Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8336
Peer-review started: March 16, 2022
First decision: May 30, 2022
Revised: June 13, 2022
Accepted: July 8, 2022
Article in press: July 8, 2022
Published online: August 16, 2022
Processing time: 137 Days and 19.2 Hours
Papillary thyroid cancer (PTC) is the most common malignant tumor of the thyroid. However, the coexistence of PTC and sarcoma in one patient is rare. In this article, we report the case of a patient who presented with both PTC and undifferentiated pleomorphic sarcoma (UPS), which has not been previously reported in the online Medline database (PubMed).
A 71-year-old man was admitted to our hospital for a mass on the right side of his neck for one month, which rapidly enlarged within 2 wk with distending pain. The patient was diagnosed with a thyroid malignancy by fine-needle aspiration and underwent total thyroidectomy and bilateral central lymph node dissection. Histology and immunohistochemistry revealed features of both PTC and UPS. The thyroid cancer 8 gene detection kit results showed BRAF and telomerase reverse transcriptase mutations. The disease progressed rapidly, and the patient died four months after surgery from extensive lung metastasis.
Our report highlights the patient’s pathological characteristics and related genetic mutations. Due to the rapid development and poor prognosis of cooccurring PTC and sarcoma, it is important for clinical physicians and pathologists to raise awareness of this type of tumor.
Core Tip: This manuscript reports the case of a patient diagnosed with coexisting papillary thyroid carcinoma and undifferentiated pleomorphic sarcoma. The coexistence of these two pathological types is extremely rare and has not been previously reported in the online Medline database. We retrospectively reviewed the clinical and pathological characteristics of this case and analyzed the related genetic mutations and possible treatments to raise awareness of this rapidly developing tumor.
- Citation: Lee YL, Cheng YQ, Zhu CF, Huo HZ. Papillary thyroid carcinoma occurring with undifferentiated pleomorphic sarcoma: A case report. World J Clin Cases 2022; 10(23): 8336-8343
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8336.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8336
Thyroid malignancies include primary thyroid cancer, sarcoma, lymphoma, and secondary malignant cancer. Primary thyroid cancer is the most common thyroid malignancy and is categorized as papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), anaplastic thyroid cancer (ATC), or medullary thyroid cancer (MTC)[1]. While the prognosis of PTC and FTC is good, the prognosis of MTC and ATC is poor. The incidence rate of primary thyroid sarcoma (PTS) is low[2], and this disease category includes angiosarcoma, fibrosarcoma, leiomyosarcoma, hemangioendothelioma and undifferentiated pleomorphic sarcoma (UPS). UPS is a type of sarcoma without defined mesenchymal cell differentiation. It was previously known as malignant fibrous histiocytoma (MFH) and was first named UPS in the 2013 World Health Organization Classification of Soft Tissue Sarcoma[3]. Most malignant thyroid lesions involve only one pathological type; however, some cases have been reported in which two different histological types were present simultaneously. For example, PTC occurring with metastatic uterine leiomyosarcoma[4], epithelial angiosarcoma[5], synovial sarcoma[6], or endobronchial histiocytic sarcoma[7]. In this article, we report the case of a patient with PTC cooccurring with UPS. We also retrospectively reviewed the clinical and pathological characteristics of this case and analyzed the related genetic mutations and possible treatments.
A 71-year-old man was admitted to our hospital for a mass on the right side of his neck for one month.
The mass rapidly enlarged within 2 wk with distending pain from the right shoulder to the neck. Other symptoms included irritability, tremor, and loss of weight. No hoarseness, dyspnea, or other symptoms were present.
The patient had a 10-year history of hyperthyroidism, but he had stopped taking his medications by himself and had not received any further related medical examinations.
The patient had no family history of thyroid malignancy.
Physical examination revealed a large mass on the right side of his thyroid, which was hard and did not move with swallowing.
The patient’s thyroid function, routine blood test, liver function, and renal function results were within the reference range. Acidosis, hyperlactatemia, and hypokalemia were presented, with specific results as: Serum pH, 7.31; PaCO2, 44.9 mmHg; HCO3-, 22.8 mmol/L; AG, 16.9; lactic acid, 4.8 mmol/L; K+, 3.3 mmol/L.
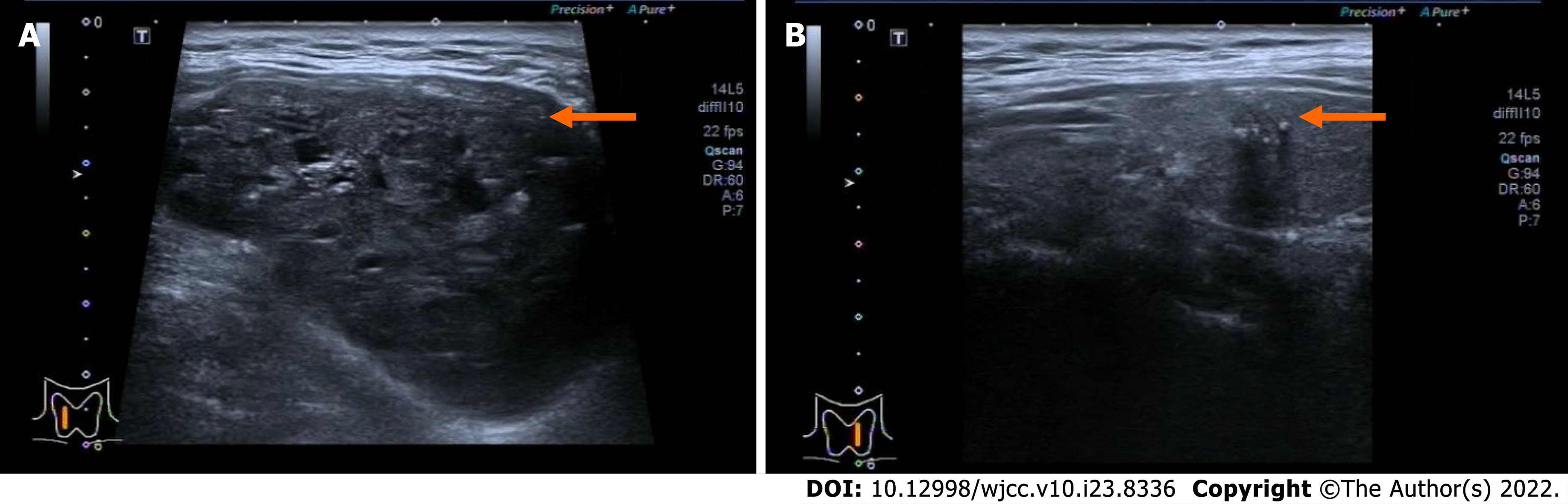
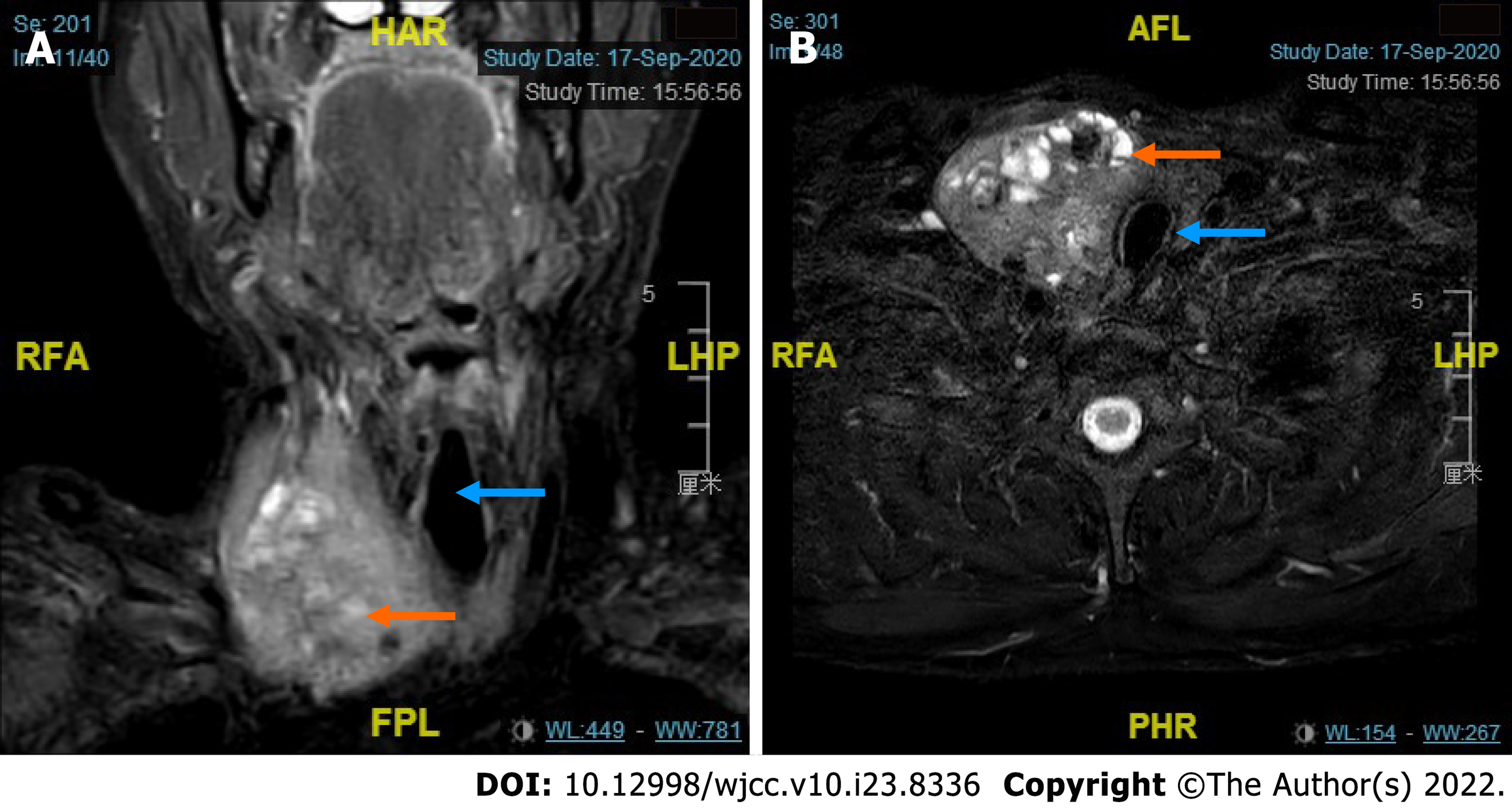
Ultrasound showed bilateral thyroid nodules. The right-side nodule was 58 mm × 41 mm × 67 mm and was classified as Thyroid Imaging Reporting and Data System (TI-RADS) 4A. The left-side nodule was 6.4 mm × 8 mm × 8 mm in size and was classified as TI-RADS 4C (Figure 1). Magnetic resonance imaging revealed that the mass was obviously pushing the trachea from right to left (Figure 2). Fine-needle aspiration showed only bilateral thyroid malignancy. Further pathological diagnosis required more thyroid tissue for confirmation.
Gene mutation analysis was also performed using a thyroid cancer 8 gene detection kit (fluorescent PCR), which detects oncogenic mutations (BRAF, NRAS, HRAS, KRAS, and TERT) and chromosome rearrangements (CCDC6-RET, PAX8/PPAR, and EVT6-NTRK3). The patient had BRAF and TERT mutations.
Considering all the information above, the patient was diagnosed with bilateral thyroid malignancy.
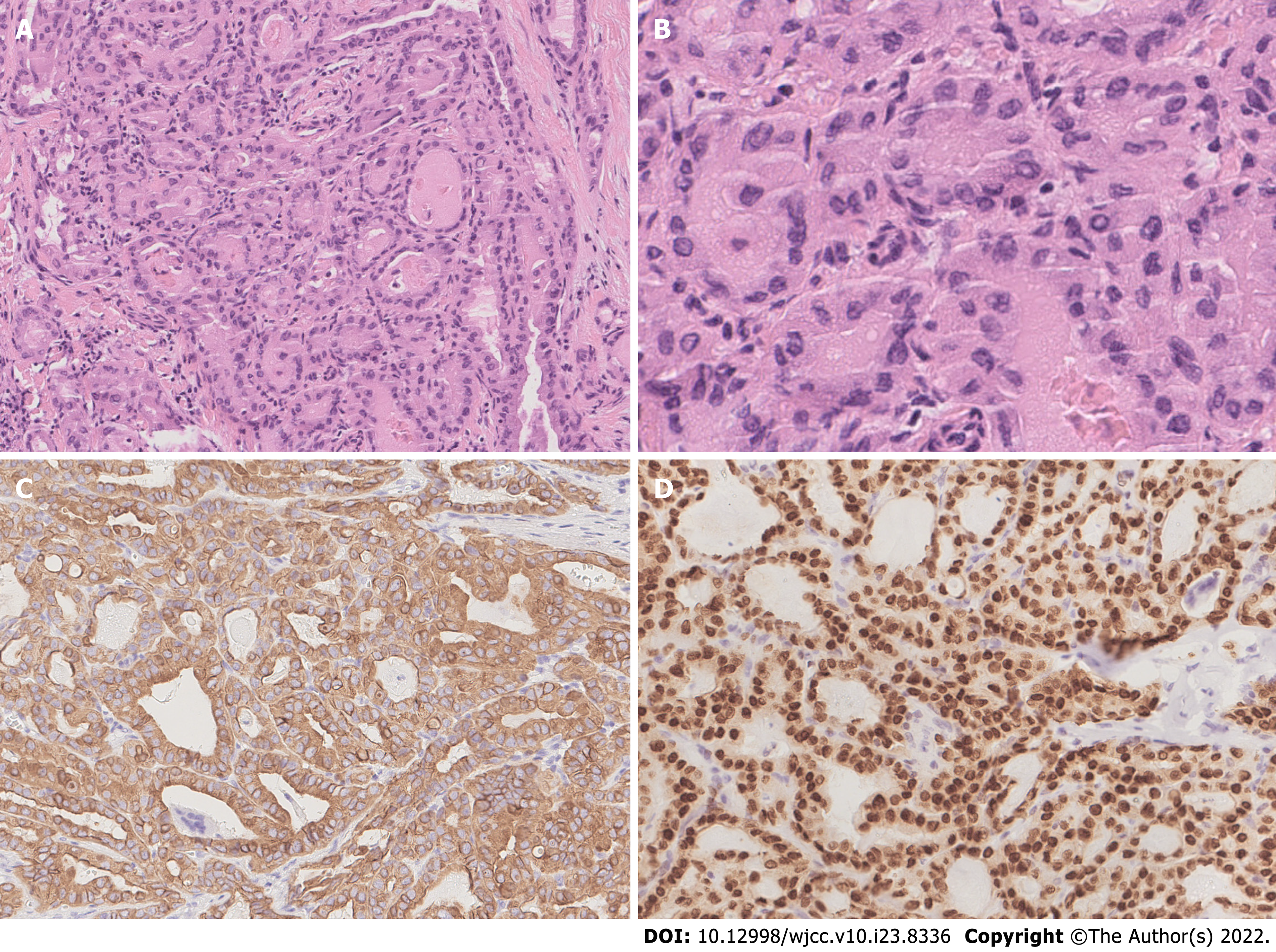
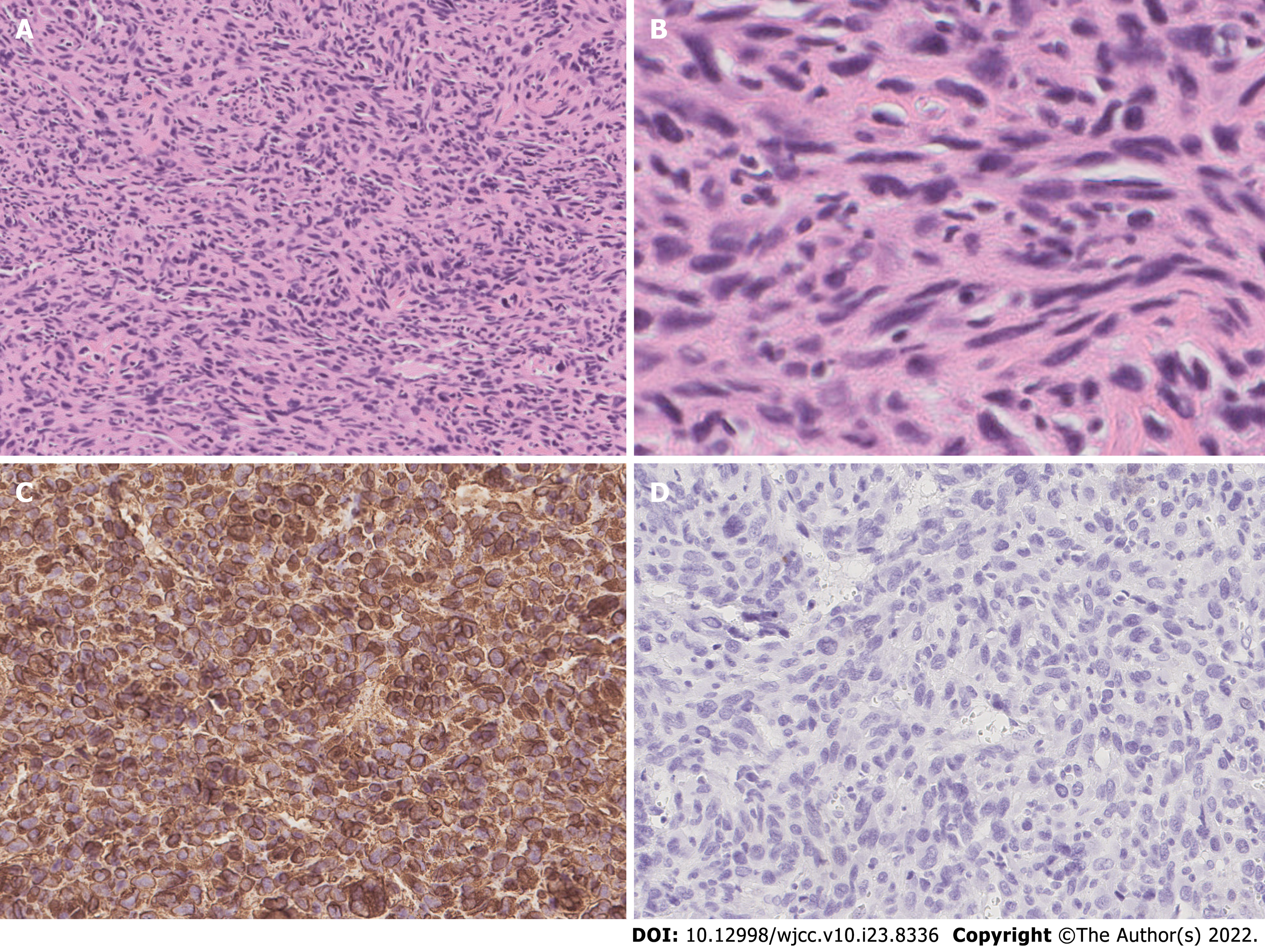
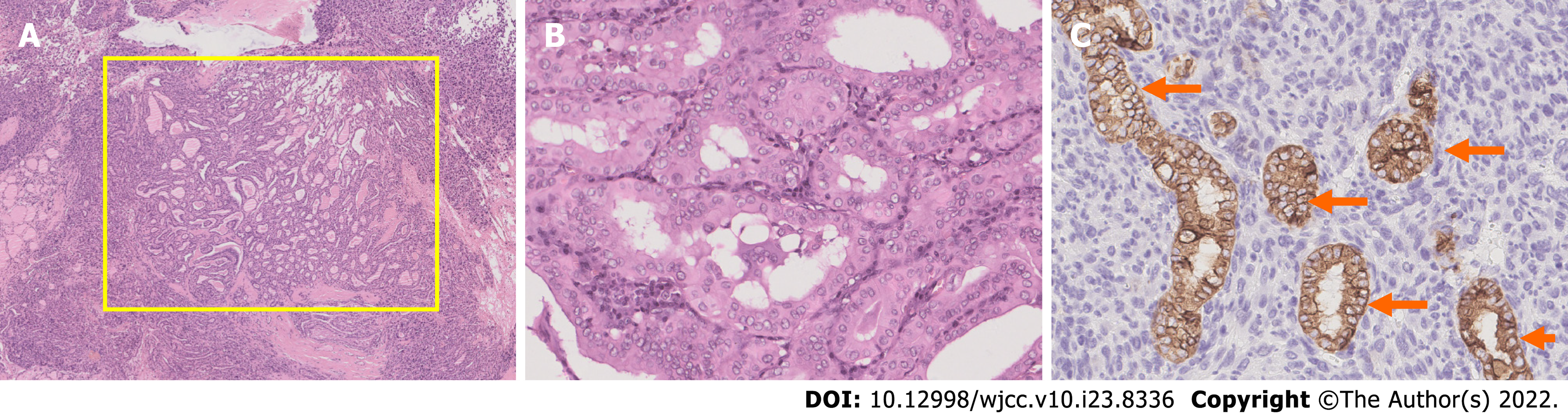
The patient underwent total thyroidectomy with bilateral central lymph node dissection. The pathology of the left nodule showed tumor cells with nuclear membrane irregularities arranged in a follicular pattern (Figure 3), and immunohistochemistry showed positive staining for CK19, MC, Gal-3, and TTF1, which matched the characteristics of PTC. The pathology of the right nodule showed two distinctive components. One component comprised spindle-shaped, round, or ovoid cells with increased mitotic activity, and immunohistochemistry showed diffuse positivity for vimentin and p53 with only sporadic CK19 expression, which supported the diagnosis of UPS (Figure 4). The other component of the right nodule matched the characteristics of PTC pathologically and immunohistochemically (Figure 5). Hence, combining the clinical behavior of the nodules with the pathological results, we concluded that the patient had coexisting PTC and UPS tumors.
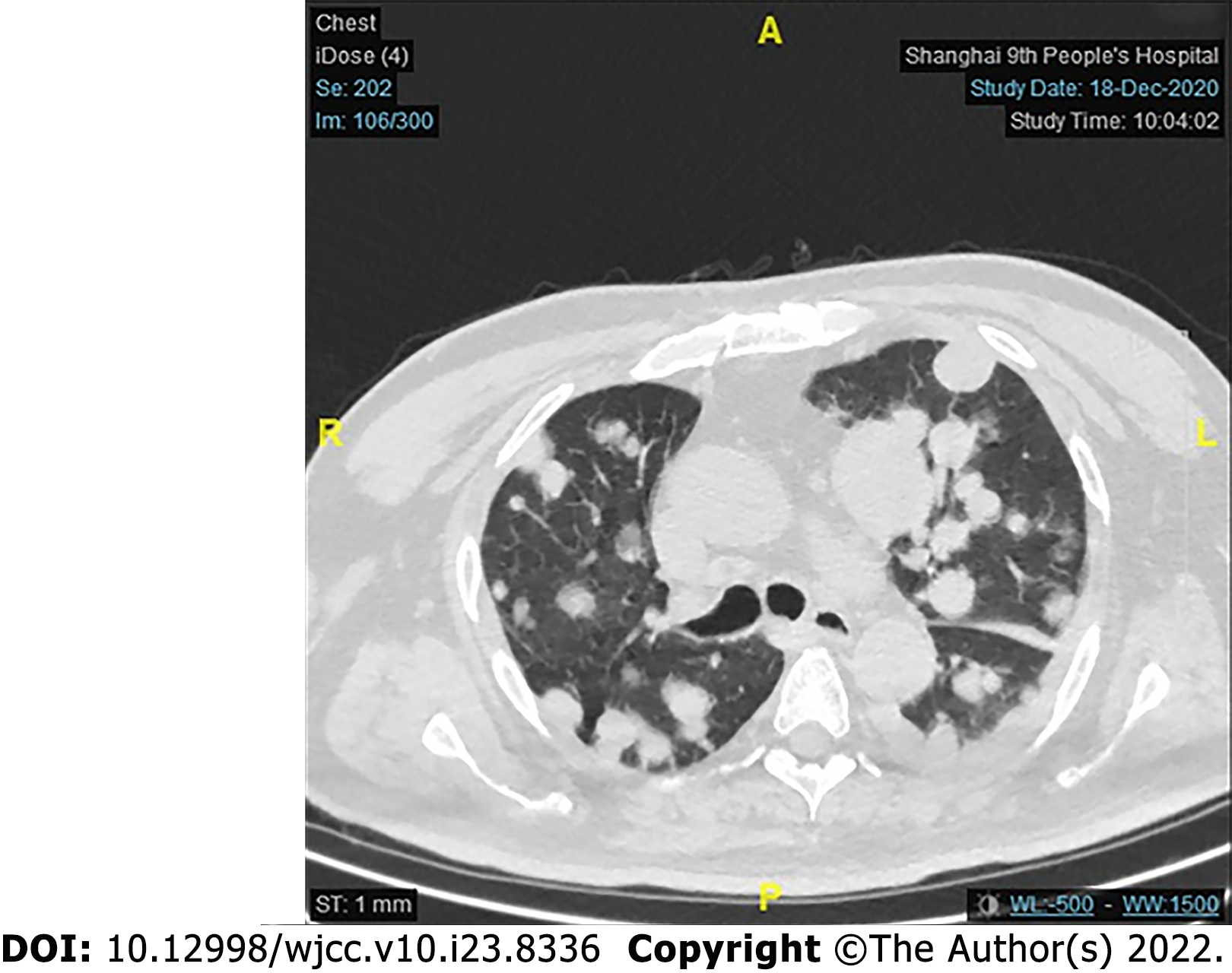
Unfortunately, for financial reasons, the patient refused to accept any further treatment. Three months after the surgery, the patient developed severe respiratory symptoms. He came to the hospital again, and serious lung metastasis was discovered (Figure 6). He died one month after the discovery of the metastasis.
To the best of our knowledge, this report is the first documented case of coexisting PTC and UPS in one tumor lesion. PTS stems from mesenchymal tissue, and the incidence of this thyroid malignancy is only approximately 0.01% to 1.5%[2]. UPS is a rare type of PTS. The morphological features of UPS are easily distinguished from those of well-differentiated thyroid carcinoma; however, the pathological features of UPS and ATC are similar on H&E staining. On immunohistochemistry, ATC is positive for epithelial markers, such as CK. However, UPS is positive for mesenchymal markers, such as vimentin, but not epithelial markers[8]. In our case, only a few cells were positive for CK; however, diffuse strong positive vimentin expression was detected. Therefore, based on the histological features and immunohistochemical results, we diagnosed the patient with coexisting PTC and UPS.
We searched the PubMed database for patients with PTS occurring with PTC using the following search terms: [(papillary thyroid carcinoma) AND (sarcoma OR angiosarcoma OR fibrosarcoma OR leiomyosarcoma OR hemangioendothelioma OR fibrous histiocytoma OR undifferentiated pleomorphic sarcoma)]. Only three patients with coexisting PTC and PTS were found in PubMed, and the type of sarcoma these patients had was epithelial angiosarcoma[5], synovial sarcoma[6] or endobronchial histiocytic sarcoma[7]. No case similar to ours was found. All patients in the three cases underwent total thyroidectomy. Aggarwal et al[7] described the case of a patient with endobronchial histiocytic sarcoma who experienced PTC. The patient underwent 5 cycles of cyclophosphamide, adriamycin, and platinum combination chemotherapy and was well after six months of follow-up. Kefeli et al[5] reported the case of a patient with thyroid angiosarcoma arising in follicular-variant PTC but did not report follow-up data. Nicola et al[6] reported the case of one patient who had PTC with synovial sarcoma. The patient underwent total thyroidectomy and was free of disease postoperatively. We also found a Chinese case report written by Wang et al[9] that described a patient with coexisting PTC and UPS. In this patient, UPS was detected 4 years after thyroidectomy for PTC, and in the UPS tissue, a small papillary lesion was observed, which was defined as tumor-to-tumor metastasis, an uncommon situation in which one tumor metastasizes to a second biologically unrelated primary tumor at the same site[10]. Our patient already presented with both PTC and UPS when it was first discovered, so we could not determine whether tumor-to-tumor metastasis occurred in our case.
The test for 8 gene mutations in this patient included oncogenic mutations (BRAF, NRAS, HRAS, KRAS, and TERT) and chromosome rearrangements (CCDC6-RET, PAX8/PPAR, and EVT6-NTRK3). In our case, the patient had BRAFV600E and TERTC228T gene mutations. The BRAFV600E mutation overstimulates the mitogen-activated protein kinase (MAPK) pathway. The MAPK pathway regulates the expression of several genes associated with proliferation and differentiation[11]. BRAF kinase acts as an intracellular signal transducer in the MAPK pathway. Therefore, the BRAFV600E mutation results in high BRAFV600E kinase activity and can lead to uncontrolled cell proliferation and metastasis[12]. The influence of BRAF on patient outcomes is still inconclusive. Some researchers have pointed out that the BRAFV600E mutation is associated with worse outcomes among PTC patients than other mutations and is also significantly associated with lymph node metastasis, extrathyroidal extension, an advanced disease stage, and a higher recurrence rate[13]. However, some other researchers demonstrated that PTC patients with the BRAFV600E mutation did not have a significantly higher risk for a poor prognosis[14]. These findings suggest that an isolated BRAF mutation is probably not specific for tumor-related mortality. Since thyroid carcinoma patients occasionally have more than one molecular mutation and the behavior of tumors may result from other gene mutations, which are known to lead to aggressive characteristics, a comprehensive discussion of different genes is essential.
Mutations in the telomerase reverse transcriptase promoter (TERT), including TERTC228T or TERTC250T, were first reported in thyroid cancer in 2013 by Liu et al[15]. Many studies have been conducted to explore the relationship between a poor prognosis and TERT mutations. TERT is a component of telomerase that adds DNA repeats onto the chromosome. It resynthesizes telomeres to preserve a sufficient telomere length for continued chromosomal replication. Mutation of the TERT promoter prompts TERT transcription, and the unlimited preservation of the telomere length facilitates cancer cell immortality. TERT mutations have been observed in only 10% of well-differentiated PTCs[16] and are associated with factors such as increased age, extrathyroidal invasion, an advanced disease stage at diagnosis, and a dedifferentiated histological type. TERT is also independently associated with PTC dedifferentiation[17]. Several studies have demonstrated the synergistic effects of concomitant mutations of BRAF and TERT. Chen et al[14] reported that PTC patients with both BRAFV600E and TERT mutations had an increased risk for a poor prognosis in terms of mortality, disease persistence, and recurrence compared with patients with one gene mutation. In another report, the interaction between BRAFV600E and TERT mutations was studied by transcriptomics[18]. In this study, when the BRAFV600E mutation was present, activation of the MAPK pathway upregulated the expression of the E-twenty-six transcription factor family, which can bind to the mutant TERT promoter and increase TERT transcription[17]. These results explain why cancer-specific mortality is increased in patients with both gene mutations.
No conclusive analysis has been performed of the possible genes associated with UPS. The possible associated mutations described to date are in GNAS, TP53, K-ras, and other genes, such as CSF1R, FGFR3, KDR, APC, PDGFRA, FLT3, ERBB4, KIT, STK11, and RET[19]. Our patient had UPS with BRAF and TERT mutations. Interestingly, studies have shown that high mutant BRAFV600E kinase activity causes genetic instability, and secondary mutation of the phosphoinositide 3-kinase-Akt serine/threonine kinase (PI3K-AKT) pathway occurs, thus leading to further progression of ATC[20]. Since ATC and UPS are both poorly differentiated tumors and have similar histological behaviors, genetic instability caused by BRAF may also lead to the generation of UPS. More research is needed to identify the association between UPS and BRAF and TERT mutations. According to the American Thyroid Association guidelines, treatment for PTC includes surgery, radiotherapy, or chemotherapy. Most PTC patients respond well to surgery and survive long-term. On the other hand, although surgery is the main treatment for UPS, due to the high aggressiveness of the tumor, it is difficult to achieve a satisfactory margin of resection, and there is a high risk of local recurrence[3]. Adjuvant radiotherapy and chemotherapy are typically given after surgery, but no proven benefit has been described[3]. Patients typically die from local recurrence or distant metastasis. Due to the rarity of coexisting PTC and UPS, therapy might be challenging because no treatment recommendations have been made to date. Recently, improvements in molecular diagnostic techniques have helped scientists identify targeted treatments for these patients. These targeted drugs can block the activity of oncogenes by binding to molecules in the signaling pathway associated with cellular differentiation, thus inhibiting tumor progression. Our patient had the BRAFV600E mutation and a TERT mutation, and this pattern of molecular alteration is likely to result in tumor progression. Thus, a more aggressive treatment is needed. When the BRAFV600E mutation is present, the MAPK pathway is overactivated; thus, we hypothesize that tyrosine kinase inhibitors might be effective by suppressing this signaling pathway[1]. A combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib can be considered. This combination is approved by the FDA, and a central radiology review demonstrated an overall response rate of 69%[21]. In one patient, dabrafenib/trametinib therapy was well tolerated, and the patient lived for more than six months[22]. To date, no medications targeting TERT or telomerase have been developed.
PTC coexisting with UPS is rare and is first reported in this article. Immunohistochemical tests are crucial for differential diagnosis. Several mutations in important oncogenes, such as BRAF and TERT, may participate in the generation of this tumor. Surgery is the standard treatment for PTC and UPS; however, advanced targeted therapy should be considered for further treatment.
We express many thanks to the patient for generously authorizing us to share his rare case.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hashimoto K, Japan; Kanat O, Turkey S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388:2783-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 1019] [Article Influence: 113.2] [Reference Citation Analysis (1)] |
| 2. | Surov A, Gottschling S, Wienke A, Meyer HJ, Spielmann RP, Dralle H. Primary Thyroid Sarcoma: A Systematic Review. Anticancer Res. 2015;35:5185-5191. [PubMed] |
| 3. | Chen Q, Huang Q, Yan JX, Li C, Lang JY. Primary undifferentiated pleomorphic sarcoma of the thyroid: A case report and review of the literature. Medicine (Baltimore). 2018;97:e9927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Giannikaki E, Mantadakis E, Mamalaki E, Delides G, Samonis G. Metastatic uterine leiomyosarcoma coexisting with papillary carcinoma of the thyroid gland. Int J Gynecol Cancer. 2006;16:442-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Kefeli M, Mete O. An unusual malignant thyroid nodule: coexistence of epithelioid angiosarcoma and follicular variant papillary thyroid carcinoma. Endocr Pathol. 2014;25:350-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 6. | Nicola M, Onorati M, Bertola G, Collini P, Fascì AI, Di Nuovo F. Primary thyroid biphasic synovial sarcoma and synchronous papillary carcinoma: report of a remarkable case. Pathologica. 2018;110:106-110. [PubMed] |
| 7. | Aggarwal R, Rao S, Sarangi R. Primary endobronchial histiocytic sarcoma in a case of papillary carcinoma thyroid: a complex clinical scenario. J Bronchology Interv Pulmonol. 2014;21:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Hsu KF, Lin YS, Hsieh CB, Yu JC, Duh QY, Sheu LF, Jen YM, Shih ML. Primary malignant fibrous histiocytoma of the thyroid: review of the literature with two new cases. Thyroid. 2008;18:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Wang L, Zhou Q, Zhang Y, Zhao H, Chang H. A case of papillary thyroid carcinoma metastasized to pleomorphic undifferentiated sarcoma and a clinicopathological observation. J Clin Exp Pathol. 2016;32:696-697. |
| 10. | Das S, Chaudhary N, Ang LC, Megyesi JS. Papillary thyroid carcinoma metastasizing to anaplastic meningioma: an unusual case of tumor-to-tumor metastasis. Brain Tumor Pathol. 2017;34:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 702] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 12. | Haroon Al Rasheed MR, Xu B. Molecular Alterations in Thyroid Carcinoma. Surg Pathol Clin. 2019;12:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer. 2007;110:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 227] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Chen B, Shi Y, Xu Y, Zhang J. The predictive value of coexisting BRAFV600E and TERT promoter mutations on poor outcomes and high tumour aggressiveness in papillary thyroid carcinoma: A systematic review and meta-analysis. Clin Endocrinol (Oxf). 2021;94:731-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, Sun H, El-Naggar AK, Xing M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 470] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 16. | Kim TH, Kim YE, Ahn S, Kim JY, Ki CS, Oh YL, Kim K, Yun JW, Park WY, Choe JH, Kim JH, Kim JS, Kim SW, Chung JH. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr Relat Cancer. 2016;23:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Oishi N, Kondo T, Ebina A, Sato Y, Akaishi J, Hino R, Yamamoto N, Mochizuki K, Nakazawa T, Yokomichi H, Ito K, Ishikawa Y, Katoh R. Molecular alterations of coexisting thyroid papillary carcinoma and anaplastic carcinoma: identification of TERT mutation as an independent risk factor for transformation. Mod Pathol. 2017;30:1527-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Song YS, Yoo SK, Kim HH, Jung G, Oh AR, Cha JY, Kim SJ, Cho SW, Lee KE, Seo JS, Park YJ. Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer. Endocr Relat Cancer. 2019;26:629-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Sawazaki H, Tsujita Y, Ito Y. Malignant fibrous histiocytoma arising from the renal capsule and gene mutation screening: A case report. Urol Case Rep. 2019;27:101004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Abdullah MI, Junit SM, Ng KL, Jayapalan JJ, Karikalan B, Hashim OH. Papillary Thyroid Cancer: Genetic Alterations and Molecular Biomarker Investigations. Int J Med Sci. 2019;16:450-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 21. | Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol. 2018;36:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 613] [Article Influence: 76.6] [Reference Citation Analysis (1)] |
| 22. | Skwiersky S, Hevroni G, Singh G, Hope L, Haidary T, Salifu MO, McFarlane SI. Concurrent Anaplastic and Papillary Thyroid Carcinomas: A Case Report. Am J Med Case Rep. 2020;8:202-205. [PubMed] [DOI] [Full Text] |