Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8255
Peer-review started: December 8, 2021
First decision: June 7, 2022
Revised: June 18, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 16, 2022
Processing time: 235 Days and 18.5 Hours
The coexistence with patent ductus arteriosus (PDA), mitral valve prolapse (MVP), atrial fibrillation (AF) and hyperthyroidism is extremely rare and complex. The optimal therapeutic strategy is difficult to develop.
A 27-year-old female with PDA, MVP, AF and hyperthyroidism presented with severe dyspnea. Given that a one-stage operation for PDA, MVP and AF is high risk, we preferred a sequential multidisciplinary minimally invasive therapeutic strategy. First, PDA transcatheter closure was performed. Hyperthyroidism and heart failure were simultaneously controlled via medical treatment. Video-assisted thoracoscopic mitral valve repair and left atrial appendage occlusion were performed when heart failure was controlled. Under this therapeutic strategy, the patient’s sinus rhythm was restored and maintained. Two years after the treatment, the symptoms of heart failure were relieved, and the enlarged heart was reversed.
Sequential multidisciplinary therapeutic strategies, which take advantage of both internal medicine and surgical approaches, might be reasonable for this type of disease.
Core Tip: The coexistence of patent ductus arteriosus (PDA), mitral valve prolapse, atrial fibrillation and hyperthyroidism is extremely rare and complex. We proposed a successful sequential multidisciplinary therapeutic strategy for a 27-year-old female suffering from these four diseases in addition to severe heart failure. PDA transcatheter closure, medical treatment and thoracoscopic mitral valve repair were performed sequentially. Two years after the treatment, the symptoms of heart failure were relieved, sinus rhythm was restored, and the enlarged heart was reversed.
- Citation: Zhao CZ, Yan Y, Cui Y, Zhu N, Ding XY. Sequential multidisciplinary minimally invasive therapeutic strategy for heart failure caused by four diseases: A case report. World J Clin Cases 2022; 10(23): 8255-8261
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8255.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8255
Patent ductus arteriosus (PDA), mitral valve prolapse (MVP), atrial fibrillation (AF) and hyper
A 27-year-old female was referred to our hospital complaining of palpitations, irritability, edema of the legs and dyspnea for over ten years. She presented with severe shortness of breath and paroxysmal nocturnal dyspnea for 20 d.
The patient developed palpitations, irritability, edema of the legs and dyspnea for over ten years. She was initially diagnosed with hyperthyroidism but failed to achieve a euthyroid state. One month prior, she was diagnosed with PDA, MVP with severe mitral regurgitation (MR) and heart failure [New York Heart Association (NYHA) Classification III] while being treated for hyperthyroidism at a previous hospital. The cardiothoracic surgeons planned to perform one-stage surgery through a standard full median sternotomy when her heart failure had been controlled. She left the hospital and took 7.5 mg qd methimazole, 11.875 mg qd metoprolol, 20 mg qd furosemide and 20 mg qd spironolactone. Twenty days before presentation, she developed severe shortness of breath and paroxysmal nocturnal dyspnea. Given that her heart failure was refractory to medical therapy, she was transferred to our hospital.
The patient had no history of past illness.
The patient had no personal or family history.
Physical examination showed continuous grade 4/6 murmurs at the left second to third intercostal spaces, systolic grade 3/6 murmurs at the apex with AF, moist rales in both lower lungs and severe edema of both legs.
Laboratory examination showed elevated brain natriuretic peptide (BNP, 890.3 pg/mL) and decreased thyroid hormone (TT3 0.53 μg/L, normal 0.66-1.61 μg/L; TT4 36.3 μg/L, normal 54.4-118.5 μg/L; TSH 0.00 mIU/L, normal 0.34-5.6 mIU/L; FT3 2.45 ng/L, normal 2.14-4.21 ng/L; FT4 5.22 ng/L, normal 5.9-12.5 ng/L).
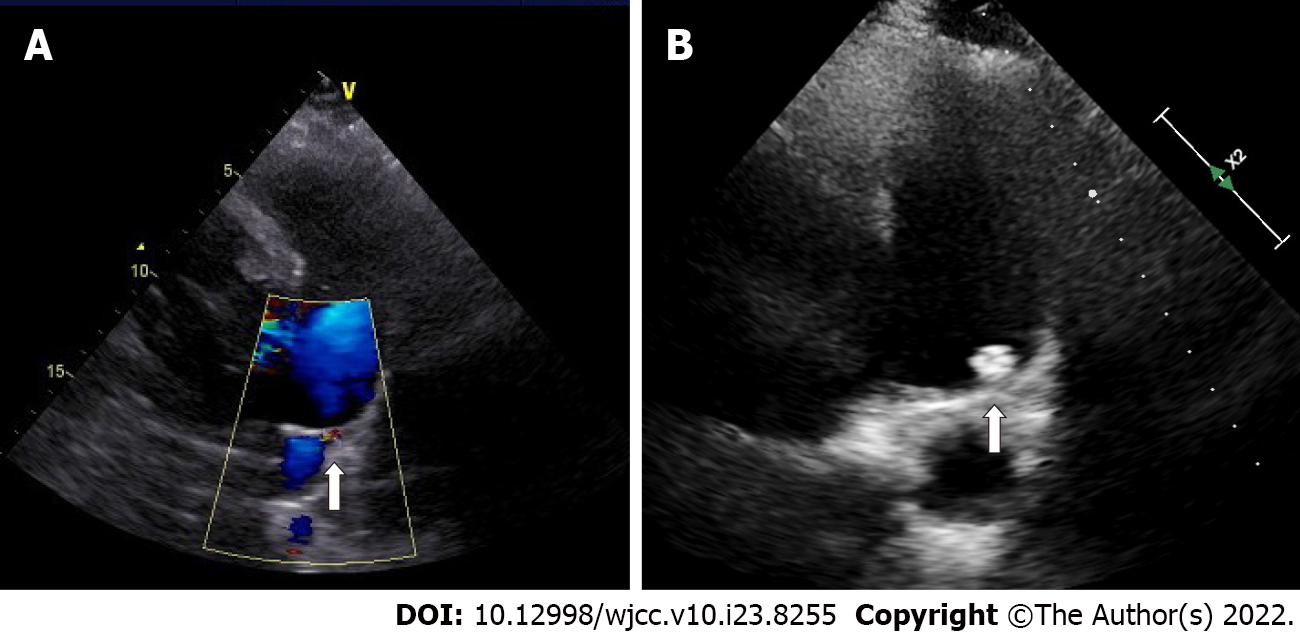
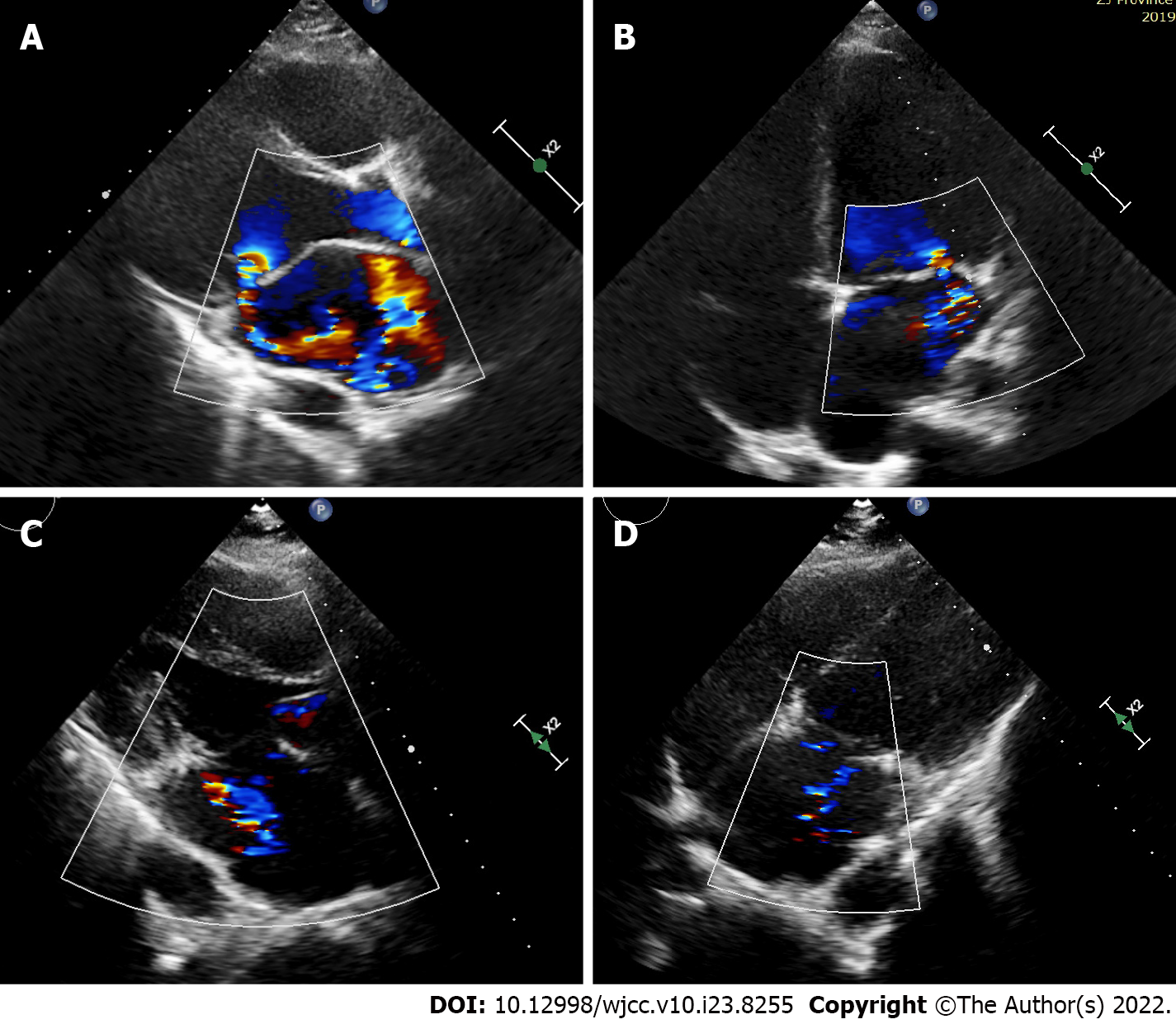
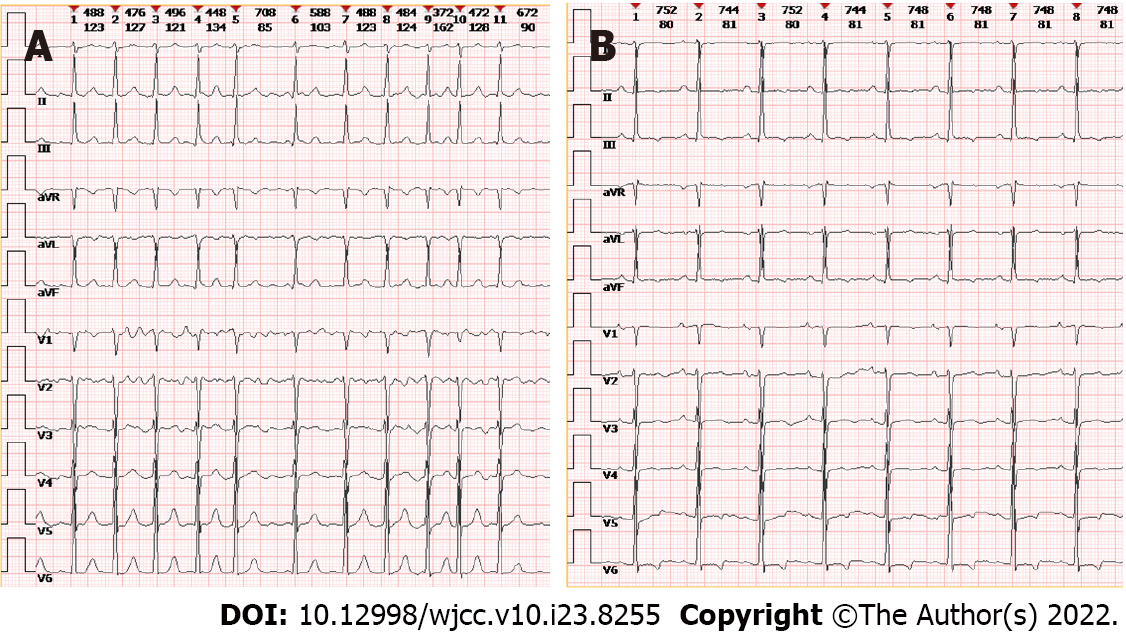
Echocardiography showed generalized cardiac enlargement, 5.1 mm PDA with a continuous shunt (Figure 1A) and anterior mitral leaflet prolapse with severe MR (Figure 2A and B). The estimated pulmonary artery systolic pressure was 68 mmHg, and the estimated right ventricular pressure was 55 mmHg. The electrocardiogram showed AF with a rapid ventricular rate (Figure 3A). Chest computed tomography scan showed pulmonary edema and bilateral pleural effusions.
The final diagnosis was PDA, MVP with severe MR, AF, hyperthyroidism, and heart failure (NYHA Classification IV).
One-stage surgery for PDA, MVP and AF is high risk due to severe refractory heart failure (NYHA Classification IV). Moreover, the patient wanted to avoid median sternotomy given that it results in a large scar on the chest. After a thorough discussion with endocrinologists, cardiologists and cardiothoracic surgeons, a sequential multidisciplinary minimally invasive therapeutic strategy was formulated. First, PDA transcatheter closure was performed. Hyperthyroidism and heart failure were controlled simultaneously via medical treatment. Video-assisted thoracoscopic mitral valve repair and left atrial appendage (LAA) occlusion were performed when her heart failure was controlled.
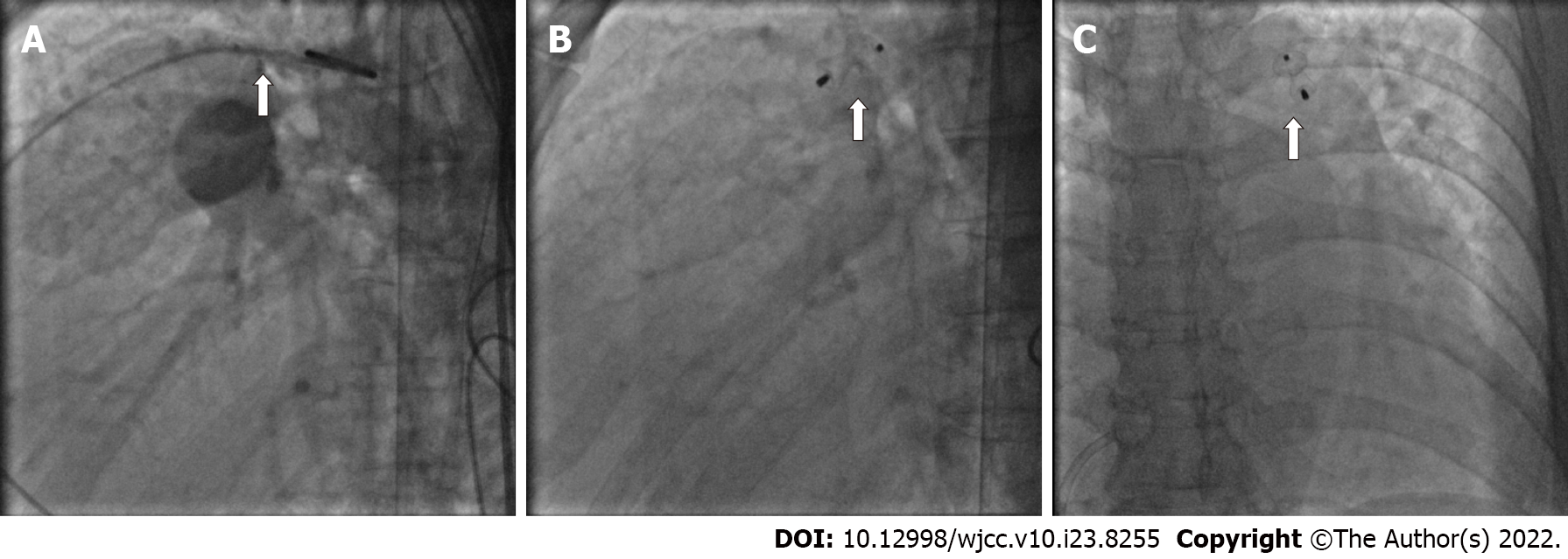
An 8-10 mm SHSMA PDA duct occluder (Shanghai Shape Memory Alloy Co. Ltd. Shanghai, China) was deployed across the PDA by catheterization through a sheath introduced from the right femoral vein under local anesthesia (Figure 4). Complete closure of the ductus was obtained immediately. As little contrast agent as possible was injected to minimize the impact on the thyroid. After catheterization, the patient was administered sacubitril valsartan sodium (25 mg bid) and a lower dose of methimazole (2.5 mg qd).
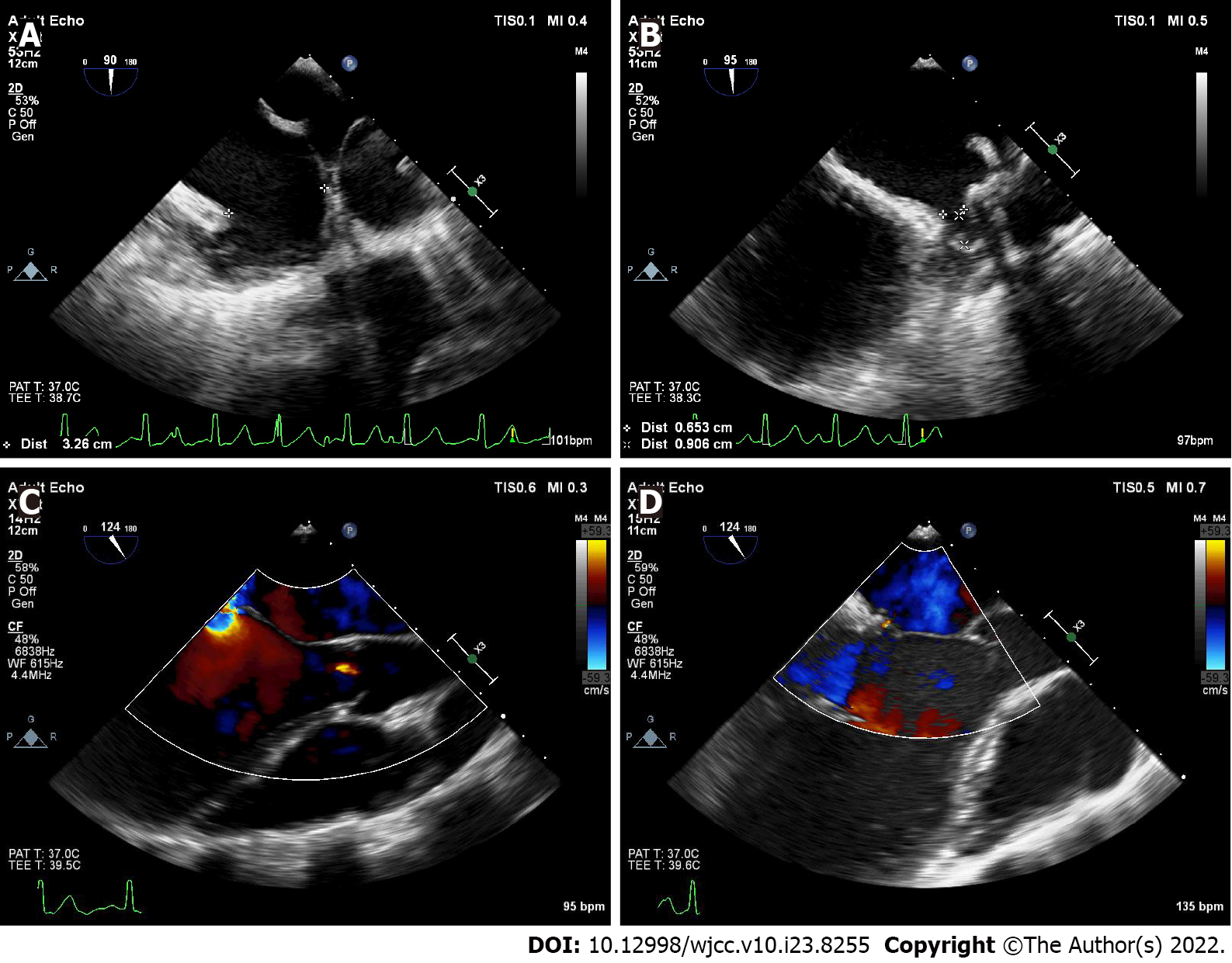
Two months later, she exhibited mild exertional dyspnea (NYHA Classification II). Video-assisted thoracoscopic mitral valve repair and LAA occlusion were then performed successfully through a minimal right infra-axillary thoracotomy using femorofemoral extracorporeal circulation. Chordal replacement was used to correct prolapse of the anterior leaflet with three artificial chordaes made of expanded polytetrafluoroethylene sutures (Gore-Tex sutures; W.L. Gore & Associates Inc, Elkton, Md). Annuloplasty ring implantation was performed (32-mm annuloplasty ring, Medtronic Inc., Minnesota, United States). The patient also underwent LAA exclusion using an LAA clip device (PM-LAA-40, Beijing Puhui Biomedical Engineering Co., Ltd, Beijing, China). After weaning from cardiopulmonary bypass, intraoperative transesophageal echocardiography was used to confirm successful LAA occlusion and mitral valve repair (Figure 5). After surgery, the dose of sacubitril valsartan sodium (50 mg bid) was increased.
Sinus rhythm was regained 3 mo after surgery (Figure 3B). Two years after the treatment, the symptoms of heart failure were relieved (NYHA Classification I), and sinus rhythm was maintained. Transthoracic echocardiography showed complete closure of the ductus (Figure 1B) and mind MR (Figure 2D). The enlarged heart gradually shrunk (Table 1). Blood BNP also notably decreased (129.1 vs 890.3 pg/mL).
| Echocardiographic time | LVEF (%) | LVIDd (mm) | LVIDs (mm) | LA (mm) | RV (mm) | RA (mm) |
| Pre-PDA closure | 57 | 59 | 41 | 50 | 30 | 45 |
| Post-PDA closure | 69 | 62 | 38 | 49 | 33 | 41 |
| Pre-surgery | 60 | 60 | 40 | 51 | 25 | 46 |
| Post-surgery | 51 | 55 | 41 | 39 | 26 | 46 |
| Three months after surgery | 62 | 48 | 32 | 39 | 23 | 44 |
| Six months after surgery | 68 | 49 | 31 | 44 | 23 | 33 |
| One year after surgery | 67 | 51 | 32 | 39 | 20 | 34 |
| Two years after surgery | 67 | 52 | 32 | 43 | 20 | 35 |
PDA, MVP, AF, and hyperthyroidism are common diseases, but the combination of these four diseases is extremely rare. No previous report has provided a therapeutic strategy for the combination of these four diseases. Minimally invasive and multidisciplinary strategies are the current trends in clinical treatment[3]. Therefore, we developed a sequential multidisciplinary minimally invasive therapeutic strategy based on the pathophysiological characteristics and main treatment methods of these four diseases. Hyperthyroidism can lead to AF and high-output heart failure. The long-standing MVP of the patient results in primary mitral regurgitation for many years, during which time the size of the left atrium and ventricle increase and the contractility of the left ventricle decreases. Mitral valve repair is recommended in symptomatic patients with MVP alone and low surgical risk[2]. PDA results in left-to-right shunt and left ventricular volume overload and remodeling, which can also lead to heart failure. Device closure is recommended, as this procedure has excellent technical success and minimal morbidity, supplanting operative ligation in adults[4]. The treatment of AF includes antiarrhythmic drugs, catheter ablation and surgical ablation. However, AF may be secondary to hyperthyroidism and severe mitral regurgitation in this case. Therefore, MVP and PDA should be treated first after hyperthyroidism is controlled via drugs.
The traditional approach for treating congenital heart disease with valvular disease is one-stage surgery with median sternotomy. A study showed that one-stage open-heart mitral valve repair, tricuspid annuloplasty, PDA direct closure and radiofrequency-modified maze procedures were successfully performed in a 73-year-old female[5]. Recently, a one-stage hybrid procedure was performed as a valuable alternative with the advantages of reducing trauma as well as recovery and hospitalization time[6-8]. However, the young female wanted to avoid median sternotomy due to its huge scarring on the chest. However, the patient had a high surgical risk due to severe heart failure. Therefore, one-stage surgery might not represent the ideal therapeutic strategy for this patient.
Transcatheter closure is the preferred treatment for adult PDA with minimal anesthetic risk and incidence of associated complications[1]. Moreover, a few reports have confirmed that transcatheter PDA occlusion can reduce the volume load of the left ventricle and functional mitral regurgitation[9,10]. Closing the PDA is likely to reverse left atrial and ventricular enlargement and will possibly provide symptom relief[11]. Based on the above reasons, transcatheter PDA occlusion combined with antithyroid drug therapy was the optimal choice as the initial treatment with low risk and trauma. As little contrast agent as possible was injected to minimize the impact on the thyroid. After PDA occlusion and optimization of drug therapy for 2 mo, her heart failure improved significantly (NYHA Classification II).
Valve repair combined with maze surgery is the standard treatment for patients with MVP and AF. However, AF may be secondary to hyperthyroidism and severe mitral regurgitation in this case. Accordingly, we did not perform the Maze procedure, which would cause substantial trauma to the left atrium. The minimal right axillary incision was selected for mitral valve repair with satisfactory cosmetic results. The LAA was simultaneously clipped to avoid thrombosis[12]. Consistent with expectations, sinus rhythm was recovered and maintained. Therefore, catheter ablation was not needed, and both the injury and additional financial expenditure of AF ablation to the patient were avoided.
Although multiple hospitalizations are needed, the multidisciplinary sequential minimally invasive therapeutic strategy can significantly reduce the surgical risk, avoid the trauma and complications of median sternotomy, and shorten the recovery time compared with one-stage surgery in this case.
Our successful experience in this patient shows that a sequential multidisciplinary therapeutic strategy, which can take advantage of both internal medicine and surgical approaches, might be reasonable for this type of disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mydam J, United States; Ong H, Malaysia S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006;114:1873-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 338] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 2. | Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 3056] [Article Influence: 764.0] [Reference Citation Analysis (0)] |
| 3. | Sugiyama Y, Sasaki M, Kouyama M, Tazaki T, Takahashi S, Nakamitsu A. Current treatment strategies and future perspectives for gastrointestinal stromal tumors. World J Gastrointest Pathophysiol. 2022;13:15-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | O'Byrne ML, Smith CL, Gillespie MJ. Device Closure of Patent Ductus Arteriosus in Adults. Can J Cardiol. 2020;36:795-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Yoshida K, Tobe S, Adachi K, Kawata M. [Adult mitral and tricuspid valve regurgitation due to patent ductus arteriosus combined with atrial fibrillation; report of a case]. Kyobu Geka. 2004;57:1127-1130. [PubMed] |
| 6. | Li Q, Lin K, Gan CP, Feng Y. One-Stage Hybrid Procedure to Treat Aortic Coarctation Complicated by Intracardiac Anomalies in Two Adults. Ann Thorac Surg. 2015;100:2364-2367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Guo H, Wang Y, Wu W, Lai Y. One-Stage Hybrid Procedure for Patent Ductus Arteriosus Combined with Other Cardiac Anomalies in Adults. Heart Surg Forum. 2018;21:E177-E178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Li CS, Lu Z, Song XR, Yan ZY. Hybrid procedure for treating adult congenital heart disease with valvular heart disease in two patients. J Cardiothorac Surg. 2019;14:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Wang Z, Chen T, Chen L, Qin Y, Zhao X. Safety and Efficacy of Transcatheter Closure of Patent Ductus Arteriosus With Severe Mitral Regurgitation in Adults. J Invasive Cardiol. 2016;28:30-33. [PubMed] |
| 10. | Watanabe N, Toyofuku M, Sato T, Shiode N, Masaoka Y, Muraoka Y, Okimoto T, Otsuka M, Tamekiyo H, Mito S, Kawase T, Kagawa Y, Senoo A, Hayashi Y. A case of adult patient ductus arteriosus with congestive heart failure and severe mitral regurgitation. Cardiovasc Interv Ther. 2011;26:278-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e637-e697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 12. | Bedeir K, Warriner S, Kofsky E, Gullett C, Ramlawi B. Left Atrial Appendage Epicardial Clip (AtriClip): Essentials and Post-Procedure Management. J Atr Fibrillation. 2019;11:2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |