Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8262
Peer-review started: January 27, 2022
First decision: March 23, 2022
Revised: April 19, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 16, 2022
Processing time: 185 Days and 17.5 Hours
Lymph node skip metastases are common in lung, breast, and thyroid cancer patients, but are rare in colon cancer patients. Specifically, lymph node skip metastases occur in 1%-3% of colon cancer patients. Previous reports have demonstrated colon cancer skip metastases involving the retropancreatic and portocaval lymph nodes and Virchow's node; however, reports involving skip metastases into the left neck lymph nodes and left shoulder skin are extremely rare, as are related reports of clinical treatment and prognosis.
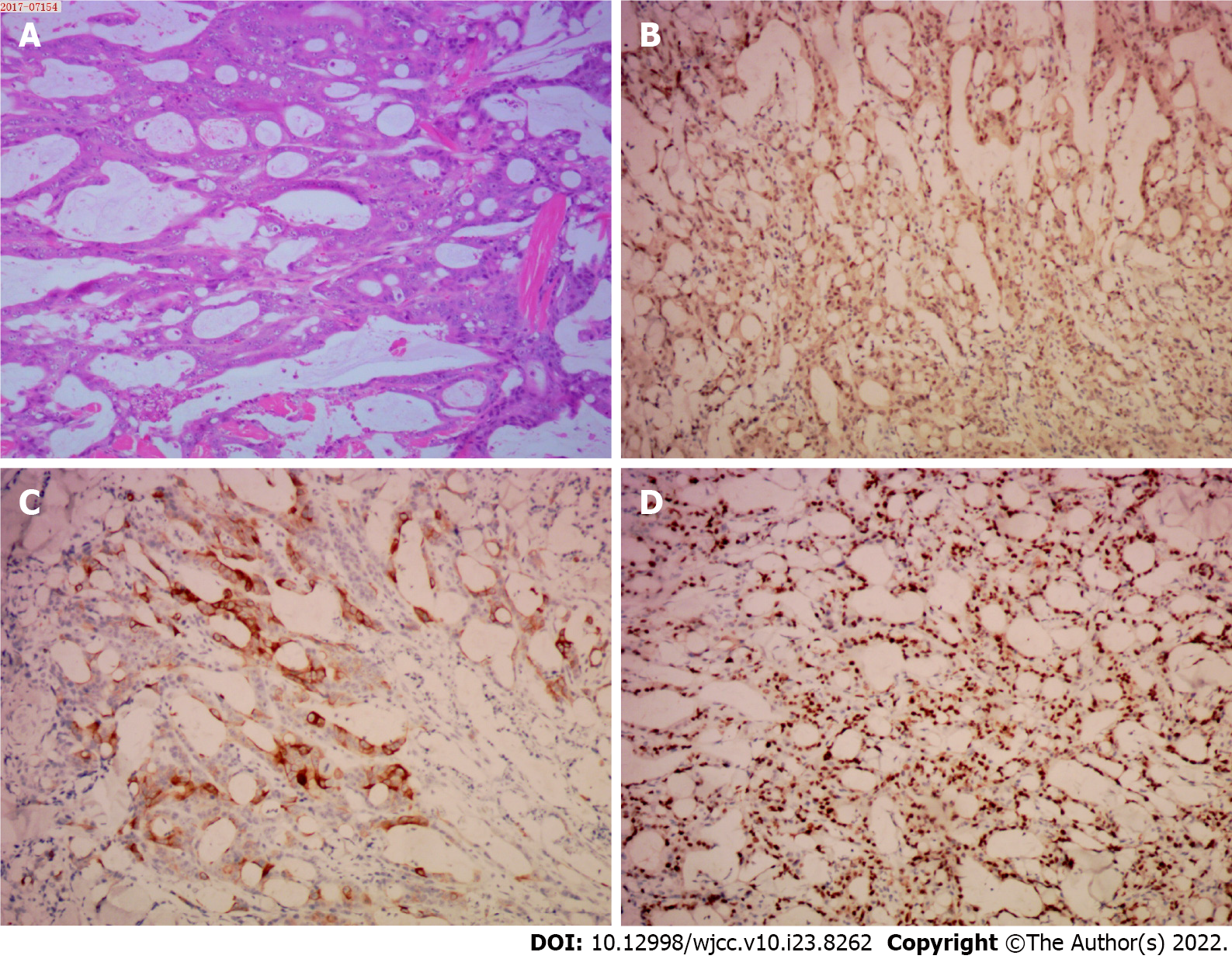
A 44-year-old Chinese man was admitted to the hospital for evaluation of persistent shoulder pain for 3 d and a cutaneous mass (3.0 cm × 2.0 cm) on the left shoulder. The left shoulder cutaneous mass was excised and bisected, revealing tissues with a fish-like appearance. The pathologic diagnosis of the cutaneous mass suggested a signature [CDX-2 (++), CK20 (++), Ki-67 (+) > 50%] of infiltrating or metastatic colorectal adenocarcinoma. An enhanced computed tomo
We report an abnormal skip metastasis involving the left shoulder skin and left neck lymph node in a patient with ascending colon adenocarcinoma. Specifically, we observed non-metastatic involvement of the lymph nodes around the tumor site but with metastases to the cervical lymph nodes. The standard surgical operations were performed to resect the cutaneous mass, tumor tissue, and cervical lymph nodes, followed by chemotherapy for eight courses. The patient is healthy with no tumor recurrences or metastases for 4 years. This clinical case will contribute to future research about the abnormal skip metastasis in colon cancers and a better clinical treatment design.
Core Tip: Colon cancer skip metastasis is rare, especially for skin skip metastasis in the left shoulder. Non-metastatic local lymph node involvement with metastasis to cervical lymph nodes suggested an unusual mechanism underlying tumor metastasis. Following resection of the primary and metastatic tumor tissues, the patient has no tumor recurrences and has a good prognosis, which may benefit future clinical treatment for colon cancer patients with skip metastases.
- Citation: Zhou JC, Wang JJ, Liu T, Tong Q, Fang YJ, Wu ZQ, Hong Q. Primary ascending colon cancer accompanying skip metastases in left shoulder skin and left neck lymph node: A case report. World J Clin Cases 2022; 10(23): 8262-8270
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8262.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8262
Tumor metastasis is commonly found in cancer patients and most correlated with cancer-related deaths[1]. In this process, the tumor cells escape from the primary tumor site and migrate into distant organs through the circulatory system. However, the evolution routes and mechanisms of these malignant cells in tumor metastasis are still unclear[2]. Furthermore, the research on developmental pathways between lymph nodes and distant organs metastases reveals the distinct genetic lineage diversity and selective evolutional routes during tumor metastasis[3]. Skip metastasis is defined as distant metastasis of lymph nodes or organs without the involvement of local lymph nodes; for colon cancers, the occurrence rate of skip metastasis is between 1% and 3%[4]. Moreover, the limited cohort size of patients with skip metastasis restrains the systemic studies regarding the pathogenesis and prognosis of colon skip metastasis[5,6]. In this case, we report a patient diagnosed with ascending colon ulcerative mucinous adenocarcinoma and skip metastasis to the left shoulder and cervical lymph nodes. Following resection of the primary and metastatic tumor lesions and oxaliplatin and capecitabine chemotherapy treatment, this patient is currently tumor-free, suggesting that colon cancer patients with skip metastasis can still achieve a good prognosis through standardized treatments.
A 44-year-old Chinese man was hospitalized in 2017 for evaluation of a mass involving the left shoulder with swelling and pain for 3 d.
No previous history of left shoulder pain or gastrointestinal diseases.
The past medical history was benign. Specifically, the patient did not have hypertension, diabetes, or cardiovascular disease.
The patient had no known allergies, a history of surgery, or a family history of colon cancer.
A 3.0 cm × 2.0 cm hard mass was noted on the left shoulder skin with local erythema and swelling, tenderness, and limited mobility, while no other abnormalities were found during the initial physical examination; palpably enlarged lymph nodes in the left neck (2.0 cm × 1.0 cm) were identified in the medical examination after surgical operation.
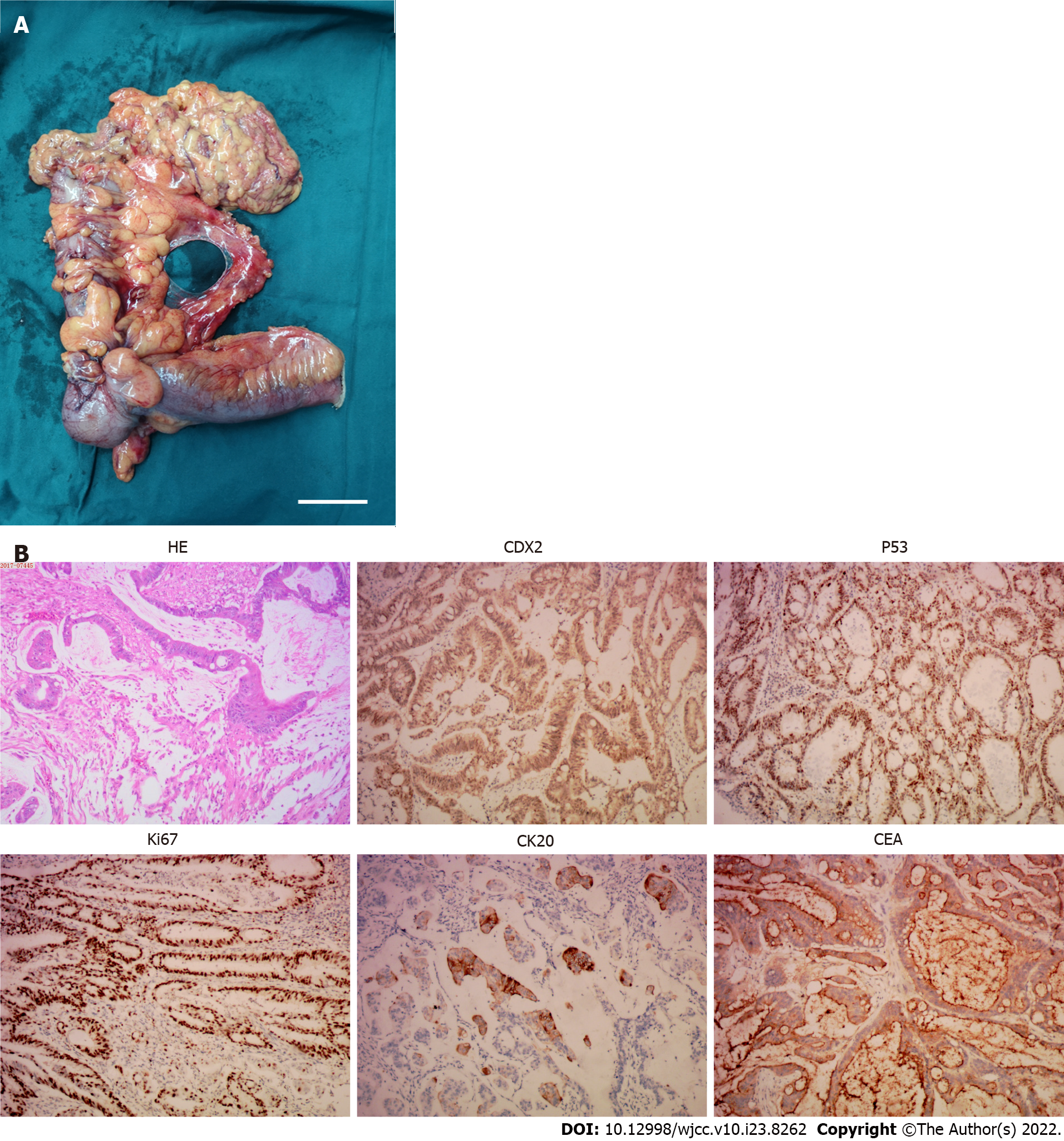
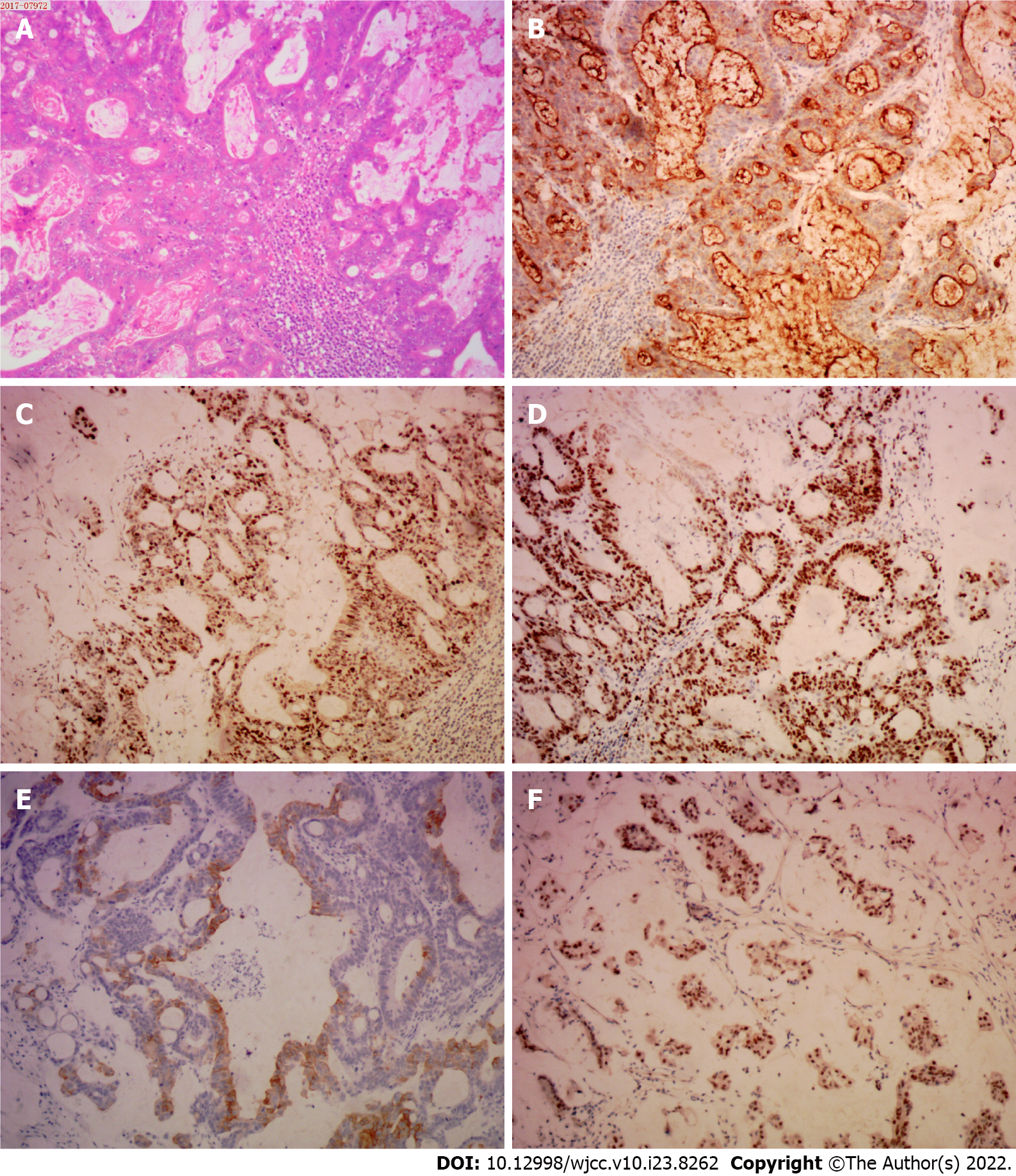
The coagulation profile, results of liver and kidney function tests, and results from routine urinalysis were all within the normal range. The routine blood test results were as follows: white blood cells, 10.01 × 109/L (normal range, 4.00-10.00 × 109/L); hypersensitive C-reactive protein, 18.28 mg/L (normal range: 0.00-5.00 mg/L); hemoglobin, 113.00 g/L (normal range: 120.00-160.00 g/L); carcinoembryonic antigen (CEA), 1.79 ng/mL (normal range: 0-2.5 ng/mL); and CA-125, 13.67 U/mL (normal range: 0.00-35.00 U/mL). The fecal occult blood test was positive (Table 1). The immunohistochemistry tests of resected cutaneous mass were CDX-2 (++), CK20 (++), and Ki-67 (+) > 50%, suggesting the infiltrating or metastatic adenocarcinoma (Figure 1); the immunohistochemistry tests of resected ascending colon tumor mass were CEA (++), P53 (+), CDX-2 (++), CK20 (+), Ki-67 (+) > 50%, confirming the ascending colon adenocarcinoma (Figure 2); the immunohistochemistry tests of cervical lymph nodes were CEA (++), P53 (++), CDX-2 (+), CK20 (+), Ki-67 (+) > 50%, which were in consistent with the previous diagnostic results (Figure 3), suggesting the skip metastasis of ascending colon cancer. The genetic testing of the tumor specimen of this patient revealed no KRAS or NRAS mutations, microsatellite stability, response to 5-fluorouracil, and the absence of PD-L1 expression.
| Data | Examination | Value | Normal range |
| 10/22/2017 | White blood cells | 10.01 × 109/L | 4.00-10.00 × 109/L |
| 10/22/2017 | Hypersensitive C-reactive protein | 18.28 mg/L | 0.00-5.00 mg/L |
| 10/22/2017 | Hemoglobin | 113.00 g/L | 120.00-160.00 g/L |
| 10/27/2017 | CEA | 1.79 ng/mL | 0-2.5 ng/mL |
| 10/27/2017 | CA-125 | 13.67 U/mL | 0.00-35.00 U/mL |
| 10/23/2017 | Fecal occult blood test | + | - |
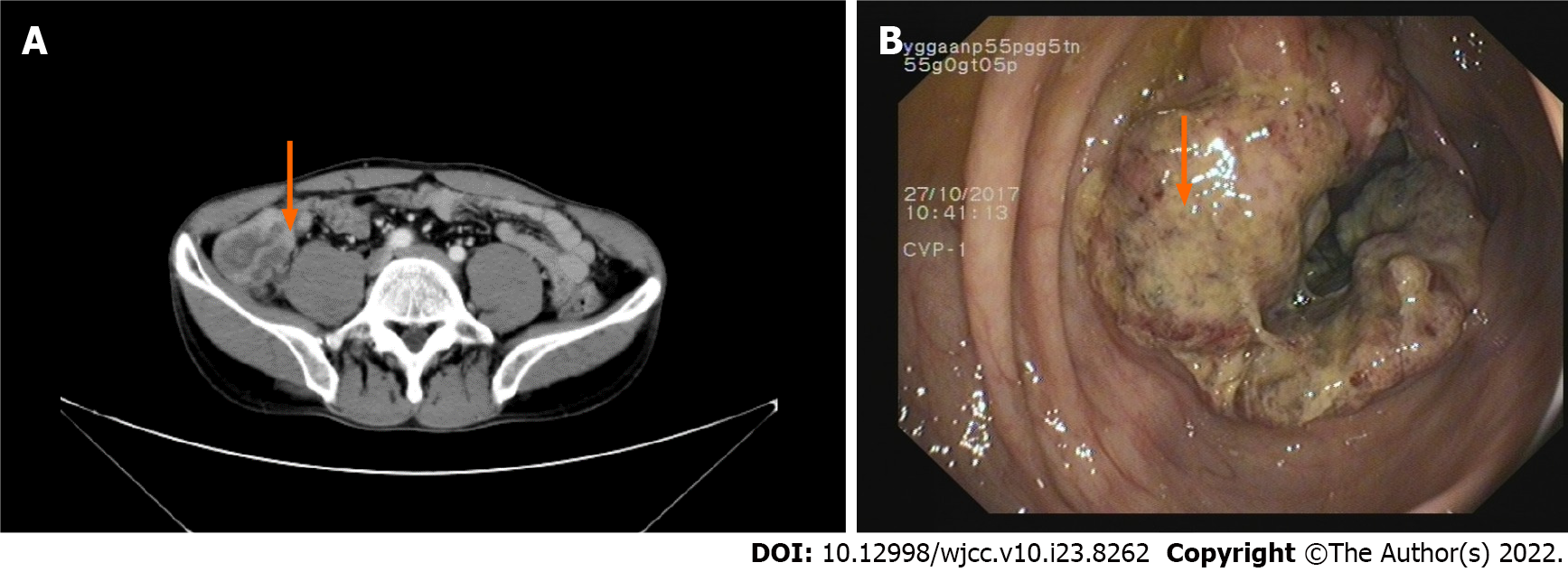
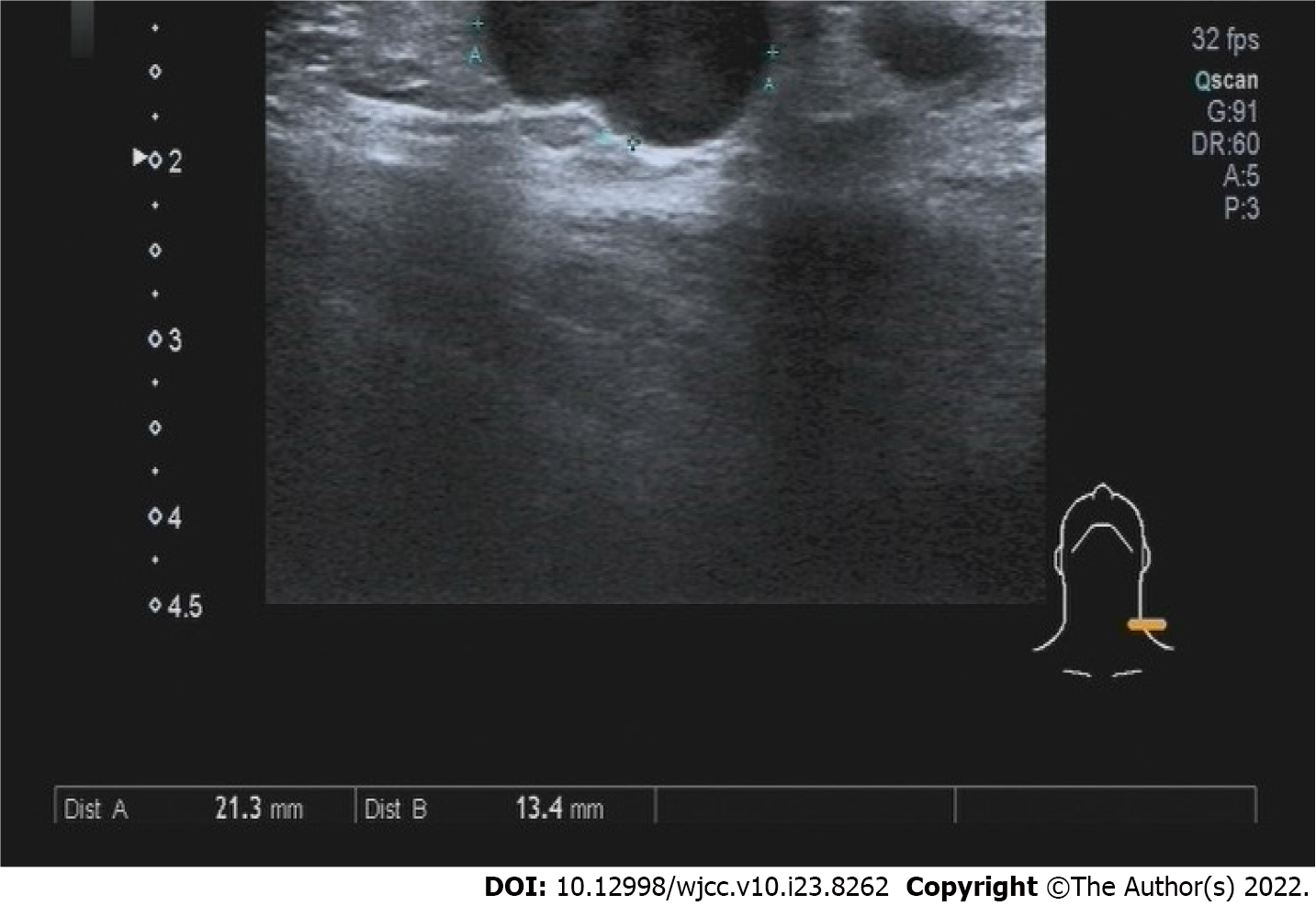
B-scan ultrasonography revealed a hypoechoic nodule on the left shoulder without tumorous features. Enhanced computed tomography (CT) of the abdomen revealed chronic appendicitis with fecal stone formation, cecal edema, and pelvic effusion (Figure 4A). Colonoscopy revealed a cauliflower-like mass in the ileocecal area involving the lumen with surface erosions and bleeding (Figure 4B). A color Doppler ultrasound of the neck revealed several hypoechoic lesions in the left neck with clear borders, while no obvious medullary echoes were observed, with the largest one being 2.13 cm × 1.34 cm (Figure 5).
The patient was diagnosed with ascending colon ulcerative mucinous adenocarcinoma with skin and cervical lymph nodes skip metastases, and classified as T4aN0M1a stage IVA, based on the American Joint Committee on Cancer stages, 8th edition[7].
This patient underwent a left shoulder skin mass resection after the initial diagnosis in October 2017, and the pathological finding of the resected mass suggested a metastatic colon adenocarcinoma. We then performed an enhanced CT of the abdomen and colonoscopy to find a potential primary tumor site and further identified and pathologically confirmed an ascending colon adenocarcinoma. This patient underwent laparoscopic radical resection of the right colon in November 2017. The postoperative medical examination revealed palpably enlarged lymph nodes in the left neck, and the following color Doppler ultrasound confirmed hypoechoic lesions in the left neck with no obvious medullary echoes, suggesting a lymph node metastasis. This patient then underwent left cervical lymphadenectomy for complete removal of the lymph nodes in November 2017, and the pathological test confirmed the metastasis of ascending colon cancer. After a multi-disciplinary team discussion, the patient received chemotherapy treatments [oxaliplatin (200 mg ivgtt d1) and capecitabine (1500 mg bid po d1-14), q3w] for eight courses, and the dose and treatment plan were adjusted based on the clinical efficacy.
The patient returned to the hospital for evaluation every 3 mo during 2018-2019, and then once a year until now (Figure 6). The results of contrast-enhanced CT and chest CT revealed no tumor recurrence or bilateral cervical swollen lymph nodes; the colonoscopy examinations revealed several benign polyps, and all were removed. The CEA and CA-125 test results have all been in normal ranges since 2018 (Table 2).
| Data | Examination | CA-125 (U/mL) | CEA (ng/mL) |
| 9/6/2018 | No abnormal CT examinations of chest and abdomen, no palpable cervical lymph nodes. | 29.9 | 1.7 |
| 12/26/2018 | No abnormal CT examinations of chest and abdomen, no palpable cervical lymph nodes. | 19.3 | 2.04 |
| 04/29/2019 | No abnormal CT examinations of chest and abdomen, no palpable cervical lymph nodes. | 21 | 1.65 |
| 10/10/2019 | No abnormal CT examinations of chest and abdomen, no palpable cervical lymph nodes. | 31.5 | 1.78 |
| 03/24/2020 | No abnormal CT examinations of chest and abdomen, no palpable cervical lymph nodes. | 23.4 | 2.02 |
| 03/31/2021 | No abnormal CT examinations of chest and abdomen, no palpable cervical lymph nodes. | 30.3 | 1.88 |
| 03/23/2022 | No abnormal CT examinations of chest and abdomen, no palpable cervical lymph nodes. | 28.9 | 1.33 |
Skip metastasis refers to metastases to distant lymph nodes without spreading into local lymph nodes[8], as has been reported in gastrointestinal tumors with cervical lymph nodes, posterior ear lymph nodes, or skin metastases[9], and nasopharyngeal cancer with inguinal lymph nodes metastasis[10]. Lymph node skin metastasis occurs with inadequate immune responses of the sinus endothelial cells or lymph follicles in the para-cancerous lymph nodes[11]. T lymphocytes, among lymphatic sinus endothelial cells, have an immune-killing effect on cancer cells that have metastasized along with the lymphatic system, and meanwhile have a filtering effect on the migration and metastasis of cancer cells[11]. When the functionality of lymphatic sinus lymphocytes is low, free cancer cells can easily metastasize to distant lymph nodes through the nearby lymph node barrier[11]. In addition, blockage of tumor thrombi in the adjacent lymphatic vessels will change the direction of lymph flow, and the cancer cells will metastasize to distant locations through the collateral circulation[12].
Only a few studies on colorectal cancer have focused on the effect of skip metastasis on patient prognosis, and the results are controversial. Amin et al[7] concluded that skip metastasis in patients with thyroid cancer has a low level of lymph node metastasis and a low local recurrence rate; while skip metastasis tumors have strong proliferative activity and high malignancy, leading to a poor prognosis. In contrast, the results from the study conducted by Shiozawa et al[8] on lung cancer skip metastasis showed that the prognosis of patients was better than those without skip metastasis. Saha et al[9] also suggested that skip metastasis did not affect postoperative survival, but further validation of the conclusion is needed. Moreover, Ho et al[10] reported an association between the number of lymph nodes resected and lymph node metastasis in 2427 patients with colorectal cancer. The 5-year survival rates of patients with lymph node metastasis in whom ≤ 7 or ≥ 18 Lymph nodes were removed were 62.2% and 75.8%, respectively (P = 0.02), which suggested that the more lymph nodes resected in patients with colorectal cancer, the better the prognosis[10].
Herein, we reported skip metastasis in a patient with ascending colon cancer. We hypothesized that due to low immune surveillance, malignant cells escaped from the immune killing effect of T lymphocytes within near lymph nodes and metastasized to left cervical lymph nodes via lymphatic reflux. Notably, we also detected metastasis to the left shoulder skin. We hypothesized that the metastatic lymph nodes of the left neck were blocked by tumor thrombi, and the malignant cells passed through the subcutaneous lymphatic network, thus causing metastasis to the left shoulder skin. During the diagnosis and treatment of this patient, the preoperative diagnosis was inaccurate. The left shoulder mass was pathologically diagnosed as an infiltrating or metastatic colon adenocarcinoma, the colonoscopy biopsy was diagnosed as an ascending colon adenocarcinoma based on the pathological evaluation without the involvement of local lymph nodes, and the imaging excluded liver or lung metastases; however, skip metastasis and other micrometastases were not sufficiently examined, leading to an incorrect preoperative diagnosis. Standard tumor markers, CT, and gastrointestinal endoscopy are insufficient to confirm the diagnosis or staging in patients with skip metastasis. Positron emission tomography (PET)-CT may be a good choice for a complete and accurate diagnosis of skip metastasis; specifically, in a patient who undergoes PET-CT examination that confirms metastasis to the left cervical lymph node, the left neck lymph node and the right colon cancer should be removed at the same time, which can save time and economic resources as well as improve the prognosis.
The oxaliplatin and capecitabine chemotherapy, which is more convenient and has fewer side effects than target-based bevacizumab therapy, was selected for treating our patient, given the tumor-free state of the patient after surgery. Presently, the patient has a good prognosis and has been tumor-free for nearly 4 years. Therefore, in our clinical case, colon cancer skip metastasis was not a contraindication to surgery. If the primary tumor and the tumor metastasis can be completely removed in conjunction with safe and efficacious treatments, a good prognosis can be achieved.
Skip metastasis in patients with colon cancer is extremely rare. The diagnosis of skip metastasis without the involvement of regional lymph nodes during primary surgery is still a challenge, which requires more comprehensive examinations for patients at the risk of skip metastasis. The impact of skip metastasis on prognosis is still controversial. We reported a good prognosis in a patient after the complete resection of primary and metastatic tumor lesions and chemotherapy. This case report will provide more evidence of skip metastasis in colon cancer and facilitate optimal clinical treatment of similar patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gnetti L, Italy; Nakaji K, Japan; Pattarajierapan S, Thailand S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Ganesh K, Massagué J. Targeting metastatic cancer. Nat Med. 2021;27:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 637] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 2. | Gui P, Bivona TG. Evolution of metastasis: new tools and insights. Trends Cancer. 2022;8:98-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Reiter JG, Hung WT, Lee IH, Nagpal S, Giunta P, Degner S, Liu G, Wassenaar ECE, Jeck WR, Taylor MS, Farahani AA, Marble HD, Knott S, Kranenburg O, Lennerz JK, Naxerova K. Lymph node metastases develop through a wider evolutionary bottleneck than distant metastases. Nat Genet. 2020;52:692-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 4. | Merrie AE, Phillips LV, Yun K, McCall JL. Skip metastases in colon cancer: assessment by lymph node mapping using molecular detection. Surgery. 2001;129:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Reddy R, Das P, Rukmangadha N, Manilal B, Kalawat T. Colonic carcinoma presenting with axillary lymphadenopathy-a very rare clinical entity. IJSR. 2017;6:112-114. |
| 6. | Suliman MS, Singh M, Ajmeri AN, Stuart DL, Teka ST. Virchow's node: a case report of an extremely rare presentation of metastasis of adenocarcinoma with mucinous features from the colon. Int J Gen Med. 2019;12:137-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4397] [Article Influence: 549.6] [Reference Citation Analysis (4)] |
| 8. | Shiozawa M, Akaike M, Yamada R, Godai T, Yamamoto N, Saito H, Sugimasa Y, Takemiya S, Rino Y, Imada T. Clinicopathological features of skip metastasis in colorectal cancer. Hepatogastroenterology. 2007;54:81-84. [PubMed] |
| 9. | Saha S, Elgamal M, Mukkamala S, Siva T, Knight P, Chang A, Pentapati S, Kaushal S, Alnounou M, Mannam S. Meta-analysis of skip metastasis after sentinel lymph node mapping in gastrointestinal cancers and its biological relevance: An international study. J Clin Oncol. 2018;15_suppl:e15698. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Ho FC, Tham IW, Earnest A, Lee KM, Lu JJ. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. BMC Cancer. 2012;12:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 11. | Ji RC. Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses. Int J Mol Sci. 2016;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Jones D, Pereira ER, Padera TP. Growth and Immune Evasion of Lymph Node Metastasis. Front Oncol. 2018;8:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |