Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8298
Peer-review started: February 6, 2022
First decision: May 30, 2022
Revised: June 9, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 16, 2022
Processing time: 175 Days and 15.7 Hours
Delayed inflammatory reactions (DIRs) in alloplast rhinoplasty are a rare complication that may occur several months to years after surgery. The exact causes and mechanisms are unclear, but several triggering factors, including infections, trauma, dental procedures, and vaccination, have been reported.
A 39-year-old male patient who had undergone augmentation rhinoplasty 8 years ago had DIRs after the administration of the first dose of the mRNA Pfizer coronavirus disease 2019 (COVID-19) vaccine. He suddenly had tender, ery
The correlation between DIRs and COVID-19 vaccination has not been reported yet and the exact mechanism is unclear. Because the uncontrolled inflammatory reactions on the nose leave serious sequelae, surgeons should be conscious of the correlation between COVID-19 vaccines and DIRs associated with nasal alloplastic implants. And further histological or microbiological studies should be performed to determine the cause of DIRs.
Core Tip: Delayed inflammatory reactions (DIRs) in alloplast rhinoplasty are rare and their correlation with coronavirus disease 2019 (COVID-19) vaccines is unclear. We present herein the first case of DIRs in alloplast rhinoplasty after the first administration of the COVID-19 vaccine. We performed surgical removal of an alloplastic implant because no improvements were observed in the patient’s condition after conservative treatment. This intervention accelerated recovery. A delayed fibrotic reaction induced by the COVID-19 vaccine may be a possible cause. Our case suggests surgeons should be aware of the correlation between COVID-19 vaccines and DIRs in alloplast rhinoplasty.
- Citation: Seo MG, Choi EK, Chung KJ. Delayed inflammatory response evoked in nasal alloplastic implants after COVID-19 vaccination: A case report. World J Clin Cases 2022; 10(23): 8298-8303
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8298.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8298
Alloplast rhinoplasty is one of the most commonly performed plastic surgery procedures[1]. Clinically, silicone implants account for the majority of alloplastic implants in rhinoplasty. Despite the proven safety of these implants, there are several complications related to these implants such as infections, extrusion, and capsular contractures[2]. Delayed inflammatory reactions (DIRs) in alloplastic implants can also occur several months to many years after alloplast rhinoplasty. Tenderness, swelling, and erythema around the nose and glabella may be noted[3]. DIRs associated with hyaluronic acid (HA) fillers are not rare, the prevalence is up to 4.25%[4]. In addition, studies on the relationship between soft tissue fillers and coronavirus disease 2019 (COVID-19) vaccines have been conducted and global recommendations have also been proposed[5]. Unlike HA fillers, the incidence of DIRs related to alloplastic implants in rhinoplasty is unknown and its exact causes and mechanisms are unclear[3]. And their correlation with COVID-19 vaccines has not been reported yet. Although the incidence of DIRs in alloplast rhinoplasty may be low, the management of DIRs is crucial because DIRs can cause serious esthetic and functional complications[3]. And increased COVID-19 vaccination rates during the COVID-19 pandemic may be a new risk factor for DIRs in alloplast rhinoplasty. In this case report, we present the first case of DIRs in alloplast rhinoplasty after the first administration of the COVID-19 vaccine. The patient who had undergone alloplast rhinoplasty with a silicone implant eight years ago developed DIRs within several days after administration of the first dose of the mRNA Pfizer COVID-19 vaccine. This case and our course of treatment will provide insights into the correlation between the COVID-19 vaccines and DIRs associated with alloplastic implants in rhinoplasty.
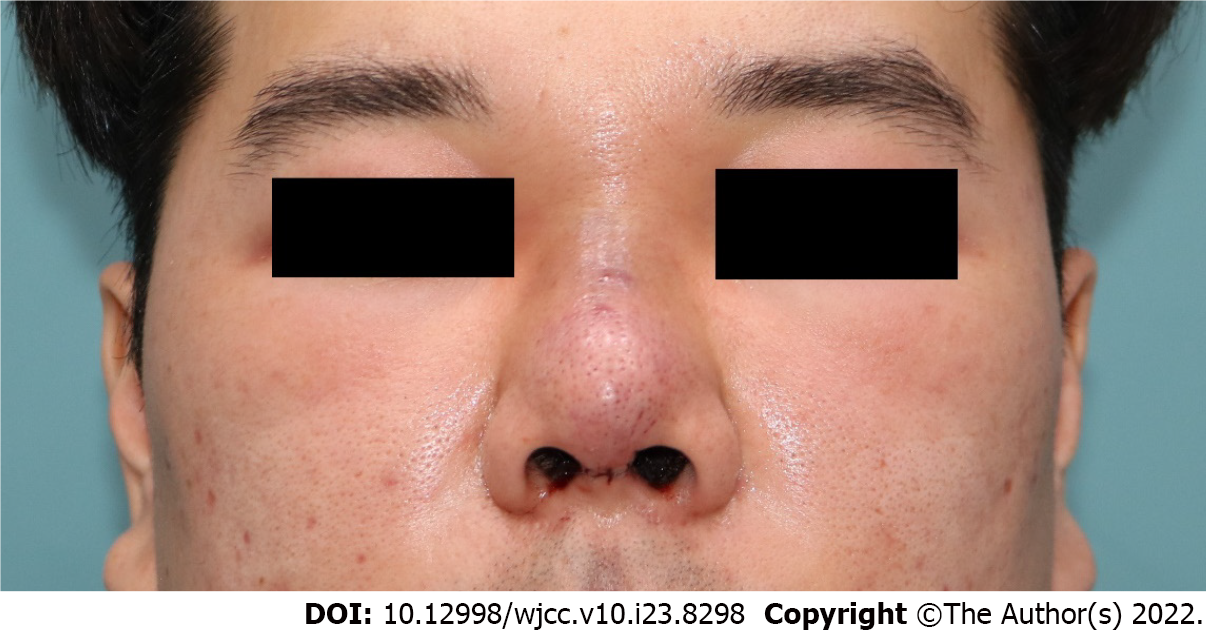
In September 2021, a 39-year-old male patient presented with tender, erythematous swelling on his glabella, the nasal dorsum, and both malar areas.
Inflammation began 6 d after the patient had received his first dose of the mRNA Pfizer COVID-19 vaccine.
He had undergone augmentation rhinoplasty with a silicone implant in 2013. After the surgery, he had not experienced any discomfort or complications related to the nasal implant. He had not undergone any dental treatment or suffered any inflammatory skin disease on the face in the six months before the vaccination.
The patient was physically fit and healthy and did not have a history of any medical comorbidities or allergies. Moreover, he had no significant familial history including a history of inflammatory skin disease.
On the day of the transfer, his body temperature was 38.2 °C. He still had tender, erythematous swelling on his glabella. The redness spread from the nasal dorsum and to both the malar areas (Figure 1). He complained of severe tenderness and discomfort in the center of his face, including his glabella. There were no other visible external lesions or discharge on the face.
The initial erythrocyte sedimentation rate was 18 mm/h (normal range, 0-20 mm/h), the C-reactive protein level was 3.805 mg/dL (normal range, 0-0.5 mg/dL), and the white blood cell (WBC) level was 15550/μL (normal range, 4000-10000/μL). The other laboratory tests included assessments of liver function, kidney function, and electrolytes, which were within the normal range.
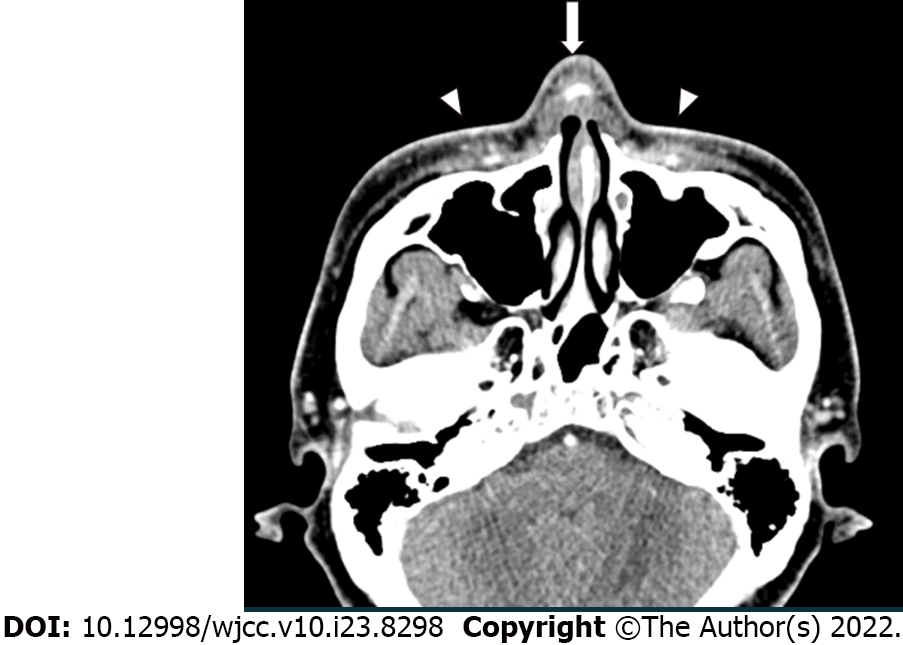
Owing to the acute symptoms, he immediately visited a nearby emergency room on the day of symptom onset and underwent facial computed tomography (CT). The non-contrast facial CT revealed that the silicone implant was located above the nasal cartilage and infiltrated subcutaneous tissue was confirmed around the silicone implant (Figure 2). There was no evidence of other foreign bodies that could cause inflammation.
During surgery, we detected inflammatory tissues and some fluid collection around the silicone implant. No other foreign bodies were detected. Other than a prior administration of COVID-19 vaccination, no other factors could be identified that might evoke this delayed inflammatory response. Bacterial culture of the fluid collection around the silicone implant and biopsy of the capsules was performed during surgery. Staphylococcus aureus without any antibiotic resistance was cultured from the fluid collection. In addition, several capsules around the silicone implant were confirmed as fibrosis and small fragments of the hyaline cartilage through biopsy.
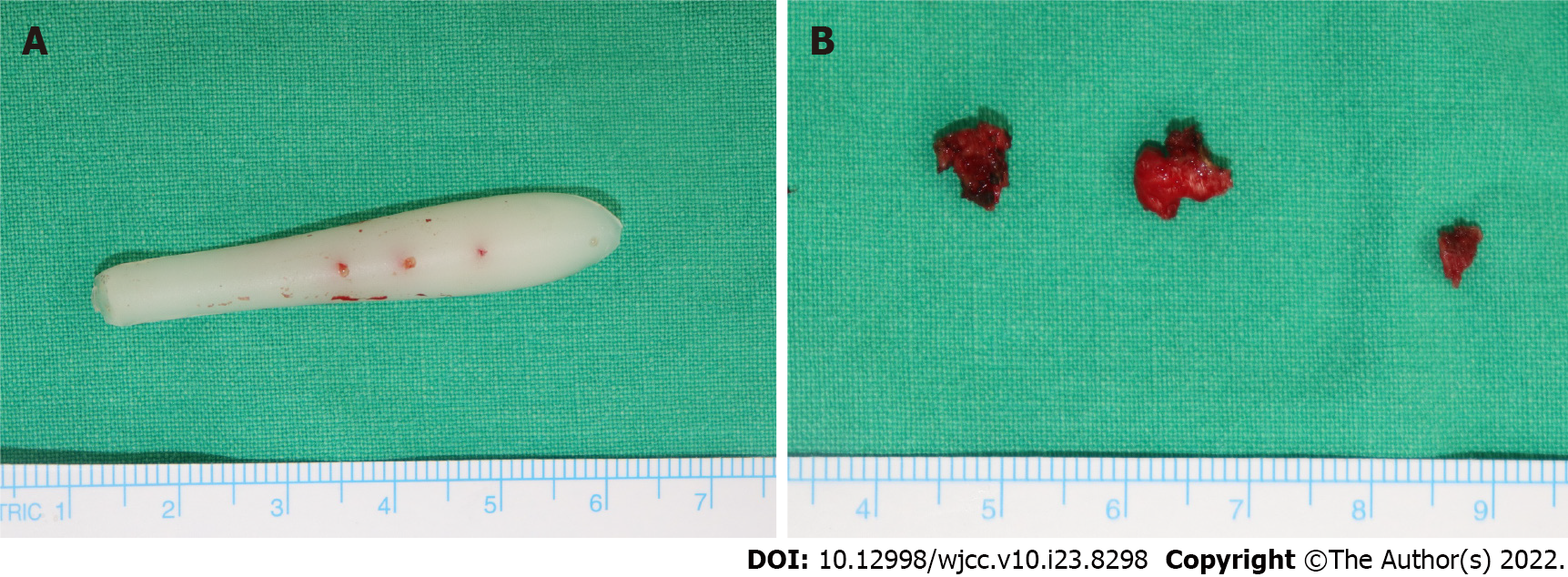
He initially received an intravenous injection of 30 mg of ketorolac tromethamine (Kerola, non-steroidal anti-inflammatory drugs) and 1 g of Cefazolin in the emergency room. As there was no improvement in his symptoms the treatment, the next day, he visited the hospital where he had undergone augmentation rhinoplasty 8 years ago. After sufficient fasting time, he underwent exploration surgery to determine the cause of inflammation and remove the nasal implant under local anesthesia. It was difficult to perform surgery because he had severe pain and anxiety, and thus, the implant removal procedure failed. He was then transferred to the Yeungnam university hospital to undergo revision surgery under general anesthesia. Revision surgery was performed the following day to debride the inflammatory tissue and remove the nasal implant under general anesthesia (Figure 3A). During surgery, bacterial culture of the fluid collection inside the wound was performed. As some capsules were formed around the implant, a biopsy of the capsules was performed (Figure 3B). After confirming that the fluid collection and implant inside the wound were sufficiently removed, wound closure was performed. We prevented hematoma formation by 16G catheters as drainage tubes and packing the merocel inside both nostrils. Intravenous administration of 1.2 g of Amoxycillin and clavulanic acid (Amocla) was performed three times per day.
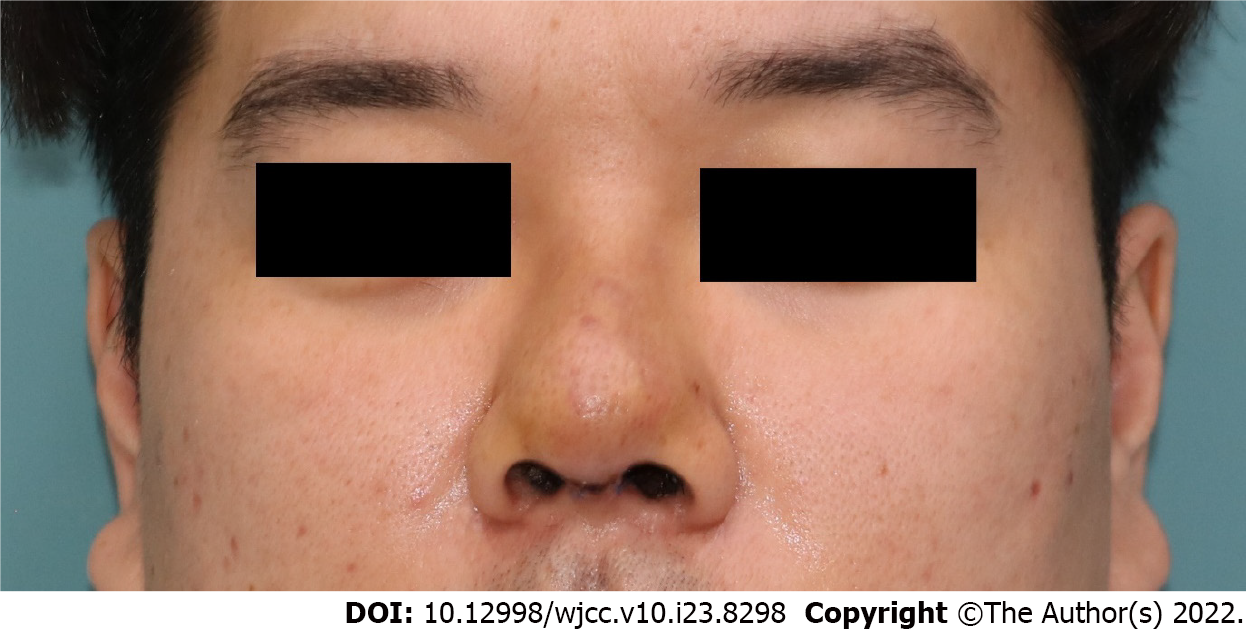
He experienced an improvement in swelling and erythema within the first day and achieved complete resolution on postoperative day 4 (Figure 4). He reported no recurrence of swelling or erythema on the nose for four months.
DIRs associated with alloplast rhinoplasty are rare, and it is difficult to predict when DIRs may occur and what causes DIRs after surgery. It is known that DIRs in HA fillers can be evoked from an immunologic stimulus, with or without the presence of bacterial infection[4]. In particular, immunizations can be potential immunologic stimulations because they can modulate immune surveillance and activate immune systems. Moreover, the COVID-19 vaccine has been reported to cause DIRs in HA fillers[6]. The symptoms of DIRs in HA fillers may appear as localized swelling, tenderness, fever, or flu-like illness[7]. As similar reactions and pathophysiological processes were observed in our case, the mRNA Pfizer COVID-19 vaccine also can be considered a triggering factor of DIRs in silicone implants used in rhinoplasty.
Other hypotheses have been proposed to explain the pathophysiology of DIRs in silicone implants used in rhinoplasty. One potential factor is infection and peri-implant biofilm development due to bacteria[3]. In our case, Staphylococcus aureus without any antibiotic resistance was cultured from the fluid collection. These results may suggest the cause of DIRs as infection in this case but the possibility is low. The test was performed during the revision surgery, so the results of the microbial culture test suggested a high possibility of contamination during the first surgery. Furthermore, the cultured bacteria were the skin flora. The initial WBC level was 15550/μL and the initial differential ratio of neutrophils was 86.9%. These findings can be related to inflammation as well and do not necessarily indicate infection. In addition, the patient had not had any inflammatory skin diseases and had not undergone dental or other aesthetic procedures that could cause infection in the six months prior to presentation.
Late seroma formation may also contribute to the etiology of DIRs[3,6]. During revision surgery, only fluid collection and inflammatory tissue around the implant due to inflammation were identified, and the amount was not large. These small clusters were not sufficient to create the mechanical dynamics required to evoke symptomatic DIRs. In particular, in dermal fillers, some adverse reactions distant from the vaccine injection site are explained by granulomatous or fibrotic reactions caused by vaccination[6]. Similarly, fibrotic reactions around the nasal implant promoted by COVID-19 vaccination may have occurred. In this case, the formation of fibrotic capsules around the nasal implant was confirmed through biopsy. Thus, delayed fibrosis around the nasal implant induced by COVID-19 vaccination may be another factor in DIRs.
As the COVID-19 vaccination rate increases, the incidence of DIRs in alloplast rhinoplasty will increase. However, vaccination is necessary because of the morbidity, mortality, and socioeconomic impact of the COVID-19 pandemic[8]. Surgeons should be conscious of the possibility and perils of DIRs in alloplast rhinoplasty. However, the management of DIRs in alloplast rhinoplasty can be quite challenging due to the mechanisms and causes of DIRs that have not yet been elucidated[7]. Patients with DIRs in nasal alloplastic implants should receive empirical antibiotics and undergo percutaneous drainage if needed[3]. Because DIRs are often transient, self-limited diseases and resolve within days to weeks, further surgical intervention is not always necessary[3,7]. If the conditions and symptoms of the patient do not improve despite conservative treatment, removal of the nasal implant via surgery might accelerate the patient’s recovery. In our case, the patient experienced an improvement in symptoms within the first day after surgery. With implant removal and total capsulectomy, curettage of the inflammatory tissue and granuloma-like lesions should be performed. The space where the implant resides should be irrigated copiously and a drain should be placed to prevent the formation of hematoma or seroma. A histological study of the capsules and additional bacterial culture tests can be useful data for determining the cause of DIRs.
The correlation between COVID-19 vaccines and soft tissue filler reactions has been well studied, and global recommendations have been proposed. In contrast, DIRs in alloplast rhinoplasty are rare, and cases related to COVID-19 vaccines have not yet been reported. Through this case, it was confirmed that the COVID-19 vaccine could be a possible factor in DIRs in nasal alloplastic implants. Surgeons should be conscious of the possibility and perils of DIRs with COVID-19 vaccination. And if DIRs occur, immediate treatment should be instituted. And additional studies should be conducted to establish clear correlations and mechanisms. Patients who undergo alloplast rhinoplasty must be educated about the risk of DIRs before COVID-19 vaccination and consent should be obtained from them.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hazafa A, Pakistan; Islamoglu MS, Turkey S-Editor: Wang DM L-Editor: A P-Editor: Wang DM
| 1. | Sharif-Askary B, Carlson AR, Van Noord MG, Marcus JR. Incidence of Postoperative Adverse Events after Rhinoplasty: A Systematic Review. Plast Reconstr Surg. 2020;145:669-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Kim IS. Augmentation Rhinoplasty Using Silicone Implants. Facial Plast Surg Clin North Am. 2018;26:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Moon KC, Lee KI, Lee JS, Kim AR, Dhong ES, Kim DW, Han SK. Late-Onset Inflammation in Asian Rhinoplasty Using Alloplastic Implants. Aesthetic Plast Surg. 2021;45:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Sarigul Guduk S. A case of delayed inflammatory filler reaction following vaccination with succesful response to colchicine. J Cosmet Laser Ther. 2021;23:52-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Gotkin RH, Gout U, Sattler S, Piansay-Soriano ME, Wanitphakdeedecha R, Ghannam S, Rossi E, Ferrariz TS, Hexsel D, Frank K, Davidovic K, Sarnoff DS, Cotofana S. Global Recommendations on COVID-19 Vaccines and Soft Tissue Filler Reactions: A Survey-Based Investigation in Cooperation With the International Society for Dermatologic and Aesthetic Surgery (ISDS). J Drugs Dermatol. 2021;20:374-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Niebel D, Novak N, Wilhelmi J, Ziob J, Wilsmann-Theis D, Bieber T, Wenzel J, Braegelmann C. Cutaneous Adverse Reactions to COVID-19 Vaccines: Insights from an Immuno-Dermatological Perspective. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 7. | Michon A. Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID-19 vaccination - A case report. J Cosmet Dermatol. 2021;20:2684-2690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg. 2020;78:185-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2872] [Cited by in RCA: 2681] [Article Influence: 536.2] [Reference Citation Analysis (0)] |