Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8115
Peer-review started: December 11, 2021
First decision: January 10, 2022
Revised: January 21, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 16, 2022
Processing time: 232 Days and 21.5 Hours
Intestinal seromuscular bladder augmentation (SMBA) surgery has produced no mucosal-related complications, but its outcomes need to be studied.
To evaluate the safety and effectiveness of SMBA in the treatment of children with neurogenic bladder.
A retrospective analysis of the clinical data of children with SMBA was performed from March 2008 to February 2018, and the data were compared with those of children receiving standard cystoplasty (SC).
In a cohort of 67 children who underwent bladder augmentation, the 46 children in the SC group had an average age of 10.6 years and a follow-up time of 36 mo, and the 21 children in the SMBA group had an average age of 7.6 years and a follow-up time of 29.7 mo. The preoperative and postoperative bladder volumes in the SMBA group were 151.7 mL and 200.4 mL, respectively, and those in the SC group were 173.9 mL and 387.0 mL, respectively. No significant difference in preoperative urinary dynamic parameters was found between the two groups, but the difference after operation was statistically significant. The main complications after SMBA were residual ureteral reflux and failed bladder augmentation, with incidences of 33.3% and 28.6%, respectively. In all 6 patients with failed augmentation in the SMBA group, ileum serom
The improvement of urinary dynamic parameters in the SMBA group was significantly lower than that in the SC group. Children with SMBA had a higher probability of patch contracture and reaugmentation, which might be related to impaired blood supply and urine stimulation, and the sigmoid colon patch should be the priority.
Core Tip: We performed a retrospective review of the mid-term outcomes of patients undergoing two bladder augmentation procedures for neurogenic bladder in a single institution. We studied a total of 67 patients who underwent standard cystoplasty (SC) or seromuscular bladder augmentation (SMBA). In the SMBA procedure, a double-layer intestinal seromuscular patch was used. We found that the improvement of urinary dynamic parameters in the SMBA group was significantly lower than that in the SC group. Children with SMBA had a higher probability of patch contracture and reaugmentation, which might be related to impaired blood supply and urine stimulation.
- Citation: Sun XG, Li YX, Ji LF, Xu JL, Chen WX, Wang RY. Outcomes of seromuscular bladder augmentation compared with standard bladder augmentation in the treatment of children with neurogenic bladder. World J Clin Cases 2022; 10(23): 8115-8123
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8115.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8115
Neurogenic bladder in children is mainly caused by spina bifida and manifests as abnormal urination, upper urinary tract dysfunction and even renal failure. Children with failed conservative treatment require bladder augmentation, which may protect upper urinary tract function, improve urodynamic parameters, and relieve urinary incontinence[1,2].
Intestinal bladder augmentation is widely used for neurogenic bladder with good results and is considered to be standard cystoplasty (SC). However, due to the mucous secretion and reabsorption function of the intestinal mucosa, many related complications occur, including bacteriuria and mucus urine, urinary tract infections, bladder stones, cancer, electrolyte disorders, acidosis, metabolic abnormalities, and vitamin B12 deficiency. In 1955, Shoemaker et al[3] performed an animal experiment in which the intestinal seromuscular patch was sutured to the bladder, and postoperative pathological examination showed that the seromuscular patch was covered by transitional epithelium. Seromuscular bladder augmentation (SMBA) can effectively expand the bladder capacity and avoid complications related to integrating the intestinal mucosa in the bladder[4] but has only been reported in a few institutions. This study retrospectively analyzed the clinical data of children who underwent SMBA at our institution from March 2008 to February 2018 and compared the data with those of children in whom SC was completed during the same period to evaluate the safety and effectiveness of SMBA in the treatment of neurogenic bladder.
Informed consent was obtained from the patients, and ethics approval was granted by the Institutional Review Board. The clinical data of children with bladder augmentation for neurogenic bladder at our institution from March 2008 to February 2018 were collected, including demographic data, clinical symptoms, urodynamic examination, perioperative management, follow-up time, and complications.
The indications for bladder augmentation were small bladder volume, bladder hypertension, low compliance, progressive deterioration of upper urinary tract function, persistent urinary incontinence that was not responsive to oral medication and intermittent catheterization[5]. The indication for ureteral replantation was high-grade reflux (grade III–V). For children with severe spinal and lower limb deformities, continent urinary stoma was recommended.
According to the different augmentation procedures, the children were divided into two groups: the SC group and the SMBA group. The parents were informed of the risks and complications of the two operations, and then they chose one procedure and signed the informed consent form. The advantages of the SC procedure were that it could effectively expand the volume, reduce pressure, and improve incontinence. The disadvantages were mucus urine, urinary tract infection, bladder stones, cancer, and metabolic abnormalities. The advantages of the SMBA procedure were that there were no mucus-related complications, and the disadvantages were postoperative urinary leakage and risk of patch contracture.
The inclusion criteria were as follows: Children who had undergone bladder augmentation for neurogenic bladder in whom the bladder augmentation procedure was SC or SMBA. The exclusion criteria were as follows: (1) Children who had undergone bladder augmentation for nonneurogenic bladder; and (2) Children who had undergone other bladder augmentation procedures.
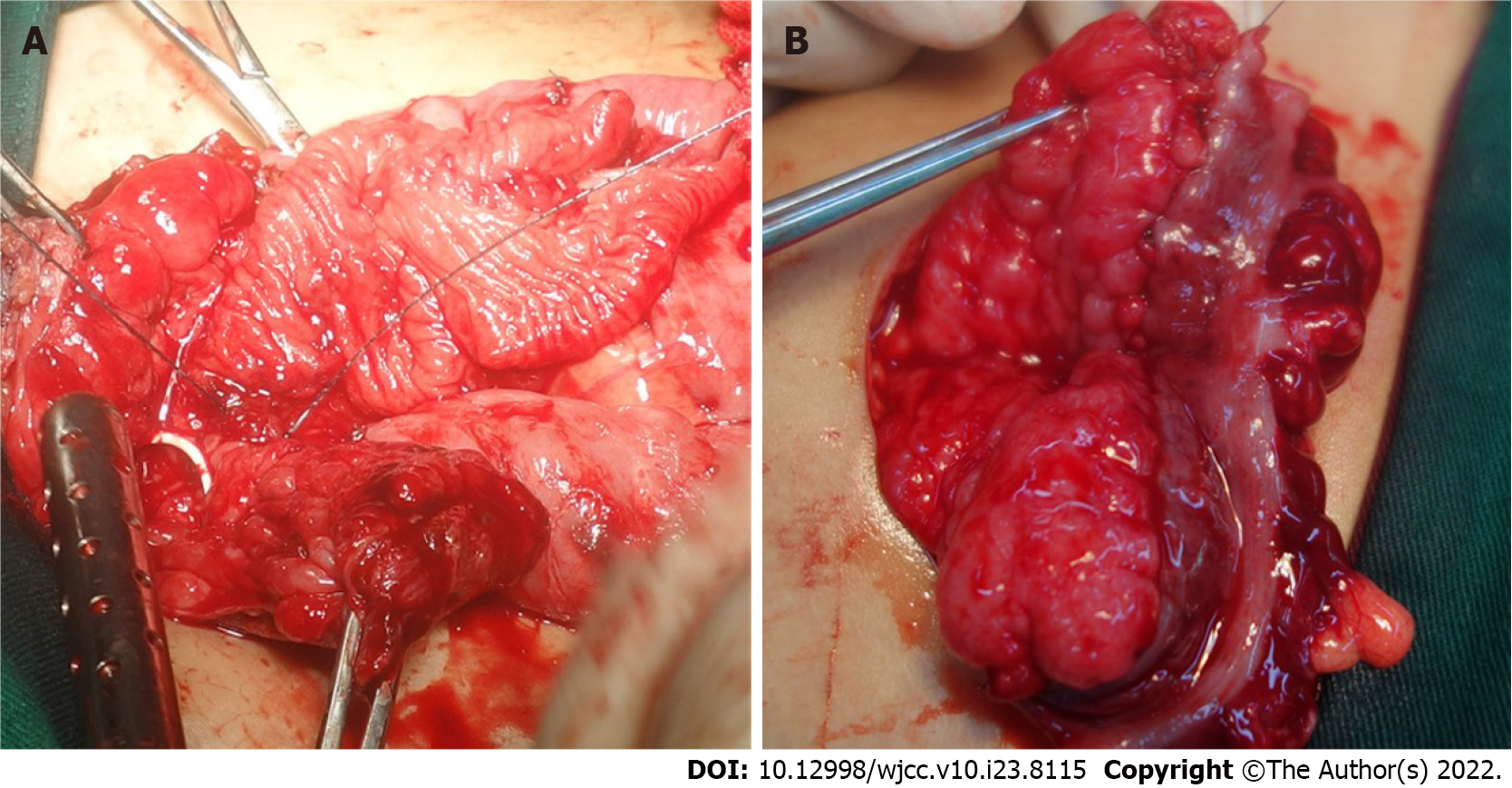
The surgical procedure of SC was as follows: the bladder was incised, 20 cm intestinal (ileum or sigmoid colon) segments were isolated and reconfigured, and then the whole layer patch was sutured with the bladder edge (Figure 1A). In SMBA, 30 cm intestinal (ileum or sigmoid colon) segments were isolated. After de-epithelialization, the double-layer intestine seromuscular patch was sutured with the bladder incisal edge (Figure 1B).
Fasting was required for 5 d after the operation, followed by a gradual return to a normal diet. The drainage catheter of the bladder was removed 2 wk after the operation, and then intermittent catheterization was commenced. Six months after surgery, the patients were followed up regarding urinary tract infection, the number of clean intermittent catheterizations (CICs) per day, anticholinergic drugs, ultrasound, urodynamic examination, and cystography, and then they were recommended for follow-up once a year. If the postoperative end-filling pressure was higher than 20 cm H2O, anticholinergic drugs [tetrodine (dose: 0.1 mg/kg/d, taken twice a day) or sorinacine (dose: 0.1 mg/kg/d, taken once a day)] were administered. CIC was recommended for each patient after surgery.
Continence or dryness was defined as occasional leakage with at most one pad per day, and quality of life was not disturbed. Urinary incontinence was defined as the leakage of urine needing more than one pad every day under regular CIC. Recurrent urinary tract infection (UTI) was defined as 3 times or more symptomatic bacteriuria with fever or pain in the kidney area. Deterioration of upper urinary tract function was defined as an increase in blood creatinine or hydronephrosis.
Few children could achieve complete continence. Most children could achieve relative continence with medication and CIC. The main difference was in the frequency of catheterization and interval between catheterizations. Herein, we recorded and compared the mean number of CICs per day without micturition to acquire continence in the two groups. The actual number of CICs was smaller because most children urinated several times a day. Generally, fewer CICs indicated a larger bladder capacity.
Definition of urodynamic parameters: (1) The bladder capacity was the maximum tolerable capacity or leak point capacity; (2) Expected bladder capacity for age (mL) = (age in years + 1) × 30; (3) Bladder compliance (mL/cm H2O) = Bladder volume difference/Detrusor pressure difference; and (4) The end-filling detrusor pressure was the maximum tolerable detrusor pressure or detrusor pressure at the leak point.
Failed bladder augmentation was defined as upper urinary tract deterioration, bladder hypertension, low volume, persistent incontinence or a high number of CICs per day, which was unresponsive to medication and CIC. After unresponsive conservative treatment, bladder augmentation can be performed again[6].
Short-term and long-term postoperative complications in the two groups were evaluated.
Data analysis was performed using SPSS software. Numerical variables were compared using t tests or Mann–Whitney U tests. Categorical variables were compared by chi square tests. Statistical significance was considered at P < 0.05.
The demographic data, age at surgery, duration of follow-up and concomitant surgery in the two groups are shown in Table 1. The sigmoid colon was used for SC in 3 cases, and the ileum was used in 43 cases. The sigmoid colon was used for SMBA in 12 cases, and the ileum was used in 9 cases.
| Total patients | Sex (male/female) | Age at surgery (yr) | Follow-up duration (mo) | Ureteral reimplantation | Continent catheterizable channel | |
| SC | 46 | 21/25 | 10.6 | 36 | 32 (70.0) | 19 (41.3) |
| SMBA | 21 | 8/13 | 7.6 | 29.7 | 18 (85.7) | 2 (9.5) |
There was massive mucus urine in the bladder after SC, and continent urinary stoma was recommended for children with SC to facilitate catheterization and irrigation. Therefore, children with continent urinary stoma (19 cases) were mainly in the SC group, and the other 2 cases were in the SMBA group.
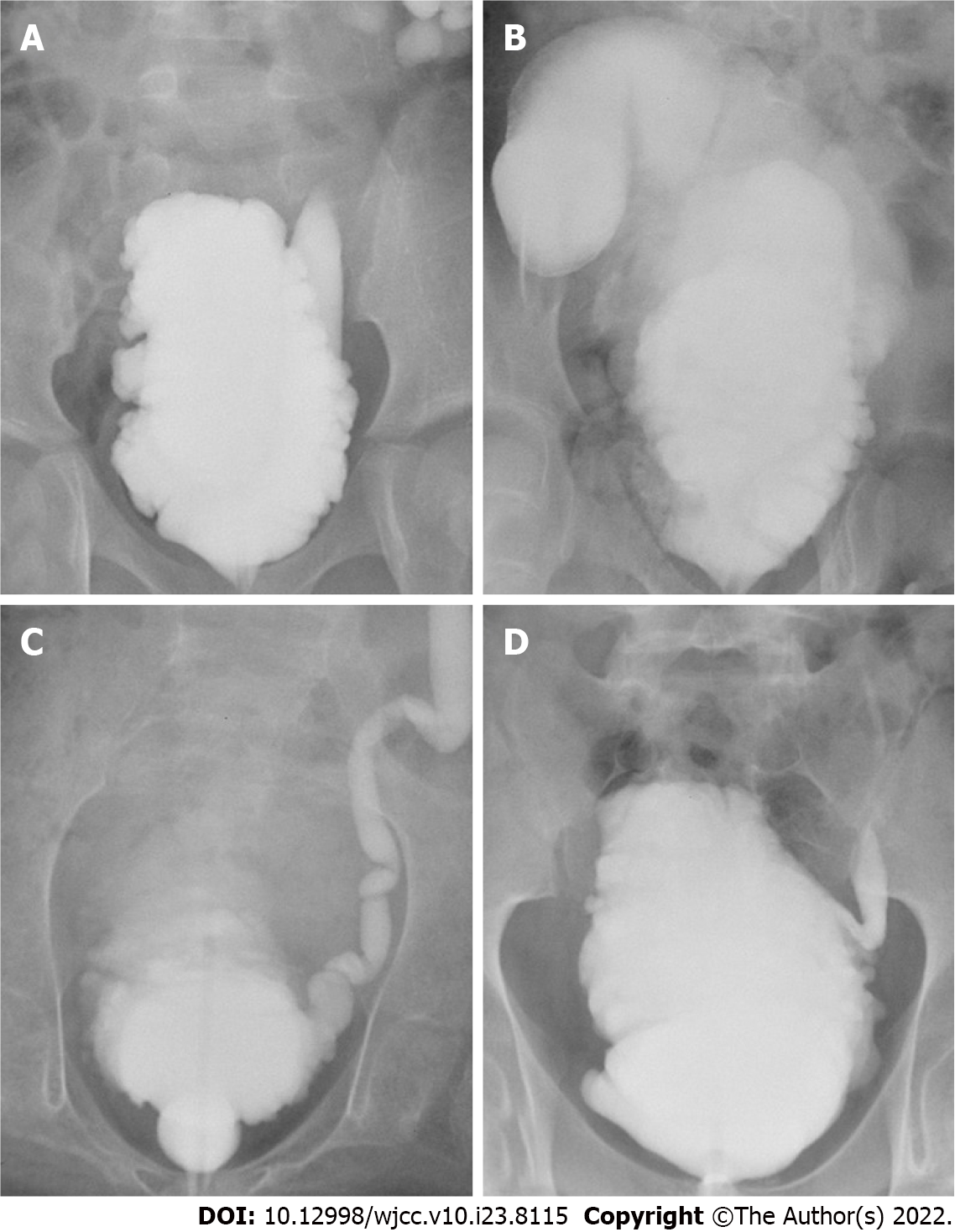
The full-thickness intestine was elastic and could significantly expand under a certain pressure, and an obvious diverticulum could be formed after surgery (Figure 2A and B). However, the elasticity of the seromuscular layer used in SMBA was limited, and the bladder did not bulge to form a diverticulum (Figure 2C and D).
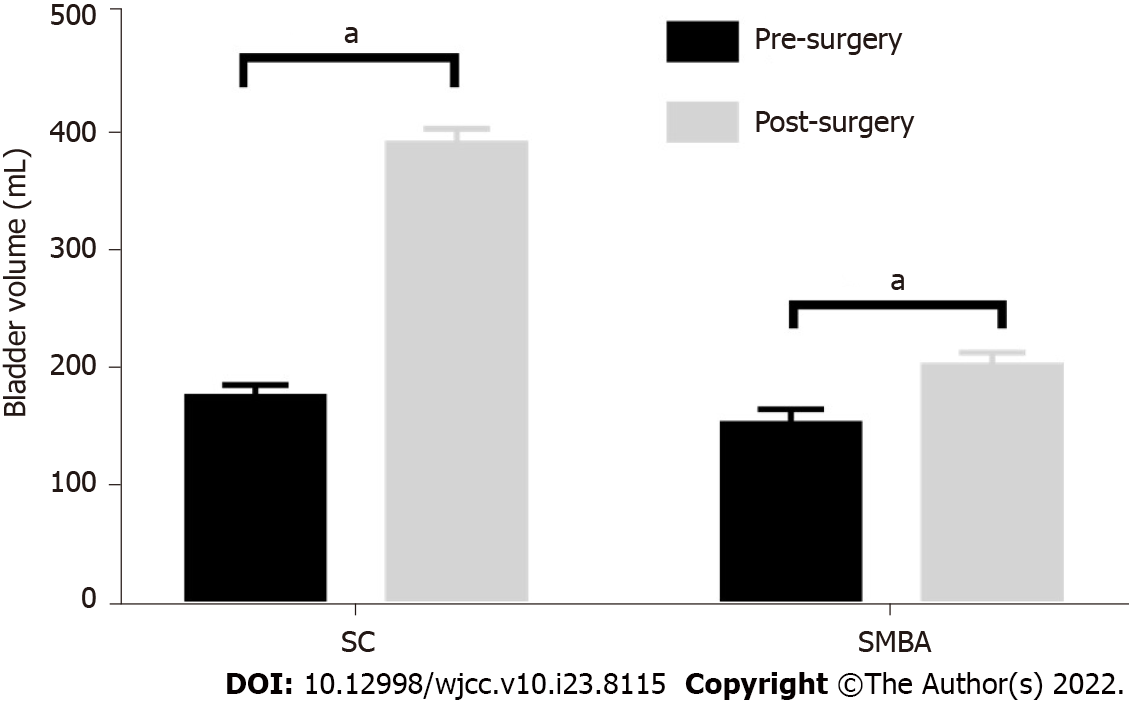
The bladder capacity, end-filling pressure and compliance of the two groups before and after surgery are shown in Table 2. The postoperative bladder volume of the two groups was significantly enlarged compared with the preoperative volume (Figure 3), but the postoperative volume of the SMBA group was significantly lower than that of the SC group (P < 0.05). The end-filling pressure in the SC group decreased significantly after the operation, and the average pressure decreased to close to 20 cm H2O, reaching a relatively safe level. The pressure in the SMBA group also decreased but was higher than the safe level, and oral anticholinergic drugs were required. The postoperative urodynamic outcomes of the SMBA group were significantly poorer than those of the SC group (Table 2), indicating the importance of the intestinal mucosa in the outcomes of bladder augmentation.
| Pre capacity (mL) | Post capacity (mL) | Pre compliance (mL/cm H2O) | Post compliance (mL/cm H2O) | Pre pressure (cm H2O) | Post pressure (cm H2O) | |
| SC | 173.9 | 387.0 | 4.8 | 26.7 | 44.9 | 20.7 |
| SMBA | 151.7 | 200.4 | 3.5 | 6.0 | 50.2 | 34.3 |
| P value | 0.26 | 0.000 | 0.19 | 0.000 | 0.28 | 0.000 |
Postoperative incontinence improved in both groups, but the number of CICs per day and the number of children treated with anticholinergic drugs in the SMBA group were greater than those in the SC group (P < 0.05) (Table 3).
| Pre incontinence | Post incontinence | Number of CICs | Oral anticholinergic drugs | |
| SC | 32 (69.6) | 3 (6.5) | 5.6 | 8 (17.4) |
| SMBA | 18 (85.7) | 7 (33.3) | 7.6 | 17 (81.0) |
| P value | 0.229 | 0.008 | 0.000 | 0.000 |
Short-term postoperative complications mainly occurred within the 1st month after surgery, including intestinal obstruction, urine leakage, and incision infection (Table 4). Both groups had two cases of incision infection caused by urine leakage. Urine leaked from the incision or drainage tube, and part of the urine accumulated under the incision and caused infection. In the SMBA group, one patient had persistent urine leakage after the operation, which was not relieved by drainage for 10 d, and then SC was performed. The reason for urine leakage in the SC group was mucus urine, resulting in frequent blockage of the drainage tube. The reason for urine leakage in the SMBA group was that the seromuscular layer was very thin, and it was difficult to anastomose the bladder and the seromuscular layer patch tightly.
| Intestinal obstruction | Leakage | Incision infection | Recurrent UTIs | Bladder calculi | Residual reflux | Reaugmentation | |
| SC | 2 (4.3) | 4 (8.7) | 6 (13.0) | 4 (8.7) | 3 (6.5) | 1 (2) | 0 |
| SMBA | 1 (4.8) | 5 (23.8) | 6 (28.6) | 2 (9.5) | 1 (4.8) | 7 (33.3) | 6 (28.6) |
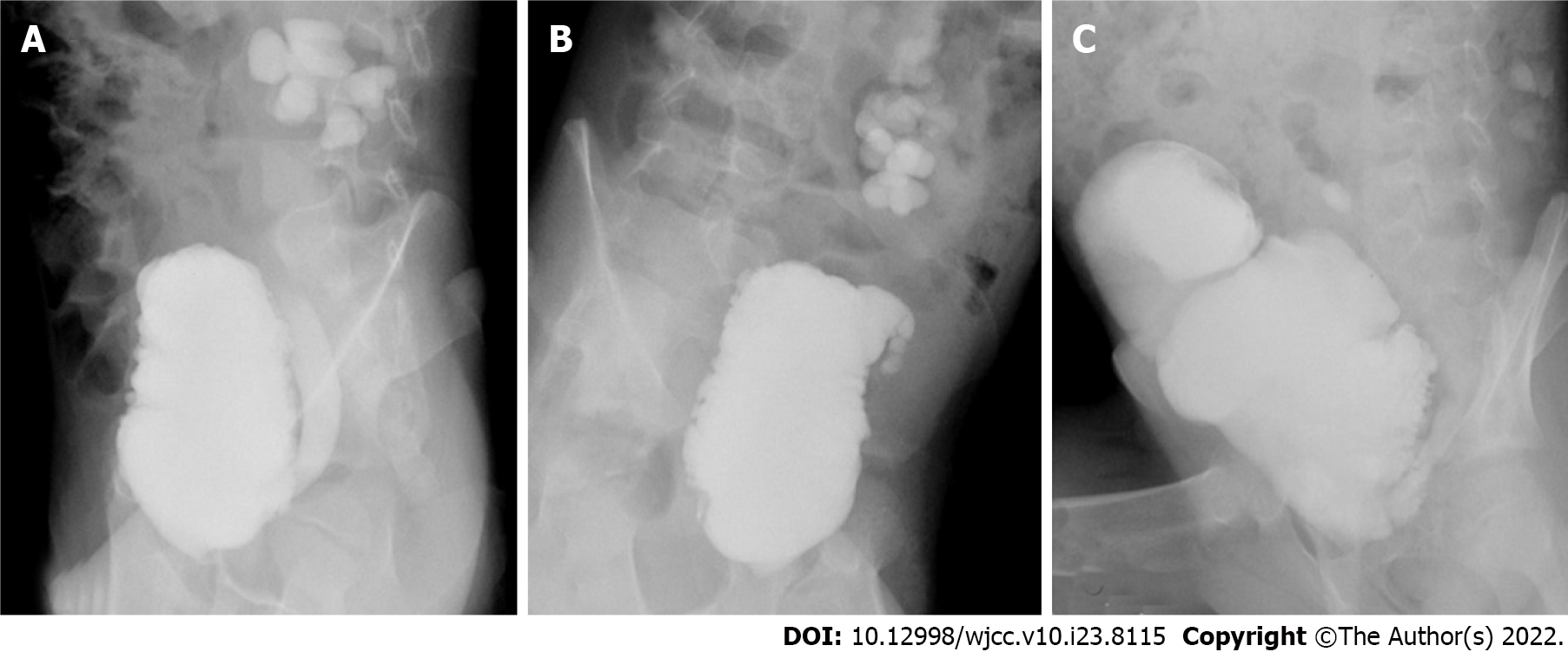
The incidence of recurrent UTIs and bladder stones in the SC group was not significantly different from that in the SMBA group, but the incidences of residual reflux and reaugmentation were significantly lower than those in the SMBA group (P < 0.05) (Table 4). Urinary tract infections were all relieved by antibiotic treatment and indwelling catheterization. Bladder stones were cured by lithotripsy using a holmium laser under a cystoscope. One patient in the SC group had undergone lithotripsy twice. In the SMBA group, 6 (28.6%) patients had reaugmentation for failed augmentation, all of whom used ileum seromuscular patches, and the SC procedure was chosen for the reoperation. The reason for failed augmentation in the SMBA group was patch contracture. As shown in Figure 4, the bladder morphology did not change significantly, and patch contracture and fibrosis were observed during reaugmentation.
Lima et al[4,7-9] reported the long-term outcomes of bladder augmentation using ileum or sigmoid seromuscular layer patches in 1998, 2001, 2004, and 2008. During the operation, a mold made of a silicone balloon was placed in the bladder, and both ureters were catheterized. It was believed that avoiding contact between urine and the patch was important for the growth of the epithelium on the patch, and postoperative maintenance of patch expansion and ureteral drainage was critical to avoid patch contracture. The postoperative bladder capacity and compliance were significantly improved, and the complications were lower than those using traditional surgery. Odeh et al[10] compared the outcomes of SMBA and SC and thought that SMBA could be used as an effective alternative. No difference was found between the two groups in urinary tract infection, incontinence, secondary operations, or spontaneous perforation. The bladder capacity was significantly increased compared with the preoperative capacity, and the incidence of bladder stones was lower.
In the present study, the postoperative maximum bladder volume, end-filling pressure and compliance in the SMBA group were improved compared with the preoperative volume, but they were significantly lower than those in the SC group. With regular CIC and anticholinergic drugs, nearly 1/3 of the patients in the SMBA group still experienced incontinence after surgery. A total of 28.6% of patients underwent SC again due to insignificant improvement in postoperative symptoms, and seromuscular patch contracture and fibrosis were observed during reoperation.
The elasticity of the seromuscular patch was inadequate, and the expansion potential of the patch under pressure was lower than that of the full-layer patch. In the SMBA group, a double-layer patch was used, and the area of the patch was smaller than that of the full-layer patch with the same length of intestine, so the postoperative capacity might be smaller. Additionally, the bladder was not filled with a silicone balloon mold to support the bladder, as reported by Lima et al[9] which might be one reason for the poor outcomes.
The theoretical basis of SMBA was that the bladder epithelium could grow to cover the surface of the intestinal seromuscular patch, but the main problem was patch contracture. Studies have shown that the velocity of circulating red blood cells and the perfusion rate after mucosectomy were reduced significantly, which was related to seromuscular patch contracture. Severely compromised microcirculation was believed to be the cause of patch contracture after SMBA, and omentopexy could not prevent contracture[11]. Abdel Hay et al[12] found in animal experiments that SMBA with a gastric seromuscular patch could produce a large compliant bladder with few complications, and histopathological examination showed that the patch urothelium grew well. They thought that the gastric blood supply was abundant, producing a blood-rich patch and facilitating the growth of the urothelium. Another animal study showed that a silicone balloon mold placed in the bladder could effectively prevent contracture of the bladder[13].
In all 6 patients with reaugmentation in the SMBA group, ileum seromuscular patches were used. During mucosectomy, the bleeding was stopped by electrocoagulation, which might damage the blood supply of the patch. The ileal seromuscular patch was thinner than the colon patch and was susceptible to urine irritation, which made it easier for the patch to contract. The outcomes of SMBA using colon seromuscular patches were better than those using ileum patches, which might be due to the relatively abundant blood supply and thicker seromuscular layer.
The main short-term complications of the two groups were urine leakage and incision infection. Most cases of incision infection resulted from urine leakage and led to delayed healing of the incision. The incidence of urine leakage and incision infection in the SMBA group was higher than that in the SC group because the seromuscular patch was thinner, and it was difficult to anastomose tightly. The long-term complications were recurrent UTIs, bladder calculi, residual reflux, deterioration of upper urinary tract function, and reaugmentation. The patients in the SMBA group did not have mucus urine, which was the main risk factor for UTIs and bladder stones[14], but there was no significant difference in the incidence of UTIs and bladder stones between the two groups. It was considered to be associated with the small bladder capacity and frequent catheterization in the SMBA group. The outcomes of the SMBA group showed no advantage in terms of complications relative to the SC group.
According to the literature and outcomes of the two groups, we summarized the key points of SMBA as follows: (1) The most important point was to preserve the blood supply of the seromuscular patch. The sigmoid colon patch should be the priority because it is thick and rich in blood supply. Sharp dissection with scissors was required to remove the mucosa during the operation, and blunt tears were avoided. Caution should be taken to stop bleeding with electrocoagulation; (2) For the double-layer seromuscular patch, the length of the intestine isolated should be longer (40 cm is recommended); (3) Placing a silicone balloon mold in the bladder and draining urine in the ureter could be attempted; and (4) Patients and their parents should be informed of the risk of patch contracture.
This study was a retrospective study, and each group had different concomitant operations, which might affect the evaluation of the outcomes. Histopathological research was not performed on the shrunken patch, and collagen deposition was not observed. Surgical procedures and postoperative management were not completely consistent with those reported in the literature, and it was difficult to determine the cause of failed bladder augmentation. In the future, prospective and controlled studies are needed to support our conclusions.
The improvement of the urodynamic parameters in the SMBA group was significantly poorer than that in the SC group. The probability of patch contracture and reaugmentation was higher, likely related to an impaired blood supply and urine irritation, and the sigmoid colon patch should be the priority.
Intestinal bladder augmentation is widely used for neurogenic bladder with good results and is standard cystoplasty (SC). However, due to the mucous secretion and reabsorption function of the intestinal mucosa, many related complications occur. To preclude the contact of urine with gastrointestinal mucosa, alternative methods have been investigated. Seromuscular bladder augmentation (SMBA) could effectively expand the bladder capacity and avoid complications related to integrating the intestinal mucosa into the bladder.
SMBA was not widely accepted and was only reported in a few institutions. Further study is needed to evaluate the safety and effectiveness of SMBA in the treatment of neurogenic bladder.
The aim of our study was to assess the outcomes of patients undergoing SC and SMBA to evaluate the safety and effectiveness of SMBA in the treatment of patients with neurogenic bladder.
This study retrospectively analyzed the clinical data of children with SMBA and compared the data with those of children with SC completed during the same period.
No significant difference was found in the preoperative urinary dynamic parameters between the two groups, but the comparison after operation was statistically significant. The main complications after SMBA were residual ureteral reflux and failed bladder augmentation. All 6 patients with failed augmentation in the SMBA group used ileum seromuscular patches for augmentation, and SC was chosen for reaugmentation. During the reoperation, patch contracture and fibrosis were observed.
The improvement of the urodynamic parameters in the SMBA group was significantly poorer than that in the SC group. The probability of patch contracture and reaugmentation was higher, likely related to an impaired blood supply and urine irritation, and the sigmoid colon patch should be the priority.
This study was a retrospective study, and each group had different concomitant operations, which might affect the evaluation of the outcomes. Histopathological research was not performed on the shrunken patch, and collagen deposition was not observed. In the future, prospective and controlled studies are needed to support our conclusions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chamie K, United States; Daneshm S, United States S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Hayashi Y, Yamataka A, Kaneyama K, Kato Y, Lane GJ, Miyano T. Review of 86 patients with myelodysplasia and neurogenic bladder who underwent sigmoidocolocystoplasty and were followed more than 10 years. J Urol. 2006;176:1806-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Zaragoza Torres RI, Galarza-Flores ME, Gómez-Castellanos JC, Barrera-de León JC. [Urodynamic changes after bladder augmentation surgery in paediatric patients with myelomeningocele due to neurogenic bladder]. Cir Cir. 2016;84:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | SHOEMAKER WC, MARUCCI HD. The experimental use of seromuscular grafts in bladder reconstruction; preliminary report. J Urol. 1955;73:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Lima SV, Araujo LA, Montoro M, Maciel A, Vilar FO. The use of demucosalized bowel to augment small contracted bladders. Br J Urol. 1998;82:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Roth JD, Cain MP. Neuropathic Bladder and Augmentation Cystoplasty. Urol Clin North Am. 2018;45:571-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Shreck E, Gioia K, Lucioni A. Indications for Augmentation Cystoplasty in the Era of OnabotulinumtoxinA. Curr Urol Rep. 2016;17:27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Lima SV, Araújo LA, Vilar FO. Nonsecretory intestinocystoplasty: a 10-year experience. J Urol. 2004;171:2636-39; discussion 2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Lima SV, Araújo LA, Vilar FO, Mota D, Maciel A. Experience with demucosalized ileum for bladder augmentation. BJU Int. 2001;88:762-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Lima SV, Araujo LA, Vilar Fde O, Lima RS, Lima RF. Nonsecretory intestinocystoplasty: a 15-year prospective study of 183 patients. J Urol. 2008;179:1113-6; discussion 1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Odeh RI, Farhat WA, Penna FJ, Koyle MA, Lee LC, Butt H, Alyami FA. Outcomes of seromuscular bladder augmentation versus standard ileocystoplasty: A single institution experience over 14 years. J Pediatr Urol. 2017;13:200.e1-200.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Cervellione RM, Hajnal D, Varga G, Rakoczy G, Kaszaki J, Keene D, Goyal A, Dickson A, Cserni T. Mucosectomy impairs ileal microcirculation and results in flap contraction after experimental ileocystoplasty. J Pediatr Urol. 2017;13:81.e1-81.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Abdel Hay S, Soliman SM, Debeky ME. Urothelial ingrowth over demucosalized gastrocystoplasty: an experimental study. BJU Int. 2002;90:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Vilar FO, de Araújo LA, Lima SV. Total bladder replacement with de-epithelialized ileum. Experimental study in dogs. Int Braz J Urol. 2004;30:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Cheng PJ, Myers JB. Augmentation cystoplasty in the patient with neurogenic bladder. World J Urol. 2020;38:3035-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |