Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8360
Peer-review started: March 16, 2022
First decision: May 12, 2022
Revised: May 24, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 16, 2022
Processing time: 137 Days and 22 Hours
Relapsing polychondritis is a rare multisystem autoimmune disease that mainly involves systemic cartilage and proteoglycan-rich tissues. If the larynx and trachea are involved, the patient’s condition deteriorates rapidly. When relapsing polychondritis becomes more advanced, the airways collapse and treatment is difficult, rendering a poor prognosis. Therefore, the diagnosis method, treatment strategy and prognosis of relapsing polychondritis with larynx and trachea involvement need to be elucidated to improve clinicians’ awareness of the disease.
A man and a woman were admitted because of breathlessness. Relapsing polychondritis was diagnosed after a series of accessory examinations. They were both treated with glucocorticoids and immunosuppressants, and underwent tracheotomy as their breathing difficulties could not be relieved by the medication.
The two cases highlight the importance of the timely diagnosis, full evaluation and initiating individualized treatment of relapsing polychondritis with larynx and trachea involvement. Laryngoscopy, bronchoscopy and pathological examination are helpful in diagnosis of this disease.
Core Tip: Relapsing polychondritis is a rare multisystem autoimmune disease, if the larynx and trachea are involved, the patient’s condition deteriorates rapidly. We here report two cases of relapsing polychondritis involving the larynx and trachea. Necessary accessory examinations, timely diagnosis and full evaluation, and initiating individualized treatment are important to reduce the mortality rate and improve patient prognosis.
- Citation: Zhai SY, Zhang YH, Guo RY, Hao JW, Wen SX. Relapsing polychondritis causing breathlessness: Two case reports. World J Clin Cases 2022; 10(23): 8360-8366
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8360.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8360
Relapsing polychondritis is a rare, multisystem autoimmune disease that mainly involves systemic cartilage and proteoglycan-rich tissues such as those of the ears, nose, larynx and trachea, articular cartilage, heart, blood vessels, inner ear, cornea, sclera and kidneys[1]. Relapsing polychondritis usually occurs in middle-aged and young people, and the peak age of onset is 40 years. About 50% of relapsing polychondritis patients have laryngeal and tracheal involvement[2,3]. The etiology and pathogenesis of relapsing polychondritis remain unclear. It is believed that its occurrence and development are related to genetic and environmental factors and immunological changes. In terms of treatment, glucocorticoids and immunosuppressants (such as methotrexate and cyclophosphamide) are mainly used[4]. If the larynx and trachea are involved, breathing difficulties occur, thereby rendering a poor prognosis. Relapsing polychondritis has various clinical manifestations, and thus, can be easily misdiagnosed and missed in clinical practice. Here, we report the clinical diagnosis and treatment of two patients with relapsing polychondritis, mainly with laryngeal and tracheal cartilage involvement. The patients were misdiagnosed in another hospital. We report their specific accessory findings to improve clinicians’ awareness of the disease.
Case 1: A 31-year-old man with a 4-mo history of hoarseness and 3-mo history of dyspnea was admitted on March 23, 2020.
Case 2: A 59-year-old woman was admitted on December 7, 2018 because of 5 mo of cough, sputum and hoarseness, in addition to breathing difficulties.
Case 1: Four months before admission, there was no obvious cause for his symptoms. One month later, after a cold, there was exacerbation of his hoarseness. At the same time, the patient developed a cough with a small amount of white sputum. Throat tuberculosis was suggested at another hospital, but antituberculosis treatment, such as rifampin and ambroxol, showed no significant improvement in his condition. The patient’s dyspnea symptoms worsened after 5 d, and stridor could be heard during calm breathing, while slight retraction of three fossae was observed.
Case 2: Five months before admission to hospital because of fatigue, the patient developed dry cough, which was obvious at night, and exhibited no obvious improvement after anti-inflammatory treatment. Cough with white sticky sputum and breathing difficulties presented 2 mo later, making her unable to lie flat at night. After treatment for bronchitis, pneumonia and asthma in other hospitals, her symptoms exhibited no improvement. The day before admission to our hospital, her breathing difficulty was exacerbated and she experienced a sudden faint.
Cases 1 and 2: The patients reported no notable past illness.
Cases 1 and 2: Personal or family medical history was unremarkable.
Case 1: No redness or congestion was found in both eyes. The nose, auricle and limb joints exhibited no swelling deformities or collapse. By indirect laryngoscopy, a well-lifted epiglottis could be identified without redness or swelling, the rima glottidis could not be seen.
Case 2: The patient exhibited obvious exhaustion, with the lungs wheezing to auscultation, the nose cartilage had collapsed and she had a saddle nose, no congestion and swelling in the eyes, and normal movement of limbs and joints. Chest CT revealed middle lobe infection in the right lung and mild dilation of the middle lobe and lower lobe of the right lung.
Case 1: Erythrocyte sedimentation rate was 65 mm/h and rheumatoid factors were normal.
Case 2: Erythrocyte sedimentation rate was 45 mm/h and rheumatoid factors were normal.
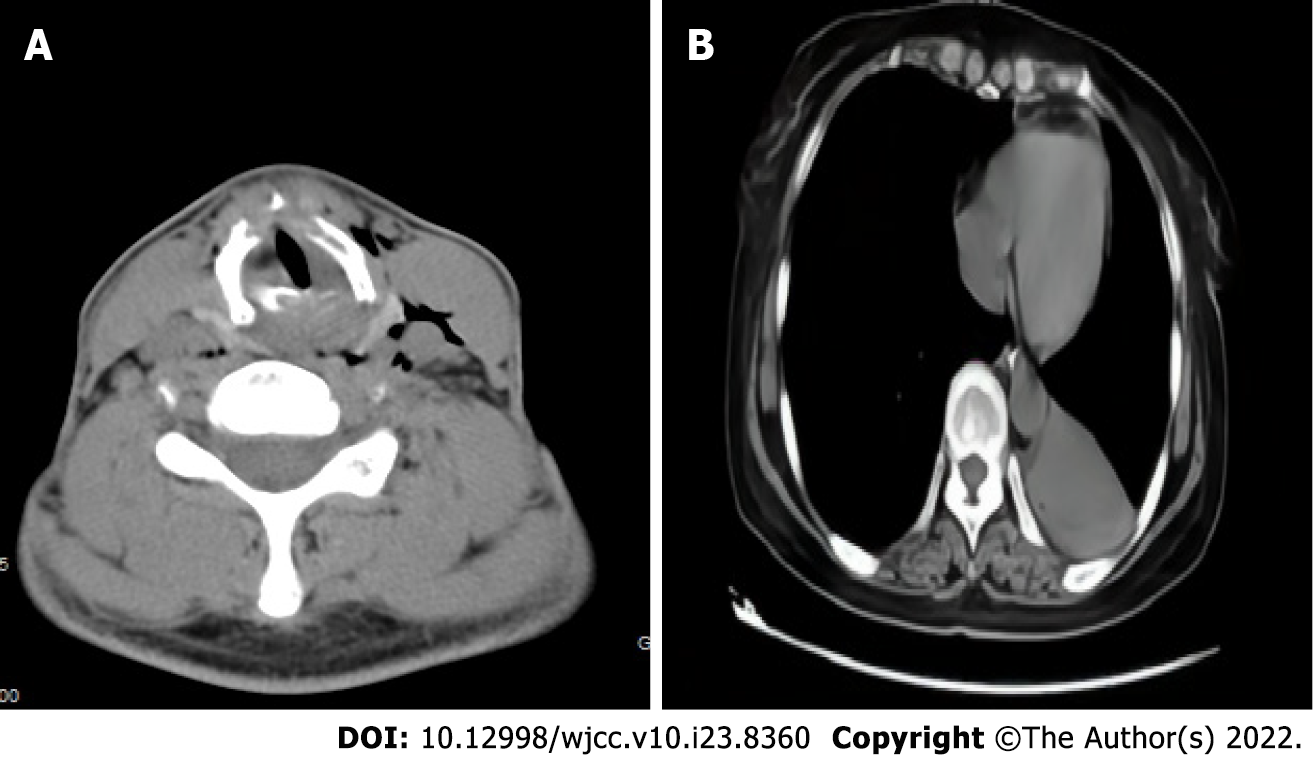
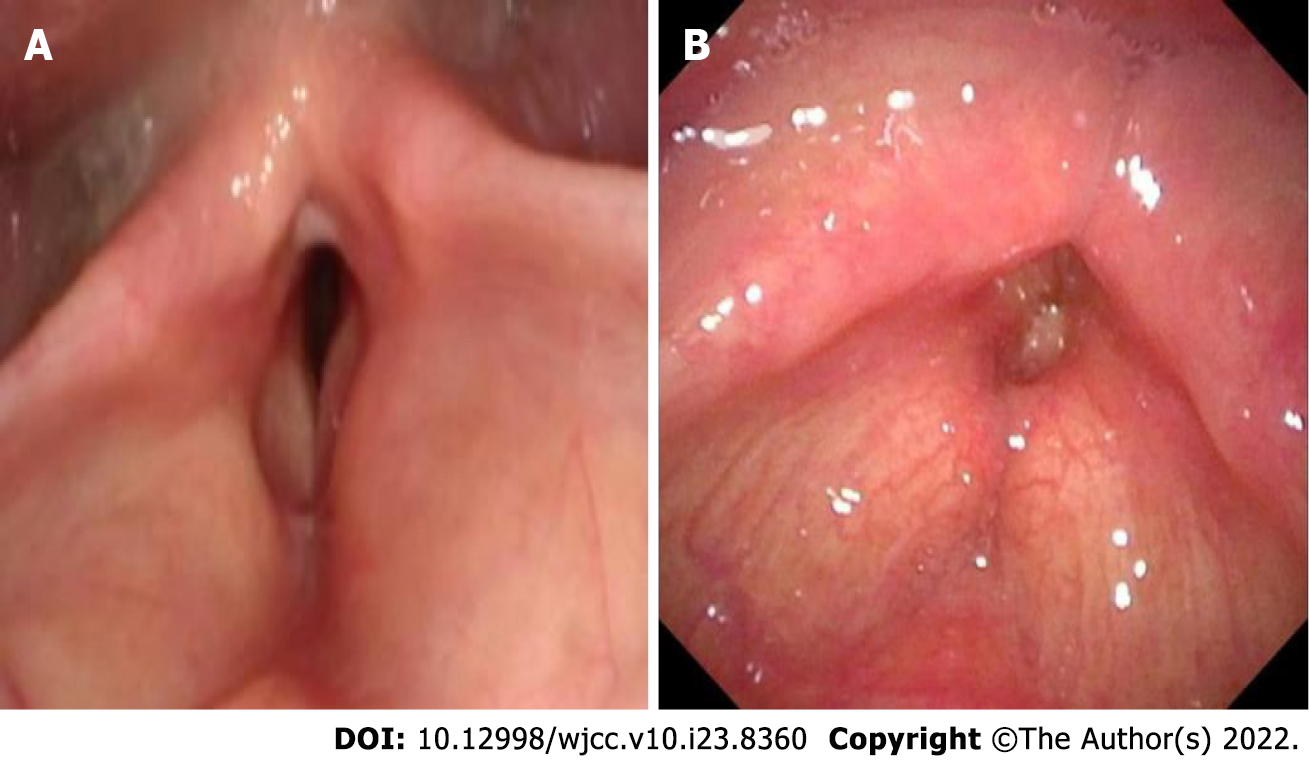
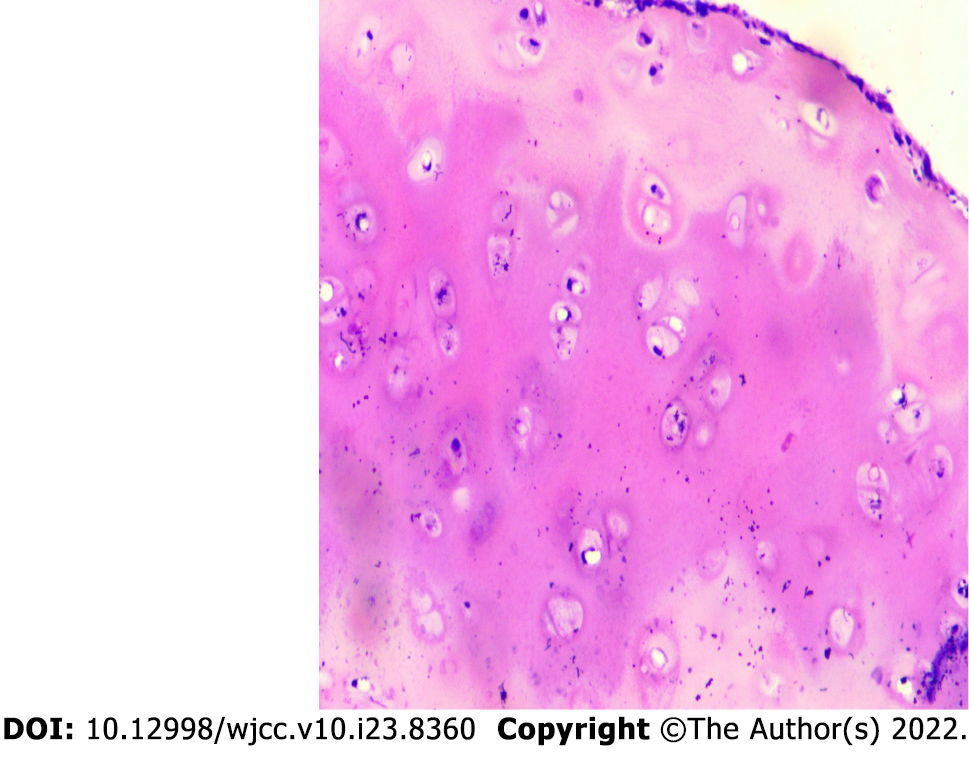
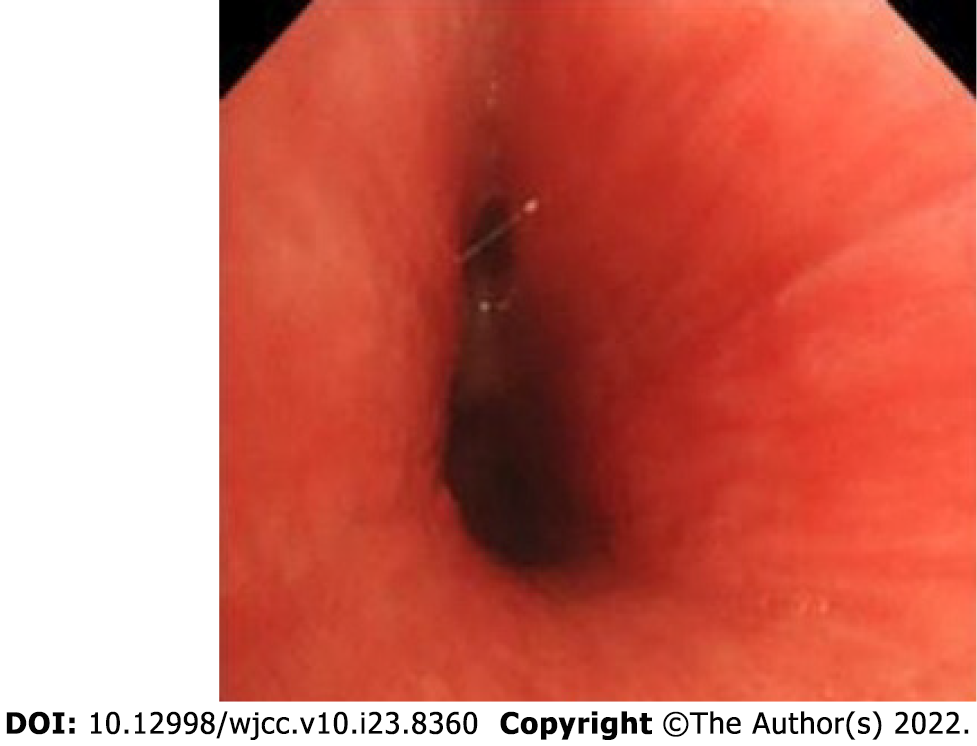
Case 1: After assessing the patient’s condition, the initial suspicion was Adam’s apple nucleus tuberculosis, so a tuberculin test, cervical and thoracic soft tissue computed tomography (CT) and electronic fiber nasolaryngoscopy were performed. Tuberculin tests were negative. Neck CT revealed stenosis of the cavum larynges with thickening of the ventricular band and vocal cords (Figure 1A). No tuberculosis foci were observed. Electronic fiber nasopharyngolarygnoscopy revealed bilateral vocal band mucosa edema and glottal stenosis (Figure 2A). So Adam’s apple nucleus tuberculosis was excluded. The pathological examination showed that fibrous tissue was visible in the cartilage, and there was chronic inflammatory cell infiltration in the fibrous interstitium (Figure 3). Congestion, edema and thickening of the middle and lower tracheal mucosa were observed by bedside bronchoscopy (Figure 4).
Case 2: On December 27, 2 wk after removing the endotracheal tube, laryngoscopy and bronchoscopy were conducted. The vocal tape mucosa was slightly thicker, the rima glottidis could not be exposed (Figure 2B), and the tracheal ring was not obvious. In addition, she had expiratory phase tracheal collapse. Chest CT revealed new atelectasis of the lower left lung (Figure 1B).
Relapsing polychondritis was considered based on the patient’s symptoms of hoarseness, presence of dyspnea and laryngeal and tracheal mucosa lesion, and the pathological results, and according to the Damiani Standard, article 2.
Relapsing polychondritis was considered based on the symptoms of dyspnea and saddle nose, the laryngoscopy and bronchoscopy results, and according to the Rose Standard.
After anti-infection and atomization treatment, the symptoms of dyspnea still exhibited no significant improvement. On March 31, a tracheotomy and biopsy were performed. Due to postoperative dyspnea, the patient had difficulty being weaned off the breathing machine, and was transferred to the intensive care unit (ICU). After diagnosis, 3 d of 0.5 g/d shock therapy was used, and low-dose methylprednisolone and mycophenolate mofetil were administered outside the hospital on a long-term basis.
After 7 d of treatment, including methylprednisolone (80 mg/d), spasmolysis, anti-infection, and correction of electrolyte disorder, the patient suddenly experienced difficulty in breathing when she inhaled. The patient was transferred to the ICU for tracheal intubation. However, there was obvious tracheal stenosis, which made intubation difficult. Finally, pipe # 5.5 was inserted. The patient was then treated with glucocorticoid combined with methotrexate (10 mg/wk). On January 21, she was discharged from hospital in improved condition. Two weeks after discharge, the patient was admitted to the hospital again with breathing difficulties. Methylprednisolone plus methotrexate were administered intravenously in combination with auxiliary endotracheal intubation. A tracheotomy was performed due to tube extraction difficulties. Long-term postoperative administration of methylprednisolone and methotrexate was proposed.
During 13 mo of follow-up, the patient was hospitalized again with dyspnea and pulmonary inflammation, during which, the patient received large-dose hormone therapy and his condition improved.
During 2 years of follow-up, due to respiratory difficulties and repeated pulmonary inflammation, the patient was hospitalized 6 times in the rheumatism department and the respiratory department.
The initial symptoms of relapsing polychondritis vary according to the affected organs. In patients with laryngotracheal involvement, relapsing polychondritis is easily confused with diseases such as Adam’s apple tuberculosis, bronchitis, and asthma due to atypical clinical symptoms, and the condition of patients can deteriorate rapidly with the involvement of the larynx and airways. After relapsing polychondritis reaches the advanced stage, the airway collapses and treatment is difficult, rendering a poor prognosis for patients. About 5% of relapsing polychondritis patients are children. Children with laryngeal and tracheal involvement of the disease are more common. It is reported that 91% of children have laryngeal involvement[5,6]. Because children’s laryngeal cavity is narrow and submucosal tissue is loose, it is easier to cause laryngeal cavity blockage and suffocation. Han et al[7] reported two cases of recurrent polychondritis in children; both of whom underwent tracheotomy due to dyspnea. Some studies have proved that patients with airway involvement are more likely to be admitted to ICU due to airway collapse and lung infection[2]. With the extension of follow-up time, patients with airway involvement have a progressively higher mortality rate[8]. In this study, both patients were admitted to the ICU due to airway collapse. Recurrent polychondritis may involve all parts of the airways. One study reported that about 50% of patients have airway involvement in the course of the disease, with 20% of patients experiencing upper airway collapse, and the potential involvement of upper and lower airway cartilage[9]. In this study, both the atmospheric and upper airways were involved. The existing diagnostic criteria are mainly based on clinical performance, as shown in Table 1[10-13]. The Damiani Standard[11] and Rose Standard[13] were referred to for diagnosis of the two patients in the present study. At present, there is no diagnostic standard sensitive enough to reach 100%[13]. Imaging examinations, such as CT, positron emission tomography–CT, tracheobronchoscopy, electronic fiber nasolaryngoscopy, and lung function and pathology could be included as auxiliary diagnostic criteria to improve early diagnostic sensitivity[14,15].
| Ref. | Symptoms | Diagnostic requirements |
| McAdam et al[10] | Bilateral auricle chondrolitis; eye inflammation; nasal chondrolitis; nonaggressive polyarthritis; larynx and/or tracheochondrolitis; cochlear implant and /or vestibular damage | Patient with ≥ 3 symptoms |
| Damiani et al[11] | A: Patients meeting ≥ 3 standards in the McAdam standard; B: > 1, plus pathological confirmation; C: lesions involving ≥ 2 anatomical sites, effective with glucocorticoids or dapsone | Patient meeting any one of the criteria |
| Michet et al[12] | Main manifestations: repeated cartilage inflammation of bilateral auricle; nasal chondrositis; larynx and/or bronchobronchial chondroitis. Secondary manifestations: inflammation of the eye; impaired hearing; impaired vestibular function; serum-negative arthritis | Diagnosed with two main manifestations or one main plus two secondary manifestations |
| Rose et al[13] | Main manifestations: repeated cartilage inflammation in both auricle; nasal cartilage; eye inflammation; larynx and/or bronchobronchial chondroitis. Secondary manifestations: hearing impairment; impaired vestibular function; serum-negative arthritis | Diagnosed with two main manifestations or one main plus two secondary manifestations |
When a patient is suffering from relapsing polychondritis mainly with laryngeal and tracheal involvement, the general and systemic treatments are the same for patients with relapsing polychondritis with involvement of other regions. Methylprednisolone is an effective drug for relapsing polychondritis, and the dose should be adjusted according to the condition of the patient. Low doses of glucocorticoid can be administered to patients with mild symptoms, while high doses should be administered, and even methylprednisolone impact treatment (1 mg/kg·daily) to patients with severe symptoms. Most patients with laryngotracheal involvement need to use methylprednisolone on a long-term basis[16]. An immunosuppressor can be used when glucocorticoid control is poor. For the short-term rapid relief of patients with dyspnea symptoms, brontracheal intubation or tracheotomy is the necessary treatment for upper respiratory collapse, but the scope and extent of tracheal collapse should be fully evaluated before surgery, and various types of tracheal catheter should be prepared. In the present study, tracheotomy was performed for Case 1 because the patient’s breathing difficulties could not be relieved. After being diagnosed with relapsing polychondritis, the patient received treatment with large doses of methylprednisolone plus immunosuppressor, which controlled the patient’s dyspnea symptoms. Treatment of Case 2 was adjusted after diagnosis, and after addition of immu
Relapsing polychondritis involving the larynx and trachea can be easily misdiagnosed or missed due to the atypical symptoms. However, the patient’s condition can deteriorate fast, and the prognosis is poor. To reduce the mortality rate and improve the prognosis of patients, clinicians should improve awa
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Otorhinolaryngology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Raposo A, Spain; Wang CR, Taiwan S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Chen N, Wang Z. Progress in the pathology and pathogenesis of Relapsing Polychondritis. Zhonghua Fengshibing Xue Zazhi. 2019;3:207-211. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Dion J, Costedoat-Chalumeau N, Sène D, Cohen-Bittan J, Leroux G, Dion C, Francès C, Piette JC. Relapsing Polychondritis Can Be Characterized by Three Different Clinical Phenotypes: Analysis of a Recent Series of 142 Patients. Arthritis Rheumatol. 2016;68:2992-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Taşlı H, Birkent H, Gerek M. Three Cases of Relapsing Polycondritis with Isolated Laryngotracheal Stenosis. Turk Arch Otorhinolaryngol. 2017;55:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Duan JN, Gao JF, Zhang LY. Progress in diagnosis and treatment of recurrent polychondritis. Zhonghua Fengshibing Xue Zazhi. 2019;5:356-360. [DOI] [Full Text] |
| 5. | Wang ZG, Cui L, Gao Y, Chen N. Clinical manifestation of child-onset relapsing polychondritis. Zhonghua Fengshibing Xue Zazhi. 2014;10:682-685. [DOI] [Full Text] |
| 6. | Fonseca AR, de Oliveira SK, Rodrigues MC, Aymoré IL, Domingues RC, Sztajnbok FR. Relapsing polychondritis in childhood: three case reports, comparison with adulthood disease and literature review. Rheumatol Int. 2013;33:1873-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Han S, Yu D, Gu TT, Liu Y, Wen LJ. [Two cases of relapsing polycondritis in childhood]. Zhonghua Er Ke Za Zhi. 2019;57:955-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Zhang L, Yun S, Wu T, He Y, Guo J, Han L, Lu J, Liu X, Yang R, Zhang S, Li T, Liu S. Clinical patterns and the evolution of relapsing polychondritis based on organ involvement: a Chinese retrospective cohort study. Orphanet J Rare Dis. 2021;16:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Ernst A, Rafeq S, Boiselle P, Sung A, Reddy C, Michaud G, Majid A, Herth FJF, Trentham D. Relapsing polychondritis and airway involvement. Chest. 2009;135:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | McAdam LP, O'Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore). 1976;55:193-215. [PubMed] |
| 11. | Damiani JM, Levine HL. Relapsing polychondritis--report of ten cases. Laryngoscope. 1979;89:929-946. [PubMed] |
| 12. | Michet CJ Jr, McKenna CH, Luthra HS, O'Fallon WM. Relapsing polychondritis. Survival and predictive role of early disease manifestations. Ann Intern Med. 1986;104:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 422] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Rose T, Schneider U, Bertolo M, Klotsche J, Casteleyn V, Biesen R, Burmester GR, Hiepe F. Observational study and brief analysis of diagnostic criteria in relapsing polychondritis. Rheumatol Int. 2018;38:2095-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kamada H, Takanami K, Toyama Y, Saito M, Takase K. 18F-FDG PET/CT Imaging of Vasculitis Complicated With Relapsing Polychondritis. Clin Nucl Med. 2020;45:e327-e328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Vitale A, Sota J, Rigante D, Lopalco G, Molinaro F, Messina M, Iannone F, Cantarini L. Relapsing Polychondritis: an Update on Pathogenesis, Clinical Features, Diagnostic Tools, and Therapeutic Perspectives. Curr Rheumatol Rep. 2016;18:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | de Montmollin N, Dusser D, Lorut C, Dion J, Costedoat-Chalumeau N, Mouthon L, Chassagnon G, Revel MP, Puéchal X. Tracheobronchial involvement of relapsing polychondritis. Autoimmun Rev. 2019;18:102353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Zhai S, Guo R, Zhang C, Yin H, Wang B, Wen S. Clinical analysis of relapsing polychondritis with airway involvement. J Laryngol Otol. 2022;1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Oryoji D, Ono N, Himeji D, Yoshihiro K, Kai Y, Matsuda M, Tsukamoto H, Ueda A. Sudden Respiratory Failure due to Tracheobronchomalacia by Relapsing Polychondritis, Successfully Rescued by Multiple Metallic Stenting and Tracheostomy. Intern Med. 2017;56:3369-3372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Yu C, Joosten SA. Relapsing polychondritis with large airway involvement. Respirol Case Rep. 2020;8:e00501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |