Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8232
Peer-review started: November 10, 2021
First decision: December 2, 2021
Revised: December 13, 2021
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 16, 2022
Processing time: 264 Days and 0.7 Hours
Autoimmune pancreatitis (AIP) is a particular type of chronic pancreatitis, and steroid treatment of AIP is effective. Spontaneous remission (SR) of AIP without steroids is relatively rare. The international consensus for the treatment of autoi
We present the cases of four patients with AIP (two females and two males) in which the AIP improved spontaneously without steroid treatment. Two patients were asymptomatic, one patient had abdominal pain with obstructive jaundice, and one patient had intermittent right upper abdominal pain. Three patients presented with localized pancreatic enlargement and one with diffuse pancreatic enlargement. In addition to the pancreatic lesions, bile duct involvement was seen in two patients, and no extra-pancreatic organ involvement was found in the other two patients. The serum IgG4 level of all patients was more than twice the normal level. After SR in the four patients, the affected pancreases exhibited three types of image features: Return to normal, progressive fibrosis, and atrophy and calcification.
The clinical features of SR in our four patients with AIP differ, but the imaging findings share some characteristics. After SR, in some cases the affected pancreas could return to normal, although some patients suffer from progressive fibrosis and atrophy as well as calcification.
Core Tip: We focus on spontaneous remission (SR) in patients with autoimmune pancreatitis due to the high risks and contraindications of steroid treatment. As with asymptomatic patients, some symptomatic patients may also have SR. Patients with localized or diffuse enlargement of the pancreas (the former may be more prone to SR), with or without involvement of the bile ducts, may have SR. After SR, the affected pancreases may return to normal, or exhibit progressive fibrosis and atrophy as well as calcification.
- Citation: Zhang BB, Huo JW, Yang ZH, Wang ZC, Jin EH. Spontaneous remission of autoimmune pancreatitis: Four case reports . World J Clin Cases 2022; 10(23): 8232-8241
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8232.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8232
Autoimmune pancreatitis (AIP) is a type of chronic pancreatitis with a hypothesized autoimmune mechanism. It is characterized by: (1) Dense infiltration of plasma cells and lymphocytes; (2) Peculiar storiform fibrosis; and (3) Occlusive phlebitis[1]. The concept of AIP was proposed by Yoshida et al[2] in 1995. Subsequent studies showed that AIP is a disease with special clinical, radiological, and serological characteristics. It is a manifestation of pancreatic involvement in IgG4-related systemic diseases that often involve the bile ducts, salivary and lacrimal glands, kidneys, and lymph nodes[3]. The international consensus for the treatment of autoimmune pancreatitis suggests that AIP patients with obstructive jaundice, abdominal pain, and back pain related to the pancreas or the bile duct should be treated with steroids. “Watchful waiting” may be appropriate in most of the asymptomatic patients, because they may improve spontaneously without steroid treatment[4]. However, in our clinical experience, we found that the clinical characteristics of AIP patients with spontaneous remission (SR) are different, and the factors that lead to SR are not clear. In addition, as far as we know, there is no published report of dynamic imaging before and after SR of AIP up to now. Therefore, we report here the clinical and imaging findings of four AIP patients with SR in order to promote more appropriate individualized treatment strategies for patients with AIP.
Case 1: A 67-year-old woman had a pancreatic lesion found accidentally by yearly ultrasound for 2 mo.
Case 2: A 55-year-old woman complained of having a pancreatic lesion detected accidentally by computed tomography (CT) for 23 d.
Case 3: A 76-year-old man suffered from right upper abdominal pain for 1 mo, and his skin and sclera were yellow for more than 10 d.
Case 4: A 78-year-old man suffered from intermittent right upper abdominal pain for 6 d without obvious cause.
Case 1: Two months preceding our examination, an abnormal pancreatic lesion was found by routine ultrasound examination in this patient. She had no discomfort. For further diagnosis, plain and enhanced MR scanning of the abdomen was performed in the outpatient department of our hospital. The diagnosis was focal AIP of the pancreatic body and tail, which required follow-up closely, to rule out a malignant tumor. In order to further clarify the diagnosis and treatment, she was admitted to our hospital.
Case 2: This patient underwent physical examination 23 d before entering our care, and abdominal plain CT showed a mass at the body and tail of the pancreas. She went to a local hospital and underwent an abdominal enhanced CT scan. The result suggested that pancreatic cancer could not be excluded. Subsequently, she visited our hospital for surgical treatment. She had no discomfort, and her weight had not changed.
Case 3: One month prior to our examination, this patient complained of right upper abdominal pain of unknown cause, accompanied by abdominal distension. He went to a primary hospital and was diagnosed with gastroenteritis, and was treated with traditional Chinese medicine. However, his symptoms did not significantly improve. Ten days ago, he had yellow skin and sclera, and dark-colored urine. He went to a local hospital where abdominal ultrasound showed that his common bile duct was dilated and his pancreatic head was enlarged. The blood test indicated that his serum total bilirubin level (TBIL) was 11.59 mg/dL, direct bilirubin level (DBIL) was 9.23 mg/dL, alanine aminotransferase level (ALT) was 164.5 U/L, and aspartate aminotransferase level (AST) was 106.7 U/L. Then he visited our hospital for further treatment. Endoscopic cholangiopancreatography (ERCP) was performed after preoperative examination, and a severe common bile duct stenosis was seen. Later, he underwent duodenal papillotomy (EST), endoscopic nasobiliary drainage (ENBD), and endoscopic pancreatic stent drainage (ERPD). After the operation, he was sent to our department for further evaluation and treatment.
Case 4: Six days prior to our examination, this patient had right upper abdominal pain without obvious cause, accompanied by intermittent vomiting. He went to a local hospital where abdominal ultrasound showed mild dilatation of the common bile duct and a solid mass in the pancreatic head. The blood test indicated that his TBIL was 9.64 mg/dL, DBIL was 3.55 mg/dL, ALT was 84 U/L, and AST was 70 U/L. Subsequently, he came to our department for further diagnosis and treatment.
Case 1: This patient had a history of allergic asthma for 6 years; she was treated with intermittent Salmeterol Xinafoate and Fluticasone Propionate aerosol. She had a 1 year history of diabetes, and used 6-9 U subcutaneous insulin and 0.5 g oral metformin three times a day. Her fasting blood glucose (FBS) was controlled at 6.20-7.20 mmol/L.
Case 2: This patient's blood sugar level increased during the course of the last year; her FBS was controlled by diet at 7.40-8.90 mmol/L.
Case 3: This patient had a history of hypertension for more than 10 years; he regularly took 10 mg nifedipine sustained-release tablets twice a day, and his blood pressure was controlled at 130-140/70 mmHg. He had a history of a prior cerebral infarction more than 10 years ago without sequelae.
Case 4: This patient had a history of hypertension for more than 7 years; he took 40 mg nimodipine three times a day, and his blood pressure was controlled at 120-130/90 mmHg. He also had abnormal liver function in the last 2 years as well as chronic gastritis in the past year without treatment.
Cases 1 and 2: These patients had no history of smoking or alcohol abuse.
Case 3: This patient smoked 20 cigarettes and drank about 20 g of alcohol per day for more than 50 years.
Case 4: This patient drank about 10 g of alcohol per day for more than 40 years. All patients had no unusual family history of disease.
Cases 1 and 2: These patients had no symptoms and no abnormalities were identified during the physical examination. All vital signs were within normal ranges.
Case 3: This patient had yellow skin and sclera, tenderness in the right upper abdomen, and no rebound pain and muscle tension, and no mass could be touched. The drainage tube of the bile duct was properly fixed on the skin and discharged dark green liquid. Vital signs were normal.
Case 4: This patient had right upper abdomen tenderness. Vital signs were normal.
Case 1: Laboratory data were as follows: Red blood cell (RBC) count, 3.69 × 1012 /L; hemoglobin (HGB), 115 g/L; white blood cell (WBC) count, 6.52 × 109 /L; C-reactive protein (CRP), 23.0 mg/L; glycosylated hemoglobin A1c (HbA1c), 6.50%; blood amylase, 33 IU/L. The indices of liver function were normal. Serum IgG4 was elevated to 530 mg/dL; antinuclear antibody and antineutrophil antibody were negative. Carbohydrate antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA), and alpha-fetoprotein (AFP) were normal.
Case 2: Laboratory data were as follows: Routine blood analysis, normal; HbA1c, 8.40%; blood amylase, 26 U/L. The indices of liver function were normal. Serum IgG4 was elevated to 407 mg/dL.CA 19-9, CEA, and AFP were normal.
Case 3: Laboratory data were as follows: RBC count, 3.23 × 1012/L; HGB, 121 g/L; WBC count, 5.51 × 109 L; CRP, 68 mg/L; blood amylase, 62 IU/L; TBIL, 9.41 mg/dL; DBIL, 4.94 mg/dL; ALT, 84 U/L; AST, 58.5 U/L; alkaline phosphatase (ALP), 356 U/L; γ-glutamyltranspeptidase (γ-GT), 168U/L. Serum IgG4 was 1310 mg/dL. CA 19-9 was 88.00 U/mL; CEA and AFP were normal.
Case 4: Laboratory data were as follows: RBC count, 2.59 × 1012/L; HGB, 100 g/L; WBC count, 3.70 × 109/L; TBIL, 2.85 mg/dL; DBIL, 1.75 mg/dL; ALP, 185 U/L; ALT, 43 U/L; AST, 41 U/L. Serum IgG4 was 590 mg/dL.CA 19-9, CEA, and AFP were normal.
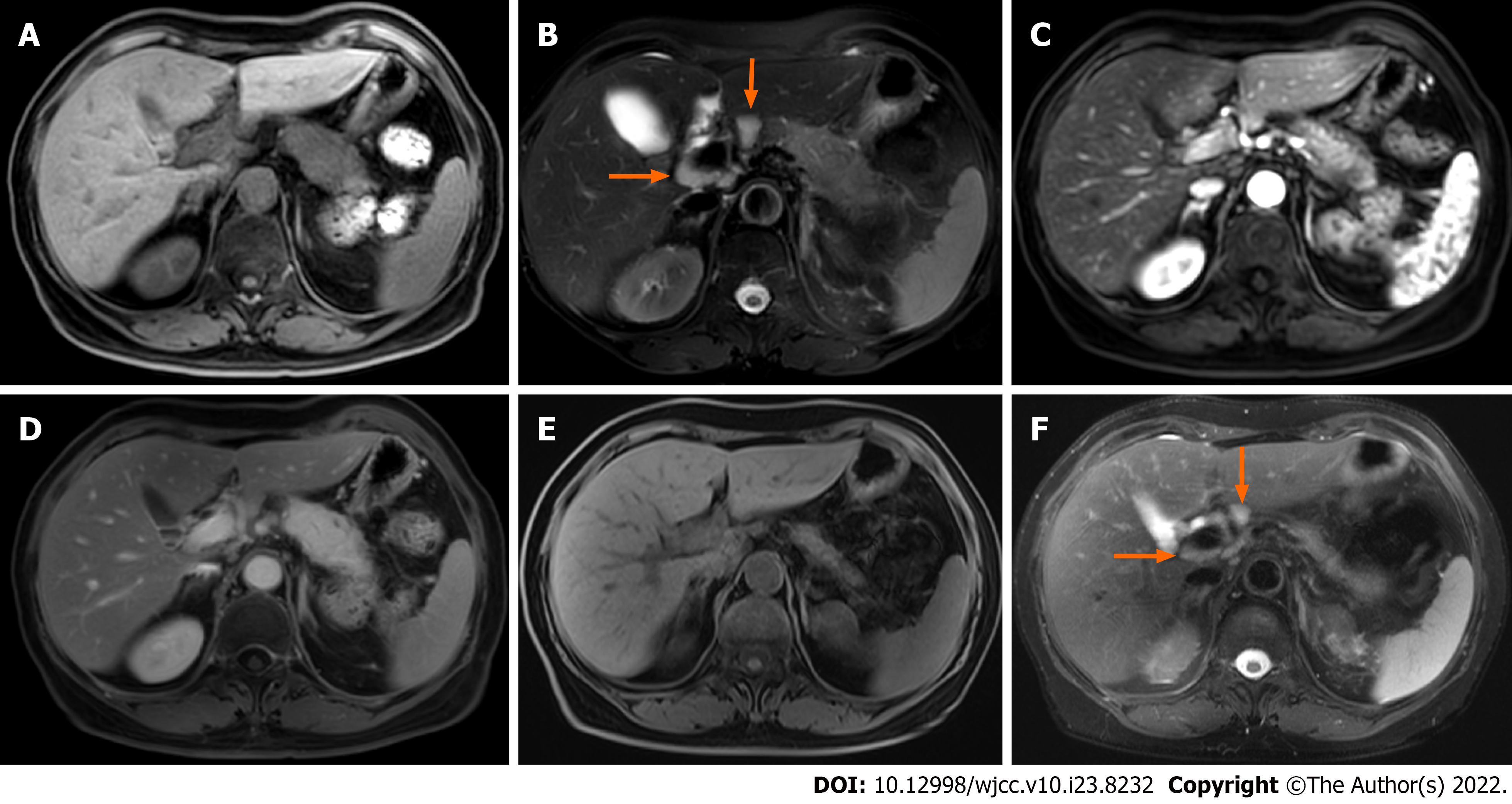
Case 1: Abdominal magnetic resonance imaging (MRI) showed obvious enlargement of the pancreatic body and tail. Compared with the pancreatic head, the lesion showed low signal intensity on T1WI, and high signal intensity on T2WI. Multiple enlarged lymph nodes were seen around the portal vein (Figure 1A and B). The lesion demonstrated decreased enhancement in the arterial phase, and progressive enhancement in the delayed phase on gadolinium-enhanced T1WI. A pseudocapsule was around the lesion and no expansion of the pancreatic duct was observed (Figure 1C and D). ECHO-3-22 needles were used to puncture the lesion under monitoring by endoscopic ultrasound. The pathology demonstrated well differentiated squamous epithelial and glandular epithelial cells, and a small number of lymphocytes and neutrophils, with no definite malignant tumor cells.
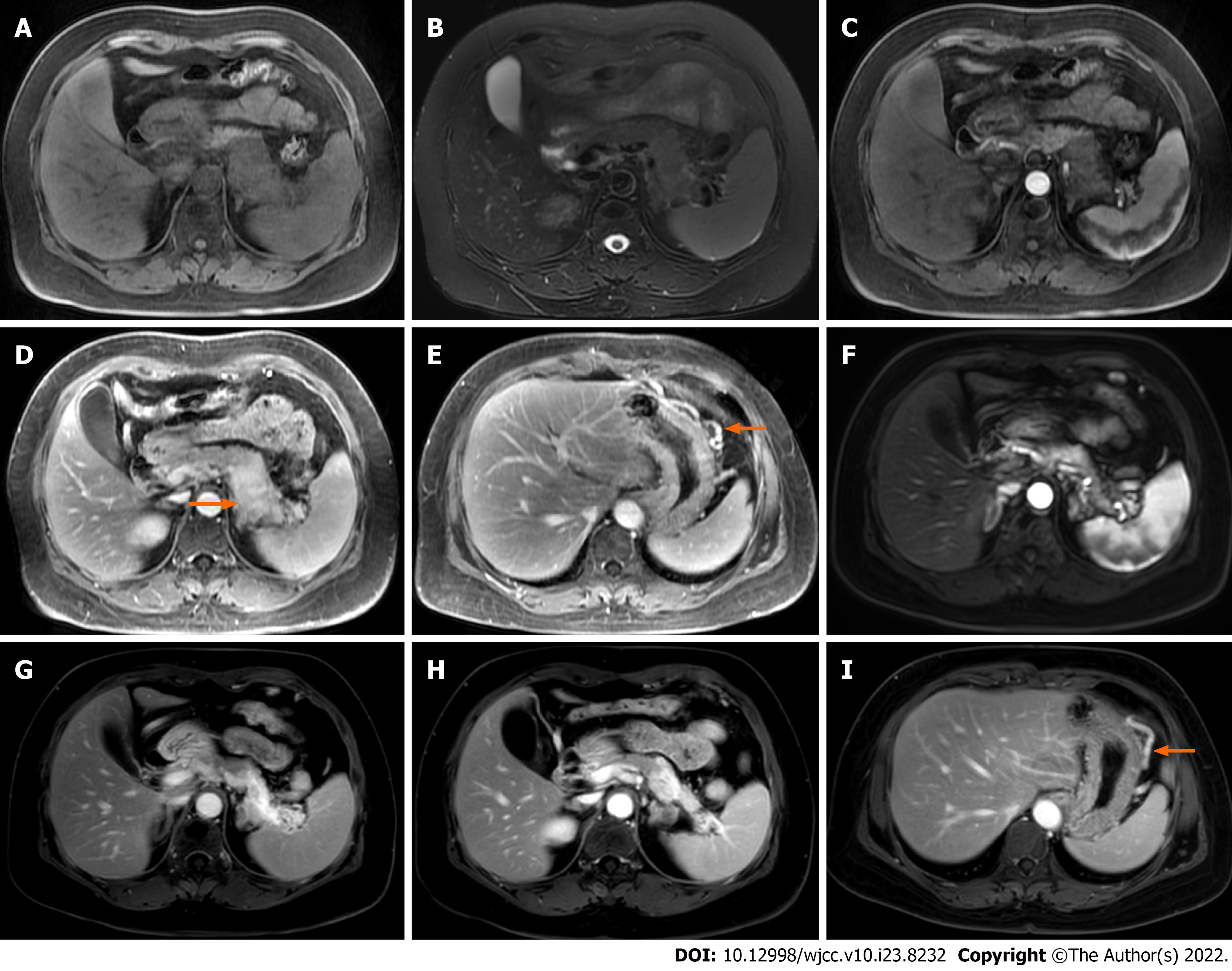
Case 2: Abdominal MRI showed obvious enlargement of the pancreatic body and tail. Compared with the pancreatic head, the lesion showed low signal intensity on T1WI, and slightly high signal intensity on T2WI (Figure 2A and B). There was gradual enhancement of the lesion, splenic vein stenosis posterior to the lesion, and dilation of the large curved side vein of the gastric body on gadolinium-enhanced T1WI. There was no dilation of the pancreatic duct (Figure 2C-E). Three ECHO-3-22 puncture needles were monitored by endoscopic ultrasound. Pathology examination demonstrated that there were a large number of lymphocytes, a small number of neutrophils, a small number of well differentiated epithelial cells, and proliferation of fibrous tissue in the enlarged pancreas. No malignant tumor cells were found.
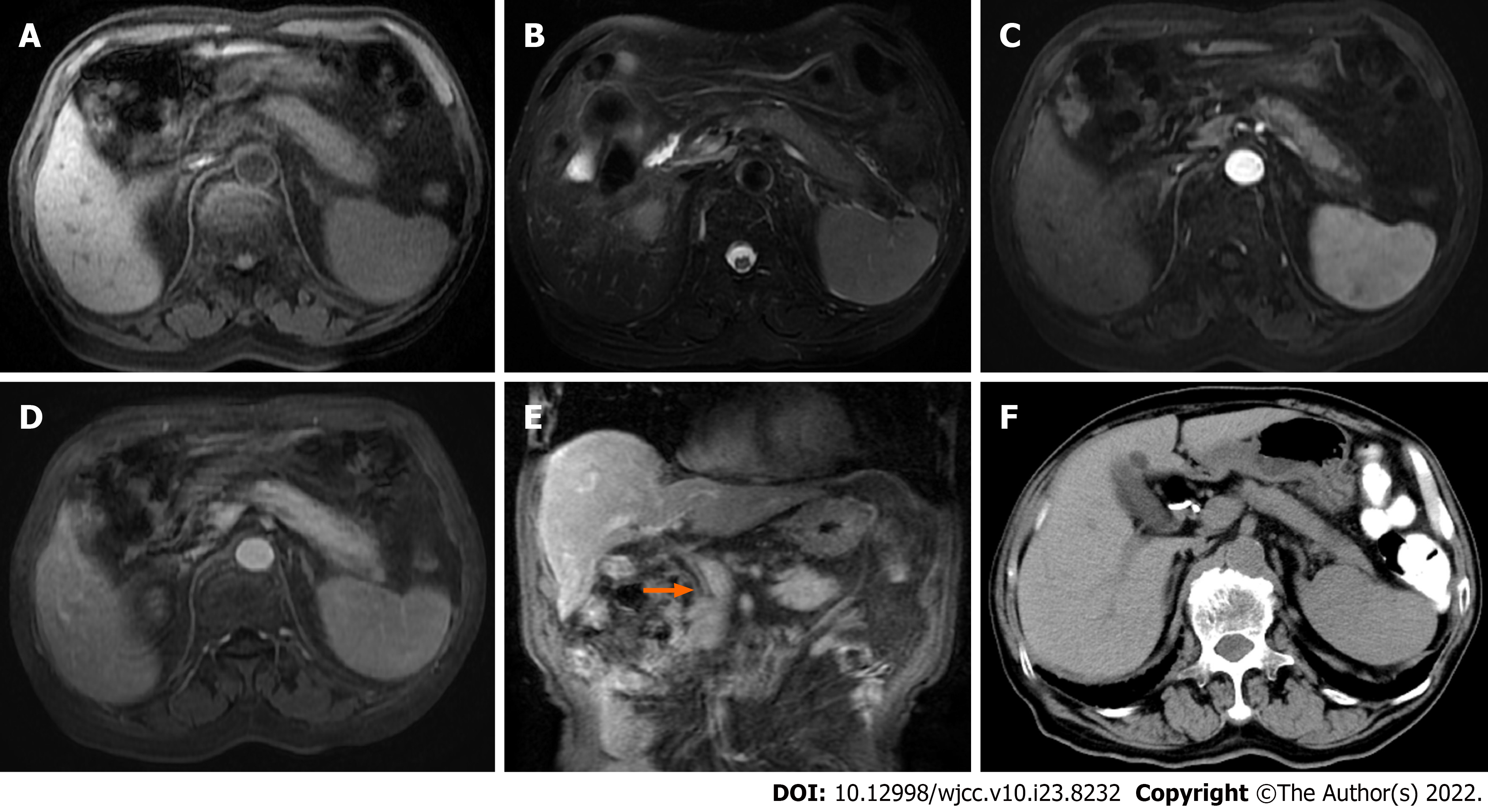
Case 3: Abdominal MRI showed diffuse enlargement of the pancreas. Compared with adjacent liver parenchyma, the pancreas showed low signal intensity on T1WI, and high signal intensity on T2WI (Figure 3A and B). On gadolinium-enhanced T1WI, a sign of progressive enhancement of the pancreatic lesion and the pseudocapsule around the pancreas was observed (Figure 3C and D); furthermore, the hepatic hilar and extra-hepatic bile duct wall was diffusely thickened and abnormally enhanced (Figure 3E).
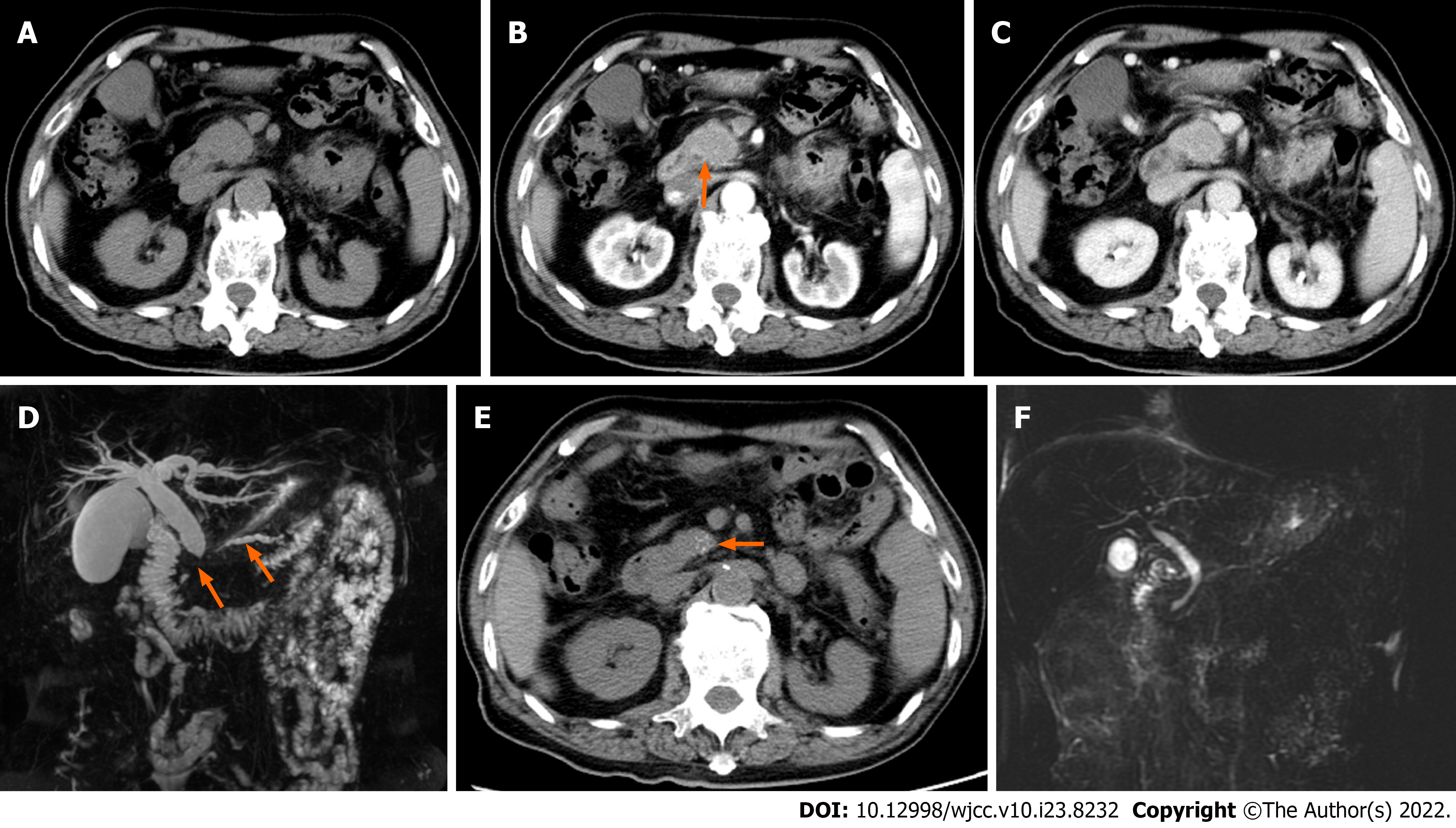
Case 4: Abdominal contrast enhanced CT showed that the pancreatic head was slightly enlarged with delayed enhancement (Figure 4A-C). There was no abnormality of the pancreatic body and tail. The bile duct wall in the pancreatic head was thickened and enhanced abnormally (Figure 4B). Magnetic resonance cholangiopancreatography showed that the main pancreatic duct was narrowed in a longer segment, and the upstream pancreatic duct was slightly dilated, with a diameter of 4 mm. Moreover, the common bile duct of the pancreatic segment was severely narrowed, and the proximal intra- and extra-hepatic bile ducts were dilated (Figure 4D). Three disposable puncture needles with COOK22G were introduced at the pancreatic head lesion with ultrasonic monitoring. Degenerative small round cells were seen, but no malignant tumor cells were found.
Cases 1 and 2: Focal AIP of the pancreatic body and tail.
Case 3: Diffuse AIP.
Case 4: Focal AIP of the pancreatic head.
Cases 1 and 2: These patients refused steroid treatment, and were followed regularly in our outpatient department.
Case 3: After the operation, this patient was treated by fasting and water prohibition, acid inhibition, and nutritional support, but he refused steroid treatment. Ten days after hospital admission, ERCP and EST and endoscopic biliary stent drainage were performed. After discharge, he took 50 mg ursodeoxycholic acid orally three times a day.
Case 4: After admission, this patient refused to use steroid and was given symptomatic treatment such as liquid food, acid suppression, and liver protection. After discharge, he received ursodeoxycholic acid (50 mg) and pancreatin enteric-coated capsules (50 mg) three times a day.
Case 1: During 15 mo following treatment, this patient had no symptoms. The HbA1c ranged from 7.20% to 8.30%. Her serum IgG4 had decreased to 162 mg/dL by the 11th month. Pancreatic swelling dissipated and the volume of the pancreas decreased significantly, and the enlarged lymph nodes were reduced obviously as observed on MRI at the 13-mo follow-up (Figure 1E and F).
Case 2: During 22 mo following treatment, this patient had no symptoms. The patient injected 6-12 U insulin subcutaneously once every night; FBS was in the range of 6.28-7.12 mmol/L. MRI follow-up at 22 mo showed that the body and tail of the pancreas were significantly reduced and more obviously gradually enhanced, which suggested that the affected pancreatic parenchyma suffered from progressive fibrosis. The stenosis of the splenic vein and the dilation of the large curvature vein of the gastric body were not improved (Figure 2F-I).
Case 3: This patient's clinical symptoms improved and his bilirubin levels decreased gradually. Twenty months after treatment, abdominal CT showed that the volume of the pancreas had decreased and the thickening of the bile duct wall had disappeared (Figure 3F).
Case 4: This patient's symptoms gradually improved. At the 12-mo follow-up, the patient’s serum IgG4 was 570 mg/dL, the pancreatic head swelling had dissipated and it appeared to contain multiple punctate calcifications (Figure 4E). There was no expansion of the bile and pancreatic ducts (Figure 4F).
AIP remission without steroid treatment has been reported occasionally, but its mechanism is not clear. In order to deepen our understanding of the SR of AIP, we searched the database through MEDLINE using keywords "autoimmune pancreatitis" or "IgG4-related disease" and "spontaneous remission" or "spontaneous resolution" or "spontaneous release" or "natural course". After excluding cases with incomplete clinical data or cases with no evidence of remission of pancreatic lesions, nine publications were ultimately examined, including four original research papers[5-8] and five case reports[9-13] (Table 1). Kamisawa et al[5] reported 11 AIP patients who were followed during the natural course of the disease. Ultimately, six patients needed steroid treatment due to disease progression, while five patients were stable or had SR of the disease. They found that there were no significant differences in the clinical characteristics between the two groups. The other three studies on SR of AIP were reported by Kubota et al[6-8] in 2007, 2009, and 2011. Multivariate analysis of factors related to the SR of AIP showed no consistent pattern. The independent predictors of SR were negative staining for IgG4 in the duodenal papilla, early manifestations of endoscopic ultrasonography (lobularity and hyperechoic pancreatic duct margin), and IgG4 seronegativity. In five case reports of AIP patients with SR, the clinical symptoms were different in each one. In these patients, the pancreas could exhibit either diffuse swelling or contain a localized mass, and serum IgG4 could be slightly or significantly increased, with or without the involvement of extra-pancreatic organs. Our four cases and the literature reports suggest that the factors responsible for the SR of AIP are not clear, and that the clinical characteristics of the AIP patients with SR are quite different. Of the four patients that we describe, two had no symptoms, one had intermittent abdominal pain, and one had abdominal pain with obstructive jaundice. Among them, three had localized enlargement, and one had diffuse enlargement of the pancreas, accompanied with bile duct involvement in two, and no extra-pancreatic organ involvement in two cases. In all the presented cases, the level of serum IgG4 was more than twice the normal level. Our four cases suggest that there are three types of image features after SR of AIP: (1) The size and density (signal) of the pancreas return to normal; (2) There may be progressive fibrosis of the pancreas; and (3) There may be atrophy and calcification of the pancreas. Previous studies had shown that the atrophy and calcification of pancreatic parenchyma accompanying AIP were often related to fibrosis and recrudescence, and may be accompanied by irreversible internal and external pancreatic secretion dysfunction[14,15]. During long-term follow-up, case 2 showed progressive fibrosis, and case 1 and case 4 showed atrophy and calcification of the affected pancreas without steroid treatment, respectively. Such imaging findings after SR of AIP have not previously been reported. With atrophy and progressive fibrosis, pancreatic endocrine dysfunction in case 1 and case 2 was aggravated and blood glucose was not well controlled. At the same time, case 2 had left portal hypertension secondary to splenic vein involvement. An MRI scan in this case indicated that the lumen of the splenic vein was narrowed and the large curved side vein of the gastric body was dilated. Juarez et al[16] reported that when AIP involved the splenic vein, blood flow was limited, resulting in left portal hypertension, which may lead to congestive splenomegaly and variceal bleeding. Therefore, these authors suggested treatment with steroids early to reduce the risk of irreversible complications. In fact, although we recommended case 2 to use steroids as soon as possible to prevent the progression of the disease, she refused. Therefore, we continued follow-up observations to determine her long-term prognosis. Of the four patients with SR of AIP, serum IgG4 was rechecked in two patients. Among these, serum IgG4 returned to normal levels in case 1, and it decreased slightly in case 4. We speculate that the change in serum IgG4 Level is not consistent with or synchronous with the imaging appearances of pancreatic lesion remissions, which is consistent with the reports of Kusano[10] and Araki[12]. It should be noted that the SR of AIP in this study is just the result of patients' rejection of steroids. Actually, a steroid therapy had been suggested for the four patients at the beginning when the final AIP diagnosis was established.
| Ref. | Age/sex | Clinical presentation | Serum IgG4 mg/dL | Type of pancreatic enlargement | Associated diseases | Treatment | Outcome |
| Ohtsubo et al[9], 2021 | 67/F | Dark urine | 674 (< 135) | Pancreatic head mass | Nothing | Biliary plastic stent | Pancreatic head mass diminished after 1month; multiple malignant lymphomas of the bile duct after 3 mo |
| Kusano et al[10], 2019 | 79/M | Dry mouth, weight loss, blood glucose control deteriorated | 1830 (4.8-105) | Diffuse pancreatic enlargement | Lymphadenopathy | Intensive insulin treatment | Pancreatic enlargement disappeared but lymph nodes persisted after 4 years; IgG4 was 623 |
| Uchida et al[11], 2007 | 54/M | Jaundice | 213 (6-140) | Pancreatic head mass | Hepatic masses | Endoscopic biliary drainage Biopsy of hepatic mass | Pancreatic masses disappeared after 3 mo; hepatic masses disappeared after 12 mo |
| Araki et al[12], 2006 | 73/F | No symptoms | 196 (4.8-105) | Pancreatic uncinate and tail mass | Nothing | Follow-up | Uncinate mass decreased but mass in the tail increased after 3.6.9 mo; IgG4 were 163-203 |
| Ozden et al[13], 2005 | 58/F | Painless jaundice | Not mentioned | Pancreatic head body and tail mass | Gallbladder | Percutaneous cholangiography and external drainage; Laparotomy and Cholecystectomy | No evidence of focal lesion in pancreas after 6 mo; IgG4 was 1040 |
In conclusion, the clinical features of the SR of AIP in our four cases are different, but the imaging findings share some characteristics. For example, all cases had localized or diffuse enlargement of the pancreas (the former is more common), with or without involvement of the bile ducts. Follow-up CT and MRI showed that after the SR of AIP, the affected pancreas could return to normal, although some patients suffered from progressive fibrosis and atrophy as well as calcification.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Secretary, Member and Consultant of Magnetic Resonance Group, Radiology Branch, Chinese Medical Association; Member of Pediatric Imaging Committee of Radiologist Branch of Chinese Medical Association; Member of Imaging Group of Colorectal Cancer Committee of Chinese Medical Association; Member of Magnetic Resonance Application Professional Committee of China Medical Equipment Association; Member of the Advisory Committee of China Medical Imaging Technology Research Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Adam CA, Romania; Innocenti T, Italy S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Madhani K, Farrell JJ. Autoimmune Pancreatitis: An Update on Diagnosis and Management. Gastroenterol Clin North Am. 2016;45:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 924] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 3. | Khandelwal A, Inoue D, Takahashi N. Autoimmune pancreatitis: an update. Abdom Radiol (NY). 2020;45:1359-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Okazaki K, Chari ST, Frulloni L, Lerch MM, Kamisawa T, Kawa S, Kim MH, Lévy P, Masamune A, Webster G, Shimosegawa T. International consensus for the treatment of autoimmune pancreatitis. Pancreatology. 2017;17:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Kamisawa T, Anjiki H, Takuma K, Egawa N, Kubota N. The natural course of autoimmune pancreatitis. Hepatogastroenterology. 2009;56:866-870. [PubMed] |
| 6. | Kubota K, Iida H, Fujisawa T, Yoneda M, Inamori M, Abe Y, Kirikoshi H, Saito S, Ohshiro H, Kakuta Y, Nakajima A. Clinical factors predictive of spontaneous remission or relapse in cases of autoimmune pancreatitis. Gastrointest Endosc. 2007;66:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Kubota K, Kato S, Akiyama T, Fujita K, Yoneda M, Takahashi H, Ogawa M, Inamori M, Abe Y, Kirikoshi H, Kobayashi N, Saito S, Hisatomi K, Matsuhashi N, Nakajima A. A proposal for differentiation between early- and advanced-stage autoimmune pancreatitis by endoscopic ultrasonography. Dig Endosc. 2009;21:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kubota K, Watanabe S, Uchiyama T, Kato S, Sekino Y, Suzuki K, Mawatari H, Iida H, Endo H, Fujita K, Yoneda M, Takahashi H, Kirikoshi H, Kobayashi N, Saito S, Sugimori K, Hisatomi K, Matsuhashi N, Sato H, Tanida E, Sakaguchi T, Fujisawa N, Nakajima A. Factors predictive of relapse and spontaneous remission of autoimmune pancreatitis patients treated/not treated with corticosteroids. J Gastroenterol. 2011;46:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Ohtsubo K, Yamashita K, Yanagimura N, Suzuki C, Tanimoto A, Nishiyama A, Takeuchi S, Iwaki N, Kawano M, Izumozaki A, Inoue D, Gabata T, Ikeda H, Watanabe M, Yano S. Multiple Malignant Lymphomas of the Bile Duct Developing after Spontaneous Regression of an Autoimmune Pancreatitis-like Mass. Intern Med. 2021;60:409-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Kusano Y. Autoimmune pancreatitis with spontaneous remission on 18F-fluorodeoxyglucose positron emission tomography/computed tomography. J Rural Med. 2019;14:110-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Uchida K, Satoi S, Miyoshi H, Hachimine D, Ikeura T, Shimatani M, Matsushita M, Takaoka M, Takai S, Ashida K, Okazaki K. Inflammatory pseudotumors of the pancreas and liver with infiltration of IgG4-positive plasma cells. Intern Med. 2007;46:1409-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Araki J, Tsujimoto F, Ohta T, Nakajima Y. Natural course of autoimmune pancreatitis without steroid therapy showing hypoechoic masses in the uncinate process and tail of the pancreas on ultrasonography. J Ultrasound Med. 2006;25:1063-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Ozden I, Dizdaroğlu F, Poyanli A, Emre A. Spontaneous regression of a pancreatic head mass and biliary obstruction due to autoimmune pancreatitis. Pancreatology. 2005;5:300-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Ikeura T, Miyoshi H, Shimatani M, Uchida K, Takaoka M, Okazaki K. Long-term outcomes of autoimmune pancreatitis. World J Gastroenterol. 2016;22:7760-7766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Madhani K, Farrell JJ. Management of Autoimmune Pancreatitis. Gastrointest Endosc Clin N Am. 2018;28:493-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Juarez LA, Gupta RR, Ruhnke GW. Gastric varices and splenic vein obstruction during steroid treatment for autoimmune pancreatitis: A case report and literature review. Medicine (Baltimore). 2018;97:e11940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |