Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8170
Peer-review started: March 5, 2022
First decision: March 27, 2022
Revised: March 30, 2022
Accepted: July 5, 2022
Article in press: July 5, 2022
Published online: August 16, 2022
Processing time: 149 Days and 4.8 Hours
Coronavirus disease 2019 (COVID-19) is a global pandemic putting the population at a high risk of infection-related health hazards, mortality and a potential failure of proper medical therapies. Therefore, it is necessary to evaluate the potential use of the existing drugs that could be used as options for the medical management of COVID-19 patients.
To evaluate the role of the H2 receptor blocker “famotidine” in COVID-19 illness.
This study was done on seriously ill COVID-19 patients admitted to the intensive care unit (ICU) from different institutes in Bangladesh. Patients were divided into famotidine treatment group “A” (famotidine 40 mg to 60 mg oral formulation every 8 h with other treatment as given), and control group “B” (treatment as given). National early warning score (NEWS)-2, and sequential organ failure assessment day-1 score was calculated to evaluate the outcome. Outcomes were evaluated by the time required for clinical improvement, characterized as duration required from enrollment to the achievement of NEWS-2 of ≤ 2 maintained for 24 h; time to symptomatic recovery, defined as the duration in days (from randomization) required for the recovery of the COVID-19 symptoms; mortality rate; duration of ICU and hospital stay; total period of hospitalization; the rate of supplementary oxygen requirement; the computed tomography (CT) chest recovery (%), the time required for the viral clearance and “NEWS-2” on discharge.
A total of 208 patients were enrolled in this study with 104 patients in each group. The famotidine treatment group had comparatively better recovery of 75% and a low mortality of 25% than the control with a recovery of 70% and a mortality of 30%. Duration of clinical improvement (group A 9.53 d, group B 14.21 d); hospitalization period among the recovered patients (group A 13.04 d, group B 16.31 d), pulmonary improvement in chest CT (group A 21.7%, group B 13.2%), and the time for viral clearance (group A 20.7 d, group B 23.8 d) were found to be statistically significant P ≤ 0.05. However, the Kaplan Meier survival test was not significant among the two study groups, P = 0.989.
According to our study, treatment with famotidine achieved a better clinical outcome compared to the control group in severe COVID-19 illness, although no significant survival benefit was found.
Core Tip: Treatment with famotidine demonstrated a comparatively better outcome in the survival rates of patients. A rapid recovery time, less duration of intensive care unit (ICU) stay among the survivors, favorable improvement in the computed tomography findings and an earlier viral clearance were observed in the famotidine treatment group which differ significantly in a t-test (P ≤ 0.05). The difference between the time to symptomatic recovery, ICU stay duration and the national early warning score-2 on discharge was not significant however, mean values were relatively less than the control. Nevertheless, survival benefit was not significant with the famotidine as an added treatment for severe coronavirus disease 2019.
- Citation: Mohiuddin Chowdhury ATM, Kamal A, Abbas MKU, Karim MR, Ali MA, Talukder S, Hamidullah Mehedi HM, Hassan H, Shahin AH, Li Y, He S. Role of H2 receptor blocker famotidine over the clinical recovery of COVID-19 patients: A randomized controlled trial. World J Clin Cases 2022; 10(23): 8170-8185
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8170.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8170
Coronavirus disease (COVID-19) or severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has rapidly developed into a pandemic since it was first reported in December 2019 in Wuhan City of China[1]. It was first detected in Wuhan City and then quickly spread all over the world. This puts the population at a high risk of infection-related health hazards and a potential failure of proper medical therapy during this pandemic[2]. Till now, no pre-or post-exposure prophylactic or definite COVID-19 medical countermeasures have been found. Clinical data suggest that famotidine may mitigate COVID-19 disease but both mechanisms of action and rationale for dose selection remain obscure. Over activation of mast cells and histamine production plays an important role in the progress of COVID-19 illness; hypothetically, this phenomenon could be inhibited by histamine target receptor activity of famotidine[3]. High-dose oral famotidine was found to be well-tolerated and associated with improved patient-reported outcomes in non-hospitalized COVID-19 cases[4]. Additionally, famotidine use in hospitalized patients was found to reduce the risk of COVID-19 mortality, lower the risk of the combined outcomes of mortality and intubation and lower levels of serum markers for severe disease[5,6].
But until now, no clinical trial has been published regarding the role of famotidine in severe COVID-19 disease. Therefore, an interventional study was carried out with famotidine therapy in patients with severe COVID-19 disease admitted in the intensive care units (ICU) of the different tertiary level institutes of Bangladesh. Notably Bangladesh has an average life expectancy of 72.59 years with easy access to healthcare facilities though availability of the healthcare management resources is not equal in all cities.
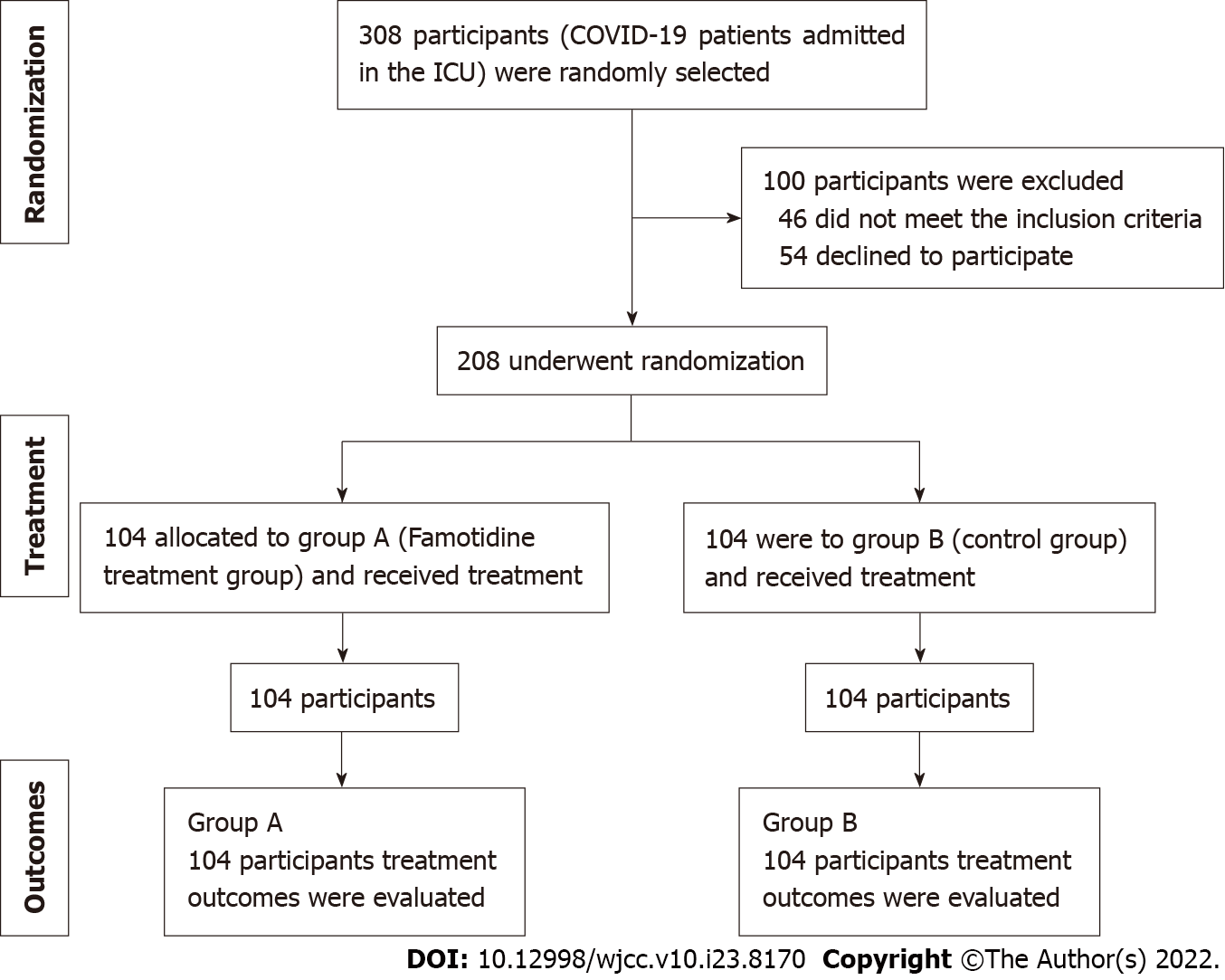
This study was designed to evaluate the effect of famotidine in severe acute respiratory distress syndrome (ARDS) caused by COVID-19. COVID-19 patients admitted in the ICU of Chattogram General hospital, M. Abdur Rahim Medical College Hospital and 250 Bed Cox’s Bazar Sadar Hospital Bangladesh from July 20, 2020 and onward were enrolled in this study. All of these institutions are tertiary level referral hospitals. The sample size was estimated to be 386, by n = z²pq/d² formula. Here z = 1.96 (at 95%CI), n = sample size, p = 0.5 (prevalence), q = 1 - p, and d = 5% (margin error at standard of 0.05) COVID-19 patients [real-time reverse transcription polymerase chain reaction (RT-PCR) positive] requiring ICU support with national early warning score 2 (NEWS-2) ≥ 5 were randomly shortlisted to be enrolled in the study. Following enrollment, patients were allocated to the study groups, A (famotidine treatment group) and B (control group). An odd-even-ratio (1:1) was applied to hospital registration number to divide the study groups. The Berlin definition was taken in consideration to define ARDS. Following primary enrollment, the cases were further confirmed by investigators. Initially, 308 patients were recruited, among them, 54 declined to enroll and 46 had uncontrolled comorbid conditions or were already hospitalized for other issues; therefore, these patients were excluded. Finally, 208 COVID-19 patients were enrolled in this study (Figure 1).
Each group contains 104 patients. Group A patients received famotidine (Famotac 20 mg oral tablet formulation) every 8 h, 30 min before the meal; 40 mg in the case of < 60 kg, and 60 mg in the case of > 60 kg body weight, and this was continued for 30 d. Other treatments included remdesivir, tocilizumab, dexamethasone, a broad-spectrum antibiotic (meropenem), proton pump inhibitor, ascorbic acid, cholecalciferol, zinc, bronchodilators and oxygen support. Additionally, treatments according to the symptomatic onset were given. Detailed clinical follow-ups that included all the vitals (temperature, pulse, respiratory rate, blood pressure, oxygen saturation, percentage of supplementary oxygen use, orientation/consciousness, chief complaints, etc) were obtained at every 24 h intervals. To evaluate the recovery, “NEWS-2” was calculated accordingly. The risk of mortality in individual cases was evaluated by sequential organ failure assessment score during admission. Regular follow-ups were obtained in every 24-h interval and noted accordingly. Outcomes were determined as: “Time to clinical improvement” characterized as the point of randomization to a maintained NEWS-2 score ≤ 2 for 24 h; “Symptomatic recovery” characterized as the time from randomization to the recovery of the COVID-19 symptoms (recovery from the major symptoms, according to the patient’s statement); mortality (%); ICU and total hospitalization duration; rate of additional oxygen usage; time required for clinical failure; on discharge NEWS-2 score; and CT chest recovery (%).
Duration of hospitalization was counted from the time from randomization to hospital discharge or "Ready for discharge" as evidenced by normal body temperature and respiratory rate, stable oxygen saturation on ambient air or ≤ 4 L supplemental oxygen. Time to clinical failure was defined as the time from randomization to the first occurrence of death, mechanical ventilation or withdrawal (whichever occurs first). The “CT chest recovery (%)” was calculated as the difference between the lung involvement in the CT findings of the initial and the CT before discharge. The CT severity score index and the average lung parenchymal involvement were calculated by an experienced radiology specialist in each case. To identify the symptomatic recovery, regular contacts were made every alternate day on the phone following discharge. A detailed history and a sample for the re-evaluation PCR were obtained during the 5th d post-discharge at a physical follow-up. The PCR was repeated at every 7 d interval if found to be positive. Time to COVID-19 recovery or viral clearance was defined as the duration (in days) from the first positive PCR to the first negative PCR that was confirmed by a repeat negative PCR after 7 d. Ethical committee approval: ERC of 250 bedded general Hospital Chattogram Ref: 980 (Date 18/07/2020). ClinicalTrials.gov ID: NCT04504240.
Patients were divided into two groups: Group A (intervention group, n = 104): famotidine (Famotac 20 mg) 40-60 mg oral tablet formulation half an hour before a meal. In the case of < 60 kg body weight famotidine 40 mg, and in the case of > 60 kg, 60 mg was given. This was continued for 30 d. Other treatments were as given: Group B (control group, n = 104): Treatment as given.
Severe COVID-19 patients requiring hospitalization under ICU (of tertiary level referral hospitals in different city of Bangladesh) with a confirmed RT-PCR were included in this study.
Patients with severe and/or uncontrolled comorbid conditions with significantly compromised organ function; patients who were hospitalized from before due to other reasons; Contraindication/possible drug interaction; pregnant patients, severely obese patients with a body mass index > 35 and critically ill COVID-19 patients already on ventilator support were not included in the study.
Statistical analyses were done by Graph Pad prism (7.2) and SPSS (V-28). Data were analyzed, mean ± SD, mean ± SEM and frequencies were calculated. Difference among the study groups were evaluated by Chi-square and a t-test. Additionally, survival benefit of the famotidine treatment was calculated by the Kaplan Meier survival analysis.
Number of patients (n) was 208; male 155 (74.5%), female 53 (25.5%); 104 patients in each group (A and B). Both the study groups have nearly similar sex distribution. Group A male 78 (75%), female 26 (25%); group B male 77 (74%), female 27 (26%). The mean age was 57.15 years, group A 57.06 years (23-83 years), and group B 57.24 years (18-85 years).
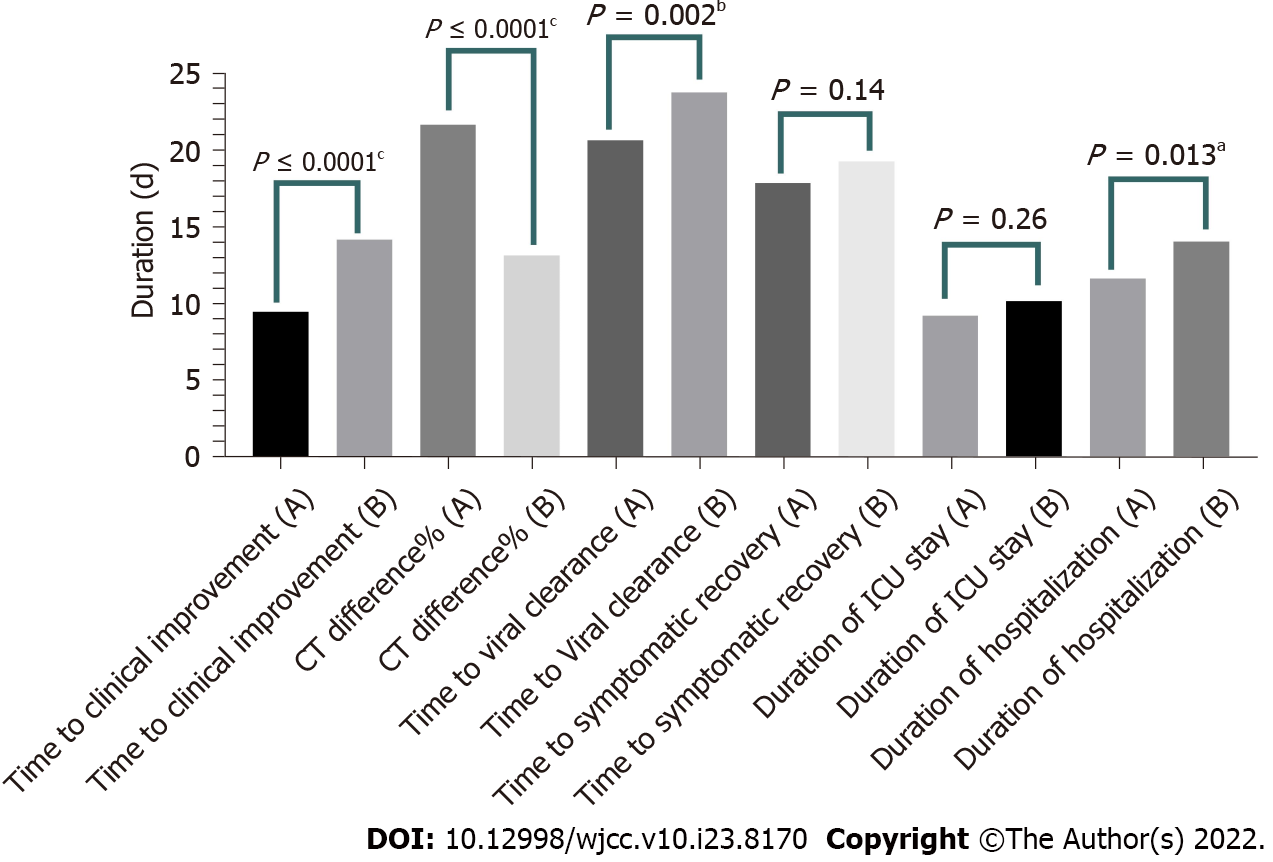
Treatment outcomes among the study groups were compared (Table 1). The recovery and death were found preferable in group A than that of group B. Recovery distribution in both study groups was comparable. The time to clinical improvement, Time to symptomatic recovery; NEWS-2 score while discharged, total ICU and hospital stay, time to viral clearance were low in the famotidine group (A). Survivors of group A experienced a reduced duration of ICU and hospital stay. Superior improvement of the CT chest findings was observed in the famotidine treatment group. Difference between the time to clinical improvement, total duration of hospitalization among the recovered patients, CT chest improvement and the time for viral clearance were statistically significant in a t-test (P ≤ 0.05). However, the difference between the time to symptomatic recovery, ICU stay duration and the time to clinical failure/death, among the groups were not significant, P ≥ 0.05. The CT chest involvement (%) during admission was high in group A and the values differ significantly among the groups. Additionally, a significantly low level (P ≤ 0.05) of P:F ratio, PaO2 and O2 saturation (finger-tip) were observed during admission in the control group than the famotidine treatment group (Table 1).
| Variables | Group A (famotidine) | Group B (control) | t-test (95%CI) | |
| Patient demographics and characteristics during hospitalization | Sex | n = 104, male: 78, female: 26 | n = 104, male: 77, female: 27 | |
| Age (yr) | 57.06 ± 14.97 (23-83) | 57.24 ± 13.87 (18-85) | P = 0.92 | |
| Body weight (Kg) | 65.9 ± 9.0 (55-93) | 66.5 ± 7.7 (53-90) | P = 0.61 | |
| BMI | 24.3 ± 5.8 (19.7-35.4) | 22.7 ± 5.0 (20.4-34.5) | P = 0.58 | |
| Comorbidity | 61 (58.7%) | 46 (44.2%) | ||
| Oxygen saturation (%) | 85.23 ± 6.961; 73-98 | 87.77 ± 8.971; 34-99 | P = 0.0243a | |
| PaO2 (mm of Hg) | 47.9 ± 6.8; 27-85 | 52.0 ± 9.8; 28-84 | P = 0.005b | |
| P:F ratio (on admission) | 62.9 ± 28.1; 38-210 | 71.3 ± 35.8; 23-192 | P = 0.015a | |
| Oxygen requirement | 19.44 ± 16.56; 4-60 L/min | 18.38 ± 10.51; 2-40 L/min | P = 0.643 | |
| CT chest (%) | 39.87 ± 14.3; 15-70 | 27.08 ± 16.7; 15-65 | P ≤ 0.0001d | |
| Respiratory rate | 29.07 ± 10; 12-55/min | 30.37 ± 6.865; 18-45/min | P = 0.299 | |
| Temperature | 100.7 ± 1.865; 98-104 °F | 101.4 ± 1.453; 98-104 °F | P = 0.398 | |
| Serum creatinine (mg/dL) | 1.2 ± 0.61; 0.5-3.3 | 1.26 ± 0.62; 0.6-3.5 | P = 0.24 | |
| Serum Bilirubin (mg/dL) | 1.62 ± 1.0; 0.2-5.0 | 1.59 ± 1.1; 0.4-5.8 | P = 0.80 | |
| Platelet count (103/mL) | 206.3 ± 995.2; 85000-400000 | 221 ± 82.6; 78000-395000 | P = 0.24 | |
| MAP (in mm of Hg) | 87.0 ± 12.27; 54-116 | 87.2 ± 11.5; 54-116 | P = 0.91 | |
| GCS score | 12.1 ± 1.7; 7-15 | 12.6 ± 1.9; 7-15 | P = 0.06 | |
| NEWS-2 score (on admission) | 8.3 ± 1.8; 3-12 | 8.7 ± 2.0; 5-13 | P = 0.11 | |
| SOFA day-1 score | 6 ± 1.68; 3-11 | 5.3 ± 1.44; 3-9 | P = 0.004b | |
| Characteristics of study group patients during discharge (recovered cases) | NEWS-2 score (on discharge; Recovered cases) | 0.89 ± 0.8; 0-2 | 1.2 ± 0.8; 0-2 | P = 0.0221a |
| Oxygen saturation (%) (on discharge; Recovered cases) | 95.9 ± 2.07; 90-100 | 96.3 ± 1.5; 93-99 | P = 0.080 | |
| Oxygen requirement (on discharge; Recovered cases) | 1.36 ± 2.2; 0-10 L/min | 1.5 ± 2.4; 0-12 L/min | P = 0.279 | |
| CT chest (%) (on discharge; Recovered cases) | 18.0 ± 7.5; 5-35 | 13.86 ± 9.9; 0-35 | P = 0.004b | |
| Respiratory rate (on discharge; recovered cases) | 19.4 ± 4.7; 12-34/min | 19.1 ± 2.2; 15-26/min | P = 0.638 | |
| Temperature (on discharge; Recovered cases) | 99.9 ± 1.6; 98-104 °F | 100.3 ± 1.7; 97.5-103.6 °F | P = 0.252 | |
| Treatment outcomes among the study groups | Recovered | n = 78 (75%), male: 58 (74.4%), female 20 (25.6%) | n = 73 (70%), male: 59 (81%), female: 14 (19%) | |
| Dead | n = 26 (25%), male: 20 (76.9%), female: 6 (23.1%) | n = 31 (30%), male: 18 (58.1%), female: 13 (41.9%) | ||
| Time to clinical improvement | 9.53 ± 5.0; 3-27 d | 14.21 ± 5.6; 6-28 d | P ≤ 0.0001d | |
| CT difference (%) (on admission and before discharge) | 21.7 ± 9.5; 8-40 | 13.2 ± 8.9; 0%-40% | P ≤ 0.0001d | |
| Time to symptomatic recovery | 17.9 ± 5.4; 8-37 d | 19.3 ± 6.3; 10-35 d | P = 0.14 | |
| duration of ICU stay | 9.28 ± 4.96; 1-27 d | 10.23 ± 7.13; 2-42 d | P = 0.26 | |
| Total duration of hospitalization | 11.73 ± 6.0; 1-30 d | 14.13 ± 7.6; 3-42 d | P = 0.013a | |
| Duration of hospitalization (recovered patients) | 13.04 ± 5.5; 4-30 d | 16.31 ± 6.1; 7-30 d | P = 0.0009c | |
| Duration of ICU stay (recovered patients) | 9.7 ± 4.6; 1-26 d | 10.6 ± 6.3; 2-26 d | P = 0.33 | |
| Time to clinical failure/death | 6.9 ± 6.1; 1-27 d | 10.4 ± 12.2; 3-42 d | P = 0.1986 | |
| Time to viral clearance (negative PCR) | 20.7 ± 5.9; 13-39 d | 23.8 ± 6.4; 13-42 d | P = 0.002b | |
According to the subgroup analysis of sex (Table 2), group A females had a shorter ICU stay duration (≤ 10 d) compared to males. This was found reversed in group B.
| Variables | Duration | Male (%) | Female (%) | ||
| Group A, ICU stay | < 10 d (71) | 51 (65.3) | n = 78 | 20 (76.9) | n = 26 |
| 11-20 d (30) | 24 (30.76) | 6 (23.1) | |||
| 21-30 d (3) | 3 (3.8) | 0 (0) | |||
| ≥ 31 d (0) | 0 (0) | 0 (0) | |||
| Group B, ICU stay | ≤ 10 d (68) | 55 (69.6) | n = 79 | 13 (52) | n = 25 |
| 11-20 d (25) | 16 (20.2) | 9 (36) | |||
| 21-30 d (9) | 8 (10.1) | 1 (4) | |||
| ≥ 31 d (2) | 0 (0) | 2 (8) | |||
| Group A (n = 101), duration of total hospital stays | ≤ 10 d (51) | 37 (47.4) | n = 78 | 14 (53.8) | n = 26 |
| 11-20 d (43) | 33 (42.3) | 10 (38.5) | |||
| 21-30 d (10) | 8 (10.3) | 2 (7.7) | |||
| ≥ 31 d (0) | 0 (0) | 0 (0) | |||
| Group B (n = 104), duration of total hospital stays | ≤ 10 d (40) | 31 (39.2) | n = 79 | 9 (36) | n = 25 |
| 11-20 d (49) | 40 (50.6) | 9 (36) | |||
| 21-30 d (13) | 8 (10.1) | 5 (20) | |||
| ≥ 31 d (2) | 0 (0) | 2 (8) | |||
| Group A (n = 78), time to clinical improvement | ≤ 10 d (50) | 31 (55.4) | n = 56 | 19 (86.3) | n = 22 |
| 11-20 d (24) | 21 (37.5) | 3 (13.6) | |||
| 21-30 d (4) | 4 (7.1) | 0 (0) | |||
| Group B (n = 73), time to clinical improvement | ≤ 10 d (21) | 20 (32.7) | n = 61 | 1 (8.3) | n = 12 |
| 11-20 d (41) | 34 (55.7) | 7 (58.3) | |||
| 21-30 d (11) | 7 (11.4) | 4 (33.3) | |||
| Group A (n = 78), recovered cases, duration of ICU stay | ≤ 10 d (49) | 33 (59) | n = 56 | 16 | n = 22 |
| 11-20 d (27) | 21 (37.5) | 6 | |||
| 21-30 d (2) | 2 (3.5) | 0 | |||
| ≥ 31 d (0) | 0 (0) | 0 | |||
| Group B (n = 73), recovered cases, duration of ICU stay | ≤ 10 d (41) | 37 (60) | n = 61 | 4 (33.3) | n = 12 |
| 11-20 d (24) | 16 (17) | 8 (66.6) | |||
| 21-30 d (8) | 8 (13) | 0 (0) | |||
| ≥ 31 d (0) | 0 (0) | 0 (0) | |||
| Group A (n = 26), expired cases; Time to clinical failure/death | ≤ 10 d (1) | 1 (4.5) | n = 22 | 0 (0) | n = 4 |
| 11-20 d (22) | 18 (82) | 4 (100) | |||
| 21-30 d (3) | 3 (13.5) | 0 (0) | |||
| Group B (n = 31), expired cases, time to clinical failure/death | ≤ 10 d (27) | 18 (100) | n = 18 | 9 (70) | n = 13 |
| 11-20 d (1) | 0 (0) | 1 (7.5) | |||
| 21-30 d (1) | 0 (0) | 1 (7.5) | |||
| > 31 d (2) | 0 (0) | 2 (15) | |||
| Group A (survived patients) (n = 78), time to symptomatic recovery | < 10 d (21) | 20 (32.8) | n = 61 | 1 (8.3) | n = 12 |
| 11-20 d (41) | 34 (55.7) | 7 (58.3) | |||
| 21-30 d (11) | 7 (11.5) | 4 (33.3) | |||
| > 31 d (0) | 0 (0) | 0 (0) | |||
| Group B (survived patients) (n = 73), time to symptomatic recovery | < 10 d (3) | 3 (3.91) | n = 61 | 0 (0) | n = 12 |
| 11-20 d (45) | 41 (67.2) | 4 (33.3) | |||
| 21-30 d (19) | 12 (19.7) | 7 (58.3) | |||
| >31 d (6) | 5 (8.1) | 1 (8.3) | |||
| Group A (n = 78) time to negative PCR recovery | 11-20 d (41) | n = 56 | n = 22 | ||
| 21-30 d (32) | |||||
| 31-40 d (5) | |||||
| Group B (survived patients) (n = 73), time to negative PCR recovery | 11-20 d (26) | n = 61 | n = 12 | ||
| 21-30 d (35) | |||||
| 31-40 d (12) | |||||
| Group A (n = 78), CT chest involvement on admission | < 20% | 26 (46.4) | n = 56 | 15 (68.2) | n = 22 |
| 21%-40% | 16 (28.6) | 5 (22.7) | |||
| 41%-60% | 11 (19.6) | 2 (9.0) | |||
| > 61% | 3 (5.3) | 0 (0) | |||
| Group B (n = 73), CT chest involvement on admission | < 20 | 26 (42.6) | n = 61 | 3 (25) | n = 12 |
| 21-40 | 22 (36) | 7 (58.3) | |||
| 41-60 | 13 (21.3) | 1 (8.3) | |||
| > 61 | 0 (0) | 1 (8.3) | |||
| Group A (n = 78), CT chest involvement during discharge | < 20 | 47 (83.9) | n = 56 | 16 (72.7) | n = 22 |
| 21-40 | 9 (16) | 6 (12.2) | |||
| Group B (n = 73), CT chest involvement during discharge | < 20 | 47 (77) | n = 61 | 8 (36.4) | n = 12 |
| 21-40 | 14 (23) | 4 (33.3) | |||
| Group A (n = 78), chest CT improvement | < 20 | 44 (74.6) | n = 56 | 19 (86.6) | n = 22 |
| 21-40 | 5 (8.5) | 3 (13.6) | |||
| 41-60 | 7 (11.9) | 0 (0) | |||
| Group B (n = 73), CT improvement | < 20 | 54 (88.5) | n = 61 | 10 (83.3) | n = 12 |
| 21-40 | 4 (6.6) | 1 (8.3) | |||
| 41-60 | 3 (4.9) | 1 (8.3) | |||
The duration of hospital stay in group A was almost similar among both sexes. Males in group B had a higher recovery within the 21-30 d period and females had a faster recovery in the 10-20 d and 21-30 d period. 8% of female patients in group B required > 31 d of ICU and hospitalization stay.
Group A gained a relatively faster hospital recovery within 10 d than group B. Similarly, a faster time to clinical improvement in group A than group B was observed within a 10 d period. Females in group A secured a clinical improvement during this time than the males, whereas, this was the opposite in group B. Both the males and females in group A had a fast symptomatic recovery < 10 d time. On the other hand, only a few male patients in group B had asymptomatic recovery within this period. Group A patients showed a remarkable recovery from the acute symptom stage during the 11-20 d period. Though the entire patient in group A gained symptomatic recovery within 30 d, some of the group B patients required > 31 d.
A better number of patients in both sexes of group A were recovered within 21 to 30 d. Although a similar number of males in both groups had a delayed viral recovery within 31 to 40 d, this number was higher in the females of group B. Group B experienced faster mortality (< 10 d) than group A. Most of the patients in group A expired within 11 to 20 d. 100% mortality was observed among the males of group B within 10 d of hospitalization. Similarly, all of the female patients died within 11 to 20 d in group A.
The recovery percentage of the CT chest among the groups was almost equivalent. Males in group A gained a remarkable recovery during the discharge. Diversely in group B, males had a lower (< 20%) and females achieved a better prognosis in the CT findings.
As stated in Table 3, Group A had a shorter hospital stay and rapid recovery, 49% of patients were discharged within 10 d time and none required > 31 d. This recovery rate was 38.5% in group B and 1.9% required > 31 d of hospitalization. Both of the study groups had experienced similar ICU stay durations. Though few patients in group B required longer ICU stays. Shorter hospital stay duration (< 20 d) was observed among early (< 40 years) and the late (> 71 years) age groups of both sides. Age influence over the study groups was analyzed and outcomes were evaluated (Table 4). The middle age group of 51-70 years was the most, and the early age group of < 20 years was the least affected. Differences in the hospital recovery were observed depending on the age group. The early (< 30 years) and the late (> 81 years) age groups had a full recovery in group A. Notably, the 51-70 year age had higher mortality in both groups.
| Variables | Age group (yr) | Group A (famotidine) | Group B (control) | ||||||
| 1-10 d | 11-20 d | 21-30 d | > 31 d | 1-10 d | 11-20 d | 21-30 d | > 31 d | ||
| Duration of hospital stay, group A (n = 104), group B (n = 104) | 11-20 | 1 | 1 | 0 | 0 | 4 | 0 | 0 | 0 |
| 21-30 | 1 | 1 | 0 | 0 | 8 | 8 | 0 | 0 | |
| 31-40 | 4 | 5 | 0 | 0 | 12 | 8 | 0 | 0 | |
| 41-50 | 7 | 8 | 1 | 0 | 4 | 8 | 4 | 0 | |
| 51-60 | 18 | 11 | 5 | 0 | 0 | 17 | 5 | 2 | |
| 61-70 | 11 | 10 | 4 | 0 | 12 | 4 | 4 | 0 | |
| 71-80 | 6 | 6 | 0 | 0 | 0 | 4 | 0 | 0 | |
| > 81 | 3 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | |
| Total (%) | 51 (49) | 43 (41.5) | 10 (9.5) | 0 (0) | 40 (38.5) | 49 (47.1) | 13 (12.5) | 2 (1.9) | |
| Duration of ICU stay, group A (n = 104), group B (n = 104) | 11-20 | 2 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| 21-30 | 2 | 0 | 0 | 0 | 12 | 4 | 0 | 0 | |
| 31-40 | 6 | 3 | 0 | 0 | 20 | 0 | 0 | 0 | |
| 41-50 | 8 | 8 | 0 | 0 | 12 | 0 | 4 | 0 | |
| 51-60 | 23 | 10 | 1 | 0 | 8 | 13 | 1 | 2 | |
| 61-70 | 17 | 6 | 2 | 0 | 12 | 4 | 4 | 0 | |
| 71-80 | 9 | 3 | 0 | 0 | 0 | 4 | 0 | 0 | |
| > 81 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | |
| Total (%) | 71 (68) | 30 (29) | 3 (3) | 0 (0) | 68 (65.4) | 25 (24) | 9 (8.6) | 2 (1.9) | |
| Age group (yr) | Total study population (%) | Over all (%) | Recovered patients (%) | Death cases (%) | |||
| Group A | Group B | Group A | Group B | Group A | Group B | ||
| 11-20 | 2 (1) | 2 (2) | 0 (0) | 2 (2.5) | 0 (0) | 0 (0) | 0 (0) |
| 21-30 | 6 (2.9) | 2 (2) | 4 (4) | 2 (2.5) | 0 (0) | 0 (0) | 4 (13) |
| 31-40 | 25 (12) | 9 (8.5) | 16 (16) | 8 (10) | 16 (22) | 1 (4) | 0 (0) |
| 41-50 | 36 (17.3) | 16 (15) | 20 (19) | 9 (11.5) | 8 (11) | 7 (27) | 12 (39) |
| 51-60 | 50 (24) | 34 (33) | 16 (15) | 24 (31) | 16 (22) | 10 (38) | 0 (0) |
| 61-70 | 49 (23.6) | 25 (24) | 24 (23) | 19 (24.5) | 20 (27) | 6 (23) | 4 (13) |
| 71-80 | 32 (15.4) | 12 (11.5) | 20 (19) | 10 (13) | 9 (12) | 2 (8) | 11 (35) |
| > 81 | 8 (3.8) | 4 (4) | 4 (4) | 4 (5) | 4 (6) | 0 (0) | 0 (0) |
| Total | 208 | 104 | 104 | 78 | 73 | 26 | 31 |
Subgroup analysis of the recovered patients against the duration of hospital stay and ICU stay, time to symptomatic recovery, time to negative PCR were evaluated (Table 5). Group A patients achieved a prompt hospital and ICU recovery within 10 d; half of them were discharged within 11-20 d time. In group B the recovery was slow. Most of the patients (66%) were discharged within 11-20 d period; patients with > 61 years experienced a longer ICU stay. Similarly, viral recovery was delayed in the control group. 41 (53%) patients in the famotidine treatment group (A) gained a negative PCR within 11-20 d, 32 (41%) within 21-30 d and 5 (6%) required > 31 d; this was 26 (36%), 35 (48%), and 12 (16%) in the group B.
| Variables | Age group (yr) | Group A (famotidine) | Group B (control) | ||||||||
| 1-10 d | 11-20 d | 21-30 d | > 31 d | Total | 1-10 d | 11-20 d | 21-30 d | > 31 d | Total | ||
| Duration of hospital stay (recovered cases), group A (n = 78), group B (n = 73) | 11-20 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 21-30 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| 31-40 | 3 | 4 | 1 | 0 | 8 | 8 | 8 | 0 | 0 | 16 | |
| 41-50 | 4 | 5 | 0 | 0 | 9 | 0 | 8 | 0 | 0 | 8 | |
| 51-60 | 13 | 8 | 3 | 0 | 24 | 4 | 8 | 4 | 0 | 16 | |
| 61-70 | 5 | 13 | 1 | 0 | 19 | 0 | 16 | 4 | 0 | 20 | |
| 71-80 | 5 | 4 | 1 | 0 | 10 | 1 | 4 | 4 | 0 | 9 | |
| > 81 | 1 | 2 | 1 | 0 | 4 | 0 | 4 | 0 | 0 | 4 | |
| Total (%) | 31 (40) | 39 (50) | 8 (10) | 0 (0) | 78 (100) | 13 (18) | 48 (66) | 12 (16) | 0 (0) | 73 (100) | |
| Duration of ICU stay (recovered cases), group A (n = 78), group B (n = 73) | 11-20 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 21-30 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| 31-40 | 6 | 2 | 0 | 0 | 8 | 12 | 4 | 0 | 0 | 16 | |
| 41-50 | 5 | 4 | 0 | 0 | 9 | 8 | 0 | 0 | 0 | 8 | |
| 51-60 | 20 | 4 | 0 | 0 | 24 | 12 | 0 | 4 | 0 | 16 | |
| 61-70 | 8 | 11 | 0 | 0 | 19 | 8 | 12 | 0 | 0 | 20 | |
| 71-80 | 6 | 3 | 1 | 0 | 10 | 1 | 4 | 4 | 0 | 9 | |
| > 81 | 1 | 2 | 1 | 0 | 4 | 0 | 4 | 0 | 0 | 4 | |
| Total (%) | 49 (63) | 27 (34.5) | 2 (2.5) | 0 (0) | 78 (100) | 41 (56) | 24 (33) | 8 (11) | 0 (0) | 73 (100) | |
| Duration of hospital/ICU stay (death cases), group A (n = 26), group B (n = 32) | 11-20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21-30 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | |
| 31-40 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 41-50 | 0 | 7 | 0 | 0 | 7 | 12 | 0 | 0 | 0 | 12 | |
| 51-60 | 1 | 8 | 1 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | |
| 61-70 | 0 | 4 | 2 | 0 | 6 | 0 | 1 | 1 | 2 | 4 | |
| 71-80 | 0 | 2 | 0 | 0 | 2 | 11 | 0 | 0 | 0 | 11 | |
| > 81 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total (%) | 1 (4) | 22 (84.5) | 3 (11.5) | 0 (0) | 26 (100) | 27 (88) | 1 (3) | 1 (3) | 2 (6) | 31 (100) | |
| Time to clinical improvement (recovered cases), group A (n = 78), group B (n = 73) | 11-20 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 21-30 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| 31-40 | 6 | 2 | 0 | 0 | 8 | 3 | 10 | 3 | 0 | 16 | |
| 41-50 | 5 | 3 | 1 | 0 | 9 | 2 | 3 | 3 | 0 | 8 | |
| 51-60 | 17 | 6 | 1 | 0 | 24 | 7 | 9 | 0 | 0 | 16 | |
| 61-70 | 10 | 8 | 1 | 0 | 19 | 6 | 11 | 3 | 0 | 20 | |
| 71-80 | 5 | 4 | 1 | 0 | 10 | 2 | 6 | 1 | 0 | 9 | |
| > 81 | 4 | 0 | 0 | 0 | 4 | 1 | 2 | 1 | 0 | 4 | |
| Total (%) | 50 (64) | 24 (31) | 4 (5) | 0 (0) | 78 (100) | 21 (29) | 41 (56) | 11 (15) | 0 (0) | 73 (100) | |
| Time to negative PCR (recovered cases), group A (n = 78), group B (n = 73) | 11-20 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 21-30 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| 31-40 | 0 | 7 | 1 | 0 | 8 | 0 | 9 | 7 | 0 | 16 | |
| 41-50 | 0 | 7 | 1 | 1 | 9 | 0 | 4 | 4 | 0 | 8 | |
| 51-60 | 0 | 12 | 11 | 1 | 24 | 0 | 8 | 5 | 3 | 16 | |
| 61-70 | 0 | 6 | 12 | 1 | 19 | 0 | 4 | 11 | 5 | 20 | |
| 71-80 | 0 | 4 | 5 | 1 | 10 | 0 | 1 | 4 | 4 | 9 | |
| > 81 | 0 | 3 | 0 | 1 | 4 | 0 | 0 | 4 | 0 | 4 | |
| Total (%) | 0 (0) | 41 (53) | 32 (41) | 5 (6) | 78 (100) | 0 (0) | 26 (36) | 35 (48) | 12 (16) | 73 (100) | |
| Time to symptomatic improvement (recovered cases), group A (n = 78), group B (n = 73) | 11-20 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 21-30 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| 31-40 | 2 | 6 | 0 | 0 | 8 | 2 | 13 | 1 | 0 | 16 | |
| 41-50 | 4 | 4 | 1 | 0 | 9 | 0 | 6 | 2 | 0 | 8 | |
| 51-60 | 11 | 12 | 1 | 0 | 24 | 1 | 11 | 3 | 1 | 16 | |
| 61-70 | 7 | 11 | 1 | 0 | 19 | 0 | 11 | 8 | 1 | 20 | |
| 71-80 | 3 | 7 | 0 | 0 | 10 | 0 | 3 | 2 | 4 | 9 | |
| > 81 | 1 | 3 | 0 | 0 | 4 | 0 | 1 | 3 | 0 | 4 | |
| Total (%) | 28 (36) | 47 (60) | 3 (4) | 0 (0) | 78 (100) | 3 (4) | 45 (62) | 19 (26) | 6 (82) | 73 (100) | |
Further analysis according to age group (Table 5) shows that group A had a 100% viral recovery within 20 d among patients < 40 years of age. The same trend was seen in 31-50 years old and > 81 years old in group B. The 41-70 years old age in group A required > 20 d for symptomatic recovery. In group B, the 51-80 years old had a delayed improvement after 31 d.
Comorbidity was present among 58.7% of patients in group A and 44.2% of patients in group B (Table 6). The comorbidities were hypertension (HTN), diabetes mellitus (DM), ischemic heart disease, chronic obstructive pulmonary disease, rheumatoid arthritis, benign prostatic hypertrophy, ischemic stroke, osteoarthritis, heart failure, hypothyroid, bronchial asthma, chronic kidney disease, inflammatory bowel disease, IBS, migraine, hepatitis-B and carcinoma. 15.8% in group A and 21.1% in group B patients had HTN. Group A patients of ≥ 2 comorbidities had a better recovery with HTN and/or diabetes than group B. Mortality was also high in group B patients with HTN or DM and two or more comorbidities than group A.
| Comorbidity, n (%) | Group A, n (%): Male 46 (75.4), female 15 (24.59) | Group B n (%): Male 33 (71.7), female 13 (28.3) | ||||||||
| Total 61 (58.7) | Recovered cases 21 (34.42) | Death cases 15 (24.59) | Total 46 (44.2) | Recovered cases 24 (52.1) | Death cases 10 (21.7) | |||||
| ≥ 2 (12) | < 2 (9) | ≥ 2 (12) | < 2 (3) | ≥ 2 (10) | < 2 (14) | ≥ 2 (8) | < 2 (2) | |||
| HTN, 22 (19) | 16 (15.8) | 6 (50) | 5 (55.5) | 5 (50) | 0 | 22 (21.1) | 8 (44.4) | 6 (42.8) | 7 (87.5) | 1 (50) |
| IHD, 11 (5.5) | 8 (7.9) | 3 (25) | 0 | 4 (33.3) | 1 (33.3) | 3 (2.8) | 1 (10) | 2 (14.2) | 0 | 0 |
| Diabetes mellitus, 28 (14) | 15 (14.5) | 6 (50) | 1 (11.1) | 8 (66.6) | 0 | 13 (12.5) | 5 (27.8) | 1 (7.1) | 6 (75) | 1 (50) |
| COPD | 5 (4.8) | 1 (8.3) | 1 (11.1) | 3 (25) | 0 | 4 (3.9) | 3 (30) | 1 (7.1) | 0 | 0 |
| BPH | 9 (8.9) | 5 (41.6) | 0 | 2 (16.6) | 2 (66.6) | 3 (2.8) | 1 (10) | 0 | 2 (25) | 0 |
| Rheumatoid arthritis | 4 (3.8) | 4 (33.3) | 0 | 0 | 0 | 2 (1.9) | 1 (10) | 1 (7.1) | 0 | 0 |
| Osteoarthritis | 5 (4.8) | 1 (8.3) | 3 (33.3) | 0 | 1 (33.3) | 1 (0.9) | 0 | 1 (7.1) | 0 | 0 |
| Hypothyroid | 3 (2.9) | 2 (16.6) | 1 (11.1) | 0 | 0 | 1 (0.9) | 0 | 1 (7.1) | 0 | 0 |
| Ischemic stroke | 2 (1.9) | 1 (8.3) | 1 (11.1) | 0 | 0 | 1 (0.9) | 0 | 1 (7.1) | 0 | 0 |
| Heart failure | 2 (1.9) | 1 (8.3) | 0 | 1 (8.3) | 0 | 2 (1.9) | 0 | 0 | 2 | 0 |
| Chronic kidney disease | 1 (1) | 0 | 0 | 0 | 1 (33.3) | 1 (0.9) | 0 | 1 (7.1) | 0 | 0 |
| Bronchial asthma | 1 (1) | 1 (8.3) | 0 | 0 | 0 | 2 (1.9) | 0 | 0 | 2 (25) | 0 |
| IBD | 1 (1) | 0 | 0 | 0 | 1 (33.3) | 0 (0) | 0 | 0 | 0 | 0 |
| IBS | 0 | 0 | 0 | 0 | 0 | 1 (0.9) | 0 | 1 (7.1) | 0 | 0 |
| Hepatitis B | 1 (1) | 0 | 1 (8.3) | 0 | 0 | 1 (0.9) | 0 | 1 (7.1) | 0 | 0 |
| Migraine | 1 (1) | 1 (8.3) | 0 | 0 | 0 | 3 (2.8) | 0 | 1 (7.1) | 2 (25) | 0 |
| Carcinoma (early) | 1 (1) | 1 (8.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
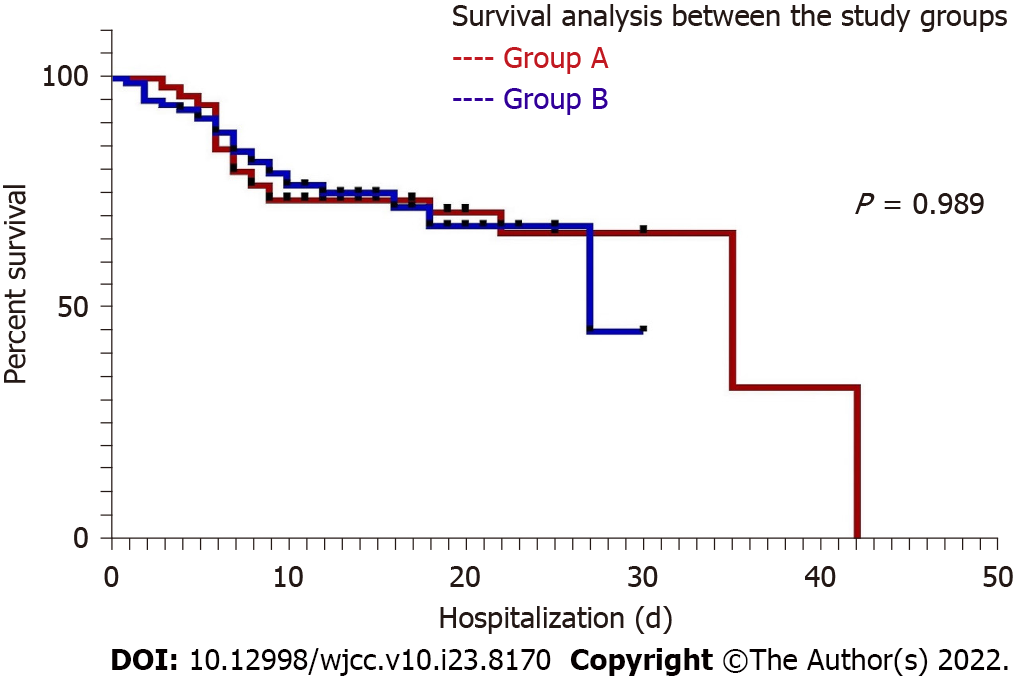
To further evaluate and compare the survival benefit with famotidine treatment, Kaplan Meier survival analysis was done. The statistical difference involving the survival among the two study groups did not show any statistical significance (P = 0.989) (Figure 2). Log-rank hazard ratio of the group A (1.003; 95%CI: 0.59-1.69); group B (0.996; 95%CI: 0.59-1.67). Median survival: group A (27; 95%CI: 0.45-1.29), group B (35; 95%CI: 0.76-2.18).
SARS-CoV–2 infection was first detected in humans during December 2019 and is known to cause COVID-19 disease[3]. Patients with COVID–19 disease can present with a variety of clinical manifestations which develop 2-14 d following exposure to the virus. These symptoms include cough, shortness of breath, fever, chills, repeated rigor, myalgia, headache, oropharyngitis, anosmia and ageusia[6,7]. More severe symptoms warranting hospital admission include difficulty breathing, a persistent sense of chest pain or compression, confusion or difficulty to arouse and central cyanosis. Of hospitalized patients, 20%-42% develop ARDS. This is the most common cause of ICU admission. The mortality rate among ICU patients is still high, 39%-72%[3,8]. Different treatment options for patients with COVID-19 to reduce morbidity, mortality and spread of the disease are an urgent global need. Trials with the repurposing of different drugs have already been published[9].
Famotidine is a potent histamine H2-receptor antagonist which has widely been used in the treatment and prevention of peptic ulcer disease. After intravenous administration, the plasma famotidine concentration-time profile exhibits a biexponential decay with a distribution half-life of about 0.18 to 0.5 h and an elimination half-life of about 2 to 4 h. Famotidine shows a low plasma protein binding (15%-22%) and steady-state drug distribution ranges from 1.0 to 1.3 L/kg. Following administration, 70% of this drug is eliminated in an unchanged form into the urine. Thus, total body and renal clearances (15 L/h) of famotidine correlate significantly with creatinine clearance. Famotidine is considered to be eliminated via glomerular filtration and renal tubular secretion[9]. Besides, famotidine is very well tolerated and free of the antiandrogenic effects infrequently reported with Cimetidine and is not associated with the altered hepatic metabolism of drugs. Thus, it a popular choice for the maintenance therapy of gastric hypersecretory disorders[10].
The idea to test the usefulness of famotidine as a medical countermeasure for COVID–19 emerged from a computational molecular docking effort to identify the papain-like protease inhibitors (PLpro) of SARS-CoV–2. In addition to processing the viral polyprotein, the PLpro from corona viruses is known to remove the cellular substrates ubiquitin. The interferon-stimulated gene 15 from host cell proteins cleaves the C-terminal end of the consensus sequence LXGG, a process termed deISGylation[11-13]. Freedberg et al[14,15] reported that results from a retrospective study tested associations between the use of famotidine and the outcome of patients with COVID-19. They classified the use of famotidine based on COVID-19 exposure within 24 h following hospital admission and maintained a follow-up for up to 30 d.
In our current study, a total of 208 ICU patients with severe COVID-19 disease were recruited. These patients were randomly divided into two groups, group A (famotidine intervention group) and group B (non-famotidine intervention group or control), where n = 104 on each side. After the intervention, group A had a recovery rate of 75% (n = 78) and a mortality rate of 25% (n = 26). On the contrary, the control group B had a relatively low recovery of 70% (n = 73) and a high mortality of 30% (n = 31) (Table 1). The time to clinical improvement, time to symptomatic recovery, duration of ICU stay and mean hospitalization duration in the famotidine treatment group were shorter than that of the control. However, all of these differences with group B were not statistically significant. Nonetheless, the time to clinical improvement, total hospitalization duration among the recovered patients, CT chest improvement (%), and duration of viral clearance of the famotidine group were statistically significant (P ≤ 0.05) when compared with the control (Table 1 and Figure 3). Though treatment with famotidine did not show a significant survival benefit against the control group in the Kaplan Meier survival analysis, P = 0.989 (Figure 2). The sex and age difference appeared to be an important concern in treatment outcome. The early and the late age groups had shown a better percentage of COVID-19 recovery in the famotidine treatment group. Females in the famotidine treatment group had a faster ICU/hospital and symptomatic recovery. Similar sex influence with a different outcome was seen with the non-famotidine treatment. Patients with comorbidities also showed a better recovery in the famotidine treatment group than the control (Table 6). Even the duration of death was prolonged among the patients who received famotidine (Table 2). Therefore, it appears that in contrast to the non-famotidine group, the famotidine intervention group had some clinical benefits in severe COVID-19 illness.
Our study and other famotidine studies suggest an association between the use of famotidine and improved outcomes among the hospitalized patients with COVID-19. This was also suggested by a series of famotidine studies with quantitative symptom tracking in non-hospitalized patients[5,16]. Samimagham et al[17] also conducted a randomized trial on the effect of famotidine on the recovery process of hospitalized COVID-19 patients in which the intervention group received standard pharmacotherapy according to the treatment protocols of the National Committee of COVID-19. The oral famotidine was given four times a day until the day of discharge for a maximum of 14 d. However, our study was specifically focused on severe COVID-19 patients which reduced the hospitalization duration and shortened ICU stay. Multiple other investigators had also conducted studies on famotidine[18-20]. Almost all of these studies, including ours, showed clinical benefits and accepted levels of tolerance of famotidine in the treatment of severe COVID-19 disease.
This study has some limitations. The small sample size is a matter of concern. Also, the exclusion of the critically ill COVID-19 patients in the ventilator support group and moderate degree of hospitalized patients might have an influence on the outcome. But to the best of our effort, we selected the study group patients, devoid of serious or uncontrolled comorbidity without compromised organ function to ensure the proper comparison and outcome among the study groups without influence.
According to this study, the famotidine treatment group demonstrated a comparatively better outcome in the survival and death rate. A rapid recovery time, less duration of ICU stays among the survivors, favorable improvement in the CT findings and an earlier viral clearance were observed in the famotidine treatment group. These values were statistically significant in a t-test. The difference between the time to symptomatic recovery, ICU stay duration and the NEWS-2 on discharge was not significant but mean values were relatively less than the control. However, the survival benefit was not significant with the famotidine treatment for severe COVID-19 disease. All these suggest that the H2 receptor blocker famotidine might have a favorable role in the prognosis of the COVID-19 illness.
Famotidine is a histamine-2 receptor antagonist that suppresses gastric acid production. In vitro, famotidine inhibits human immunodeficiency virus replication. Recently, computational methods were applied to predict structures of proteins encoded by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) genome and identified famotidine as one of the drugs most likely to inhibit the 3-chymotrypsin-like protease which processes proteins essential for viral replication. Famotidine use was associated with a reduced risk of intubation and mortality among the patients hospitalized with coronavirus disease 2019 (COVID-19). Therefore, it is necessary to evaluate the potential use of the existing drugs like famotidine that could be used as options for the medical management of COVID-19 patients.
COVID-19 is a worldwide pandemic. Hence SARS-CoV-2 is a novel virus; there is no specific medication against it. Thus, clinicians and scientists all over the world are struggling with the treatment of this disease. Besides antiviral drugs, immunosuppressive agents and symptomatic therapy like the H2 receptor blocker famotidine came to the limelight due to its role in reducing the symptoms of COVID-19 patients.
To evaluate the role of H2 receptor blocker “famotidine” in COVID-19 illness.
COVID-19 patients admitted in the intensive care unit (ICU) of Chattogram General hospital, M. Abdur Rahim Medical College Hospital, and 250 bed Cox’s Bazar Sadar Hospital Bangladesh from July 20, 2020 and onward were enrolled in this study. Patients were divided into famotidine treatment group “A” (famotidine 40 mg to 60 mg oral formulation at 8 h intervals with other treatment as given), and control group “B” (treatment as given). National early warning score (NEWS)-2 and sequential organ failure assessment day-1 score was calculated to evaluate the outcome of the patients.
(1) The recovery (75% in group A and 70% in group B and death (25% in group A and 30% in group B) were found preferable in group A than that in group B; (2) Superior improvement of the computed tomography (CT) chest findings was observed in the famotidine treatment group; (3) Among the group A survivors, the duration of ICU and hospital stays were low; (4) However, the difference between the time to symptomatic recovery, ICU stay duration and the time to clinical failure/death among the groups were not significant, P ≥ 0.05; (5) Group A achieved a reduction of hospital stay and rapid recovery; (6) Viral recovery was delayed in the control group; and (7) The Kaplan Meier survival analysis was performed. The difference involving survival among the two study groups did not show any statistical significance (P = 0.989).
The famotidine treatment group demonstrated a comparatively better clinical outcome than the control. A rapid recovery time, less duration of ICU stay among the survivors, favorable improvement in the CT findings and an earlier viral clearance was observed in the famotidine treatment group; and were statistically significant in a t-test with the control. However, survival benefit was not significant with the famotidine treatment for severe COVID-19 disease.
The results of this study will add up to an important point in treating the SARS-CoV-2 infection during this time of desperate need which will have an overall effect in the long run from every perspective.
The authors are thankful for the support and cooperation of The First Affiliated Hospital of Xi'an Jiaotong University; the Doctors and staff of the Department of Intensive Care Unit of M Abdur Rahim Medical College Hospital, Cox’s Bazar Sadar Hospital, and Chattogram General Hospital. Additionally, we are grateful to Mr. Shahed Bin Siddique, Associate Professor, at the University of Chittagong to perform the Bio-statistics review of this research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Bangladesh
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bozkurt HS, Turkey; Karnyoto AS, China; Morris DL, Australia S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Xie J, Ding C, Li J, Wang Y, Guo H, Lu Z, Wang J, Zheng C, Jin T, Gao Y, He H. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol. 2020;92:2004-2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 2. | Zheng SQ, Yang L, Zhou PX, Li HB, Liu F, Zhao RS. Recommendations and guidance for providing pharmaceutical care services during COVID-19 pandemic: A China perspective. Res Social Adm Pharm. 2021;17:1819-1824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 3. | Malone RW, Tisdall P, Fremont-Smith P, Liu Y, Huang XP, White KM, Miorin L, Moreno E, Alon A, Delaforge E, Hennecker CD, Wang G, Pottel J, Blair RV, Roy CJ, Smith N, Hall JM, Tomera KM, Shapiro G, Mittermaier A, Kruse AC, García-Sastre A, Roth BL, Glasspool-Malone J, Ricke DO. COVID-19: Famotidine, Histamine, Mast Cells, and Mechanisms. Front Pharmacol. 2021;12:633680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Janowitz T, Gablenz E, Pattinson D, Wang TC, Conigliaro J, Tracey K, Tuveson D. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. 2020;69:1592-1597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 5. | Mather JF, Seip RL, McKay RG. Impact of Famotidine Use on Clinical Outcomes of Hospitalized Patients With COVID-19. Am J Gastroenterol. 2020;115:1617-1623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 6. | Mohiuddin Chowdhury ATM, Karim MR, Mehedi HH, Shahbaz M, Chowdhury MW, Dan G, He S. Analysis of the primary presenting symptoms and hematological findings of COVID-19 patients in Bangladesh. J Infect Dev Ctries. 2021;15:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251-2261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1729] [Cited by in RCA: 1737] [Article Influence: 347.4] [Reference Citation Analysis (0)] |
| 8. | Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID–19). [cited 5 March 2022]. Available from: https://stacks.cdc.gov/view/cdc/88624. |
| 9. | Chowdhury A, Shahbaz M, Karim M, Islam J, Dan G, Shuixiang H. A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients. Eurasian J Med Oncol. 2021;5:63-70.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Langtry HD, Grant SM, Goa KL. Famotidine. An updated review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in peptic ulcer disease and other allied diseases. Drugs. 1989;38:551-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Báez-Santos YM, St John SE, Mesecar AD. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 606] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 12. | Daczkowski CM, Dzimianski JV, Clasman JR, Goodwin O, Mesecar AD, Pegan SD. Structural Insights into the Interaction of Coronavirus Papain-Like Proteases and Interferon-Stimulated Gene Product 15 from Different Species. J Mol Biol. 2017;429:1661-1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Mielech AM, Chen Y, Mesecar AD, Baker SC. Nidovirus papain-like proteases: multifunctional enzymes with protease, deubiquitinating and deISGylating activities. Virus Res. 2014;194:184-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Freedberg DE, Wang TC, Abrams JA. Famotidine and Coronavirus Disease 2019. Gastroenterology. 2021;161:360-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Freedberg DE, Conigliaro J, Wang TC, Tracey KJ, Callahan MV, Abrams JA; Famotidine Research Group. Famotidine Use Is Associated With Improved Clinical Outcomes in Hospitalized COVID-19 Patients: A Propensity Score Matched Retrospective Cohort Study. Gastroenterology. 2020;159:1129-1131.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 16. | Bozkurt HS, Bilen Ö. Gastrointestinal symptoms in COVID-19 could be associated with severe lung involvement and increased readmission rates. Eur J Inflamm. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Samimagham HR, Hassani Azad M, Haddad M, Arabi M, Hooshyar D, KazemiJahromi M. The Efficacy of Famotidine in improvement of outcomes in Hospitalized COVID-19 Patients: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Zhou J, Wang X, Lee S, Wu WKK, Cheung BMY, Zhang Q, Tse G. Proton pump inhibitor or famotidine use and severe COVID-19 disease: a propensity score-matched territory-wide study. Gut. 2021;70:2012-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Yousefi H, Mashouri L, Okpechi SC, Alahari N, Alahari SK. Repurposing existing drugs for the treatment of COVID-19/SARS-CoV-2 infection: A review describing drug mechanisms of action. Biochem Pharmacol. 2021;183:114296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Sun C, Chen Y, Hu L, Wu Y, Liang M, Ayaz Ahmed M, Bhan C, Guo Z, Yang H, Zuo Y, Yan Y, Zhou Q. Does Famotidine Reduce the Risk of Progression to Severe Disease, Death, and Intubation for COVID-19 Patients? Dig Dis Sci. 2021;66:3929-3937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |