| 75. | Pacifico L, Martino MD, Catalano C, Panebianco V, Bezzi M, Anania C, Chiesa C. T1-weighted dual-echo MRI for fat quantification in pediatric nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17:3012-3019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Guaraldi G, Besutti G, Stentarelli C, Zona S, Nocetti L, Loria P, Ballestri S, Losi L, Torricelli P, Ligabue G. Magnetic resonance for quantitative assessment of liver steatosis: a new potential tool to monitor antiretroviral-drug-related toxicities. Antivir Ther. 2012;17:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Koelblinger C, Krššák M, Maresch J, Wrba F, Kaczirek K, Gruenberger T, Tamandl D, Ba-Ssalamah A, Berger-Kulemann V, Weber M, Schima W. Hepatic steatosis assessment with 1H-spectroscopy and chemical shift imaging at 3.0 T before hepatic surgery: reliable enough for making clinical decisions? Eur J Radiol. 2012;81:2990-2995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Bhat V, Velandai S, Belliappa V, Illayraja J, Halli KG, Gopalakrishnan G. Quantification of Liver Fat with mDIXON Magnetic Resonance Imaging, Comparison with the Computed Tomography and the Biopsy. J Clin Diagn Res. 2017;11:TC06-TC10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, Changchien C, Brenner DA, Sirlin C, Loomba R. Utility of magnetic resonance imaging vs histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 439] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 80. | Schwimmer JB, Middleton MS, Behling C, Newton KP, Awai HI, Paiz MN, Lam J, Hooker JC, Hamilton G, Fontanesi J, Sirlin CB. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology. 2015;61:1887-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 81. | Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, Brunt EM, Kleiner DE, Doo E, Van Natta ML, Lavine JE, Neuschwander-Tetri BA, Sanyal A, Loomba R, Sirlin CB; NASH Clinical Research Network. Agreement Between Magnetic Resonance Imaging Proton Density Fat Fraction Measurements and Pathologist-Assigned Steatosis Grades of Liver Biopsies From Adults With Nonalcoholic Steatohepatitis. Gastroenterology. 2017;153:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 82. | Pickhardt PJ, Graffy PM, Reeder SB, Hernando D, Li K. Quantification of Liver Fat Content With Unenhanced MDCT: Phantom and Clinical Correlation With MRI Proton Density Fat Fraction. AJR Am J Roentgenol. 2018;211:W151-W157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 83. | Guo Z, Blake GM, Li K, Liang W, Zhang W, Zhang Y, Xu L, Wang L, Brown JK, Cheng X, Pickhardt PJ. Liver Fat Content Measurement with Quantitative CT Validated against MRI Proton Density Fat Fraction: A Prospective Study of 400 Healthy Volunteers. Radiology. 2020;294:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |