Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8097
Peer-review started: March 7, 2022
First decision: April 5, 2022
Revised: April 13, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 16, 2022
Processing time: 147 Days and 4 Hours
Hepatic encephalopathy (HE) is a neurocognitive condition in cirrhosis leading to frequent hospitalizations. Nonselective beta-blockers (NSBBs) are the mainstay of pharmacologic treatment in cirrhotic patients. We hypothesized that since NSBBs decrease cardiac output and portal flow, the decreased metabolic filtering process of liver parenchyma may lead to increased HE-related hospitalizations.
To evaluate the impact of NSBB administration on HE-related readmissions in cirrhotic patients.
In this retrospective cohort study, we included 393 patients admitted to Baylor University Medical Center for liver-related portal hypertension indications between January 2013 and July 2018. Independent predictors of the first HE-related readmissions were identified using Cox proportional hazards analysis. The cumulative incidence of the first HE-related readmissions between patients receiving NSBBs and not receiving NSBBs was examined using Fine-Gray modeling to account for the competing risk of death or liver transplantation.
The mean age was 58.1 ± 10.2 years and most patients fell into Child class C (49.1%) or B (43.8%). The median Model for End-Stage Liver Disease-Sodium score was 22 (IQR: 11). The cumulative incidence of the first HE-related readmissions was significantly higher in patients taking NSBBs compared to patients not receiving NSBBs (71.8% vs 41.8%, P < 0.0001). In multivariate analysis, after adjusting for demographics, markers of liver disease severity, selective beta-blocker, lactulose and rifaximin use, NSBB use [Hazard ratio: 1.74 (95%CI: 1.29-2.34)] was independently associated with the first HE-related readmissions over a median follow-up of 3.8 years.
NSBB use is independently associated with increased HE-related readmissions in patients with cirrhosis, regardless of liver disease severity.
Core Tip: In this study, we evaluated the impact of nonselective beta-blocker (NSBB) administration on hepatic encephalopathy (HE)-related readmissions in patients with Child B or C cirrhosis. After adjusting for markers of liver disease severity, NSBB use was independently associated with the first HE-related readmissions. NSBB use was also an independent predictor of HE-related admissions per person-month.
- Citation: Fallahzadeh MA, Asrani SK, Tapper EB, Saracino G, Rahimi RS. Nonselective beta-blocker use is associated with increased hepatic encephalopathy-related readmissions in cirrhosis. World J Clin Cases 2022; 10(23): 8097-8106
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8097.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8097
Hepatic encephalopathy (HE) is a reversible neurocognitive disorder seen in patients with advanced liver disease[1,2]. It is observed in up to 60% of patients with cirrhosis and is associated with frequent hospitalizations and decreased survival[3-5]. The incidence of HE-related hospitalizations is rising in the United States and imposes a significant economic burden on the healthcare system[6]. In a large population-based cohort, nonselective beta-blocker (NSBB) use was independently linked to HE development[7]. However, the mechanism is unclear.
NSBBs are the mainstay of pharmacologic treatment for portal hypertension and in the prevention of variceal bleeding in cirrhosis[8]. NSBB administration results in reduced cardiac output through inhibition of β1 receptor and splanchnic vasoconstriction via antagonism of the β2 receptor, leading to decreased portal inflow[9]. NSBB use may be associated with decreased survival in patients with refractory ascites, increased risk of acute kidney injury, and decreased transplant-free survival in patients with prior spontaneous bacterial peritonitis[10-13]. However, its role in the development of HE-related complications is not known.
We hypothesized that NSBB use contributes to decreased metabolic filtering process of the liver parenchyma, by way of decreased portal inflow (i.e., similar to spontaneous portosystemic shunting), resulting in a secondary increase in HE-related hospitalizations independent of liver disease severity.
In this observational, retrospective, single-center study, we examined all adults with cirrhosis following a liver-related hospitalization between January 2013 and July 2018. A hospitalization was considered liver-related if the primary or secondary cause of hospitalization was a portal hypertension-related complication such as HE, ascites, variceal bleeding, or hepatorenal syndrome. Patients were considered to have HE on hospital admission only if they had signs of overt HE (i.e., HE grades II-IV) according to the International Society for Hepatic Encephalopathy and Nitrogen Metabolism and West Haven criteria, respectively[14,15].
Our primary aim was to examine the association between the use of NSBBs and subsequent HE-related readmissions. Our secondary aim was to identify factors that were predictive of HE-related admissions.
We collected information about demographics (age, gender), liver disease [etiology of liver disease, history of hepatocellular carcinoma, esophageal varices (EV) and transjugular intrahepatic portosystemic shunt (TIPS)], physical examination findings in particular heart rate, presence of HE (with or without lactulose +/- rifaximin) and ascites as well as biochemical values including serum creatinine, total bilirubin, international normalized ratio, serum albumin, aspartate aminotransferase, alanine aminotransferase, platelet count and white blood cell count during each admission. The patients were divided into two groups according to whether they were receiving NSBB or not on the first liver-related hospitalization. We also gathered data about selective beta-blocker (SBB) use on the first liver-related hospitalization. Model for End-Stage Liver Disease (MELD), MELD-Sodium (MELD-Na) and Child-Turcotte-Pugh (CTP) scores were calculated for all patients during their first admission. Hospital course and outcome variables (i.e., recurrent HE, death or liver transplantation) were determined during the follow-up period. Patients with a change in their NSBB or SBB status after the first liver-related hospitalization were not included in our study.
Continuous data with normal distribution are reported as mean ± SD while continuous data with non-normal distribution are reported as median and ranges (minimum to maximum) or interquartile ranges (IQRs). Independent-samples t-test and Mann-Whitney U test were used for group comparisons for variables with normal and non-normal distribution, respectively. Categorical data are reported as counts and percentages. Group comparisons for categorical data were made with the χ2 test.
All analysis began at the landmark time of discharge from the index hospitalization. Cumulative incidence function using Fine-Gray modeling was used to compare the incidence of first HE-related readmissions between the NSBB and no-NSBB groups while taking competing risk of death or liver transplant into account.
Univariate and multivariate Cox regression analyses were done to identify independent predictors of the first HE-related readmissions. Backward elimination technique with P < 0.10 for entering the model and P < 0.05 for staying in the model was utilized.
To include all of the HE-related admissions, we also determined independent predictors of HE-related hospitalizations per person-month. Due to the overdispersion and right-skewed distribution of this outcome variable, a negative binomial generalized regression model was employed. In this model, total person-months of follow-up was implemented as the offset variable and the follow-up period ended with death, liver transplantation, or end of the study period. The results for the negative binomial generalized regression model were reported as adjusted incidence rate ratios (IRRs) with 95%CIs that represent the relationship between HE-related admissions per person-month and a predictor while considering other covariates.
To further explore the association of NSBB use and HE-related readmissions, multivariate Cox regression analysis and negative binomial generalized regression model were performed in different subgroups including NSBB vs SBB, ascites, EV, MELD-Na score, lactulose and rifaximin subgroups.
A P value < 0.05 was considered to be statistically significant. We performed the statistical analyses using SPSS 21 (SPSS Inc) and R statistical software, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). The study was approved by the institutional review board. The statistical review of the study was performed by a biomedical statistician Giovanna Saracino, PhD.
There were 393 patients with a mean age of 58.1 ± 10.2 years and 144 (36.7%) had ascites. The median MELD-Na score was 22 (IQR: 11) and most patients fell into CTP class C (49.1%) or B (43.8%) (Table 1). A total of 143 patients (36.4%) were treated with NSBBs (nadolol, propranolol, or carvedilol) for either prevention of gastrointestinal bleeding or when used for cardiac indications. Nadolol was the most common NSBB used in this study (46.2%) followed by propranolol (43.4%) and carvedilol (10.5%) (Table 2). In the SBB comparison group, 39 patients were given either metoprolol (89.7%) or atenolol (10.3%).
| Whole group (n = 393) | NSBB group (n = 143) | No-NSBB group (n = 250) | P value | |
| Age, yr | 58.1 ± 10.2 | 57.5 ± 8.8 | 58.4 ± 11.0 | 0.392 |
| Gender, male | 229 (58.3) | 88 (61.5) | 141 (56.4) | 0.323 |
| Heart rate, bpm | 84 (44-161) | 76 (44-122) | 87 (50-161) | < 0.0014 |
| Etiology of liver disease1 | 0.323 | |||
| Hepatitis C virus | 129 (32.8) | 55 (38.5) | 74 (29.6) | |
| Alcoholic | 124 (31.6) | 38 (26.6) | 86 (34.4) | |
| NASH | 49 (12.5) | 17 (11.9) | 32 (12.8) | |
| Cryptogenic | 51 (13.0) | 21 (14.7) | 30 (12.0) | |
| Other causes | 56 (14.2) | 23 (16.1) | 33 (13.2) | |
| CTP score | 0.0543 | |||
| A | 28 (7.1) | 13 (9.1) | 15 (6.0) | |
| B | 172 (43.8) | 71 (49.7) | 101 (40.4) | |
| C | 193 (49.1) | 59 (41.3) | 134 (53.6) | |
| MELD score | 19 (6-40) | 17 (6-39) | 19 (6-40) | 0.024 |
| MELD-Na score | 22 (6-40) | 20 (6-39) | 22.5 (6-40) | 0.0054 |
| History of hepatocellular carcinoma | 29 (7.4) | 8 (5.6) | 21 (8.4) | 0.313 |
| History of esophageal varices | 154 (39.2) | 84 (58.7) | 70 (28.0) | < 0.0013 |
| History of TIPS | 47 (12.0) | 17 (11.9) | 30 (12.0) | 0.973 |
| Presence of HE | 323 (82.2) | 113 (79.0) | 210 (84.0) | 0.223 |
| Lactulose use | 285 (72.5) | 107 (74.8) | 178 (71.2) | 0.443 |
| Rifaximin use | 208 (52.9) | 81 (56.6) | 127 (50.8) | 0.263 |
| Presence of ascites | 144 (36.7) | 50 (35.0) | 94 (37.8) | 0.583 |
| International normalized ratio | 1.5 (1-14) | 1.4 (1-4) | 1.6 (1-14) | 0.0034 |
| Platelet count, × 10-3/mm3 | 84 (4-515) | 72 (15-280) | 95 (4-515) | 0.0024 |
| White cell count, × 10-3/mm3 | 6.8 (0.2-51.9) | 5.7 (1.3-43.7) | 7.7 (0.2-51.9) | < 0.0014 |
| Creatinine, mg/dL | 1.3 (0.3-33.0) | 1.4 (0.4-33.0) | 1.3 (0.3-9.3) | 0.594 |
| Total bilirubin, mg/dL | 2.7 (0.2-137.0) | 2.5 (0.2-43.0) | 3.1 (0.3-137.0) | 0.034 |
| Serum Albumin, g/dL | 2.7 (1.0-5.0) | 2.8 (2.0-5.0) | 2.6 (1.0-5.0) | 0.024 |
| Aspartate aminotransferase, U/L | 59 (3-4048) | 51 (8-677) | 67 (3-4048) | < 0.0014 |
| Alanine aminotransferase, U/L | 39 (10-1180) | 34 (13-538) | 41 (10-1180) | 0.024 |
| Number of patients | Total daily dose (mg) | |
| Nonselective beta-blocker (n = 143) | ||
| Nadolol | 66 (46.2) | 20 (20-80) |
| Propranolol | 62 (43.4) | 30 (10-80) |
| Carvedilol | 15 (10.5) | 12.50 (6.25-50.00) |
| Selective beta-blocker (n = 39) | ||
| Metoprolol | 35 (89.7) | 50.00 (12.50-200.00) |
| Atenolol | 4 (10.3) | 50 (25-100) |
The median follow-up time was 3.8 years (IQR: 4.1 years). There were 187 patients (47.6%) who had HE-related readmissions during the follow-up period. The median time between the first admission and future readmission was 1.9 mo (IQR:4.7 mo). Ninety-six patients (24.4%) died and 50 patients (12.7%) received a liver transplant during the study period. The leading causes of death was sepsis [n = 32 (33.3%)] followed by cirrhosis and its complications [n = 24 (25.0%)], respiratory failure [n = 17 (17.7%)] and multi-organ failure [n = 8 (8.33%)]. The remaining 15 (15.6%) patients died of other causes.
The NSBB group had significantly lower heart rate, MELD-Na score, and platelet count compared with the group not receiving NSBB therapy (Table 1). Further, patients on NSBB therapy had significantly higher rates of presence of EV in comparison with patients not receiving treatment with NSBBs (58.7% vs 28%, respectively).
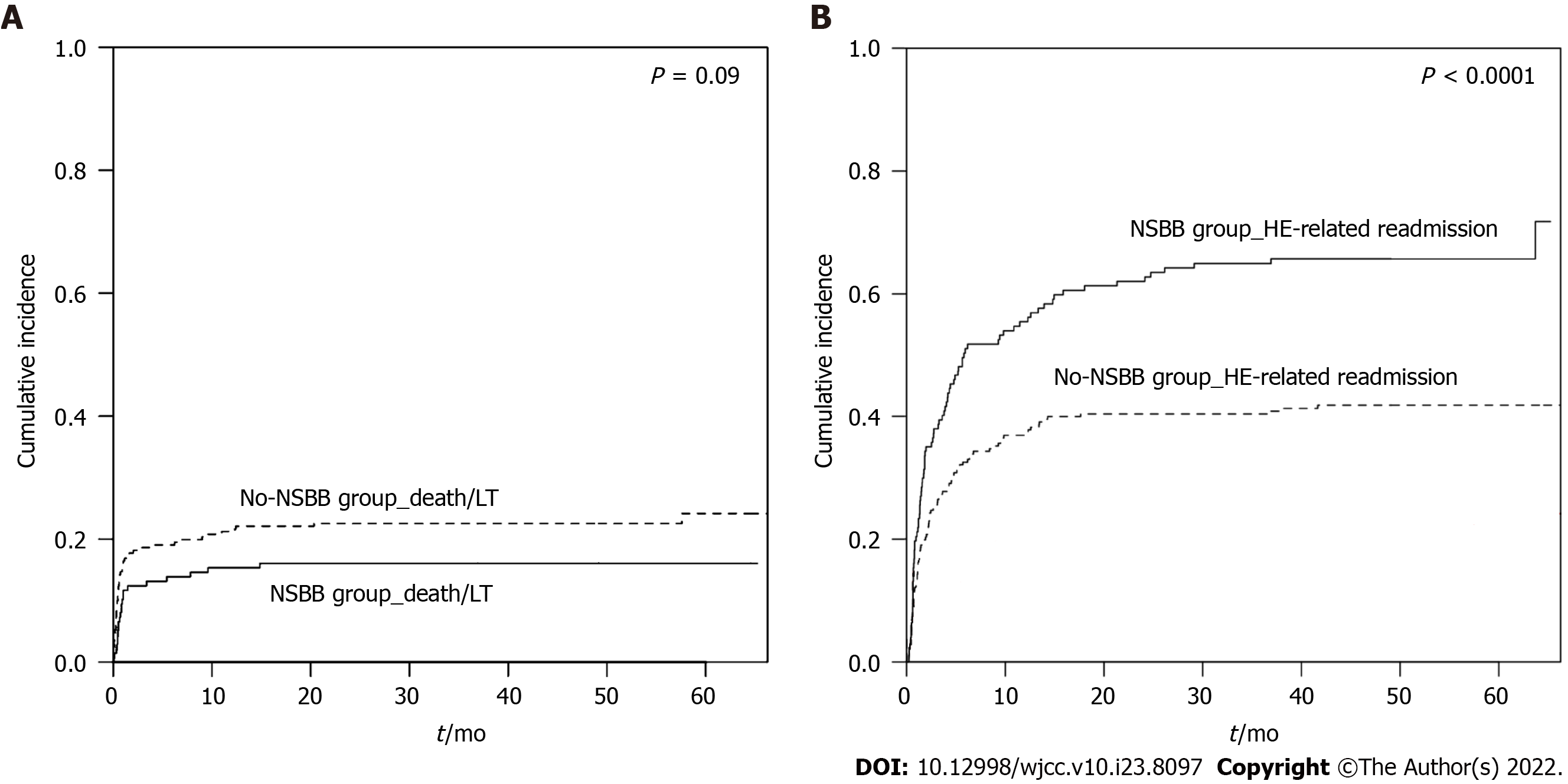
Ninety-one patients (63.6%) in the NSBB group and 96 patients (38.4%) in the no-NSBB group experienced HE-related readmission (P < 0.001). The cumulative incidence of the first HE-related readmissions within 5.5 years was significantly higher in patients taking NSBB compared with patients who were not prescribed NSBB (71.8% vs 41.8%, respectively; P < 0.0001) (Figure 1). The mean time to the first HE-related readmission was not significantly different between the two groups (5.3 mo vs 4.6 mo in NSBB and no-NSBB groups, respectively, P = 0.5). Furthermore, no significant difference was observed between the two groups regarding the mortality rate [n = 32 (22.4%) in the NSBB vs n = 64 (25.6%) in the no-NSBB groups, P = 0.5]. Stratifying the patients according to SBB use, 18 patients (46.2%) with SBB use and 169 patients (47.7%) without SBB use experienced HE-related readmission (P = 0.9).
Results of the univariate analysis of factors associated with HE-related rehospitalization are shown in Table 3. After adjustment of demographic characteristics and surrogate markers of liver disease severity, NSBB use was the only independent predictor of the first HE-related readmissions [HR: 1.74 (95%CI: 1.29-2.34)]. This effect was not seen in patients taking SBBs. To further explore this, multivariate Cox regression model was employed in different subgroups of our patients including NSBB vs SBB, ascites, EV, MELD-Na score, lactulose, and rifaximin subgroups. NSBB use remained an independent predictor of the first HE-related readmissions in all of these subgroups (Supplementary Tables 1-9).
| Variable | Unadjusted | Adjusted | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age, yr | 1.00 (0.99-1.02) | 0.93 | ||
| Gender, male | 1.03 (0.77-1.38) | 0.82 | ||
| MELD-Na score (reference: MELD-Na score < 15) | ||||
| 15 ≤ MELD-Na score ≤ 24 | 1.26 (0.88-1.79) | 0.21 | ||
| 25 ≤ MELD-Na score ≤ 34 | 0.89 (0.58-1.36) | 0.60 | ||
| MELD-Na score > 34 | 0.38 (0.09-1.55) | 0.18 | ||
| History of EV, presence of | 1.24 (0.93-1.66) | 0.15 | ||
| History of TIPS, presence of | 1.34 (0.89-2.01) | 0.16 | 1.48 (0.98-2.25) | 0.065 |
| NSBB use, presence of | 1.81 (1.35-2.41) | < 0.001 | 1.74 (1.29-2.34) | < 0.001 |
| SBB use, presence of | 0.90 (0.55-1.46) | 0.66 | ||
| Lactulose use, presence of | 1.28 (0.89-1.82) | 0.18 | ||
| Rifaximin use, presence of | 0.88 (0.66-1.18) | 0.40 | ||
| Ascites, presence of | 1.10 (0.81-1.48) | 0.55 | ||
| Platelet count, × 10-3/mm3 | 0.996 (0.994-0.999) | 0.008 | 0.997 (0.995-1.000) | 0.07 |
To further explore our findings, we examined the association of NSBB use with all HE-related admissions. NSBB use was an independent predictor of HE-related admissions per person-month [IRR: 1.50 (95%CI: 1.08-2.07)] alongside other variables including MELD-Na score, history of TIPS, lactulose use and platelet count (Table 4). Similar findings were present with liver-related admissions as the outcome variable. This effect was not observed in patients on SBBs. To further investigate this, negative binomial generalized regression model was performed in different subgroups of our patients including NSBB vs SBB, ascites, EV, MELD-Na score, lactulose and rifaximin subgroups. NSBB use remained an independent predictor of HE-related admissions per person-month in all of these subgroups (Supplementary Tables 10-18).
| Variable | IRR (95%CI) | P value | B value |
| Age, yr | 0.99 (0.98-1.01) | 0.19 | -0.010 |
| Gender, male | 1.10 (0.82-1.48) | 0.54 | 0.094 |
| MELD-Na score | 1.05 (1.03-1.08) | < 0.001 | 0.052 |
| History of EV, presence of | 0.98 (0.72-1.35) | 0.92 | -0.016 |
| History of TIPS, presence of | 1.93 (1.24-3.01) | 0.003 | 0.660 |
| NSBB use, presence of | 1.50 (1.08-2.07) | 0.015 | 0.403 |
| SBB use, presence of | 0.81 (0.50-1.31) | 0.40 | -0.208 |
| Lactulose use, presence of | 1.47 (1.00-2.15) | 0.048 | 0.384 |
| Rifaximin use, presence of | 0.73 (0.53-1.00) | 0.050 | -0.319 |
| Ascites, presence of | 1.26 (0.93-1.72) | 0.14 | 0.233 |
| Platelet count, × 10-3/mm3 | 0.997 (0.995-1.000) | 0.03 | -0.003 |
Our results demonstrate that patients treated with NSBBs experienced a significantly higher rate of the first HE-related readmissions compared to patients who did not receive NSBBs. Additionally, NSBB group patients had significantly higher cumulative incidence of the first HE-related readmissions compared to patients in the no-NSBB group. Finally, NSBB use was an independent predictor of HE-related admissions per person-month. These findings were persistent even after adjustment for markers of liver disease severity. NSBB use is associated with incident HE[16,17]; however, these data now extend prior research to show that NSBB use is associated with an increased burden of HE-related readmissions.
There is controversy in the literature regarding the effect that NSBB use has on HE development. Prior studies in early-stage cirrhotic patients reported beneficial to no effect of NSBB on HE development. This includes a cohort of 28 responders to propranolol ± isosorbide mononitrate therapy[18], a randomized trial of propranolol in 20 CTP class A patients[19], and a cohort of 82 patients with cirrhosis[20]. Conversely, other reports indicate an increased risk of HE in NSBB users. In a prospective cohort study of 218 patients with cirrhosis, both NSBB/SBB use was independently associated with a higher rate of minimal HE diagnosis[17]. In a population-based cohort study of 1979 cirrhotic patients, NSBB use was a significant risk factor for incident HE[16]. They hypothesized that NSBB use is a proxy for high-risk varices and severe portal hypertension. The possible explanations for these contradictory research reports of NSBB on HE development are small patient populations, different demographic data, severity of the liver disease, and duration of follow up.
Our data make two major contributions. First, we showed that adjusting for disease severity with granular patient-level data, NSBB use was clearly associated with recurrent HE. Second, our data raise the complexity of the discussion substantially. Our competing risk regression shows that NSBBs are associated with a slightly lower risk of death, confirming the benefits of NSBB use[21,22]. While longer survival avails patients of more opportunity for readmission, our data also shows that NSBBs are associated with a higher burden of readmissions per person-month. Taken together, these data clarify the tradeoffs of NSBB therapy. Therefore, physicians need to be vigilant about NSBB prescription and intensifying therapy.
We showed that NSBB use in decompensated cirrhotic patients (CTP classes B and C) with high MELD-Na scores increases the risk of HE-related admissions during long-term follow-up. Although no clear explanation for the increase in HE-related admissions with NSBB use can be made, specific comments can be described. Krag et al[23] proposed the ‘window hypothesis’, a certain time frame during the natural course of cirrhosis that only within which NSBB use has a beneficial effect on mortality. The same concept can be true regarding the effect of NSBBs on HE development. In cirrhotic patients with mild to moderate portal hypertension, NSBBs counteract the hyperdynamic cardiovascular state and decrease portal hypertension[23,24]. This likely results in a beneficial effect on HE as it counteracts the most likely pathophysiologic mechanism of HE development; shunting of ammonia towards the brain. This can also be the explanation for the slightly increased survival of the patients taking NSBBs in our study.
With cirrhosis progression, patients develop severe portal hypertension that results in increased cardiac output and decreased systemic vascular resistance. NSBB administration in this stage compromises the systemic perfusion pressure that can ultimately decrease hepatic perfusion[13,23-25]. This will result in increased blood ammonia level shunting systemically to the brain in the context of severe portal hypertension, resulting in the development of HE and increased HE-related readmissions over time.
Our study has certain limitations. Although retrospective in nature, having a relatively large sample size with long-term follow up mitigates the study design. We did not know whether any of the patients had a liver-related hospitalization or a previous HE episode before inclusion in the study. However, adjusting for disease severity did not change the results. Although the exact start and end dates and compliance with NSBB and SBB use in all cases and hence the association between duration of NSBB use and risk of HE-related readmissions were not determined, stable estimates across a variety of subsets showed similar results. Furthermore, blood pressure, ammonia levels, precipitant factors for HE, association of NSBB use with different overt HE grades, indications/contraindications for NSBB use and diuretic/proton pump inhibitor use were not explored in our study. Due to limited sample size for an individual NSBB medication and lack of a universal dose-conversion guideline for different NSBBs, a meaningful analysis exploring the association of different doses of NSBBs and risk of HE-related readmissions could not be performed in our study. Although HVPG measurements would have been useful, obtaining HVPG data in a retrospective fashion on our patients was impractical. However, future trials could help delineate the exact HVPG level at which HE readmissions occur in relation to heart rate and NSBB use. Therefore, we believe that our study provides a foundation to guide future prospective trials, allowing pharmacologic comparisons to further delineate the association between NSBB, SBB and HE-related readmissions.
In conclusion, we show that NSBB use is independently associated with increased HE-related readmissions in patients with cirrhosis, regardless of liver disease severity or biochemical abnormalities. Further prospective studies are needed to determine the impact of NSBBs on HE and other portal hypertension complications.
Hepatic encephalopathy (HE) is a cirrhosis complication leading to frequent hospitalizations and imposes a significant economic burden on the healthcare system. Nonselective beta-blockers (NSBBs) are the mainstay of pharmacologic treatment for portal hypertension and in the prevention of variceal bleeding in cirrhosis. The role of NSBBs in the development of HE-related complications is not known.
We hypothesized that since NSBBs decrease cardiac output and portal flow, the decreased metabolic filtering process of liver parenchyma may lead to increased HE-related hospitalizations. If there is a signal that NSBB use is associated with HE-related hospitalizations, further multicenter trials are warranted to explore the impact of NSBBs on HE and other portal hypertension complications.
The main objective of this study was to evaluate the impact of NSBB administration on HE-related readmissions in cirrhotic patients.
We performed an observational, retrospective, single-center cohort study including 393 patients with cirrhosis admitted to Baylor University Medical Center for liver-related portal hypertension indications between January 2013 and July 2018. Independent predictors of the first HE-related readmissions were identified using Cox proportional hazards analysis. The cumulative incidence of the first HE-related readmissions between patients receiving NSBBs and not receiving NSBBs was examined using Fine-Gray modeling to account for the competing risk of death or liver transplantation.
In a cohort of patient with mostly Child class C (49.1%) or B (43.8%) cirrhosis, the cumulative incidence of the first HE-related readmissions was significantly higher in patients taking NSBBs compared to patients not receiving NSBBs (71.8% vs 41.8%, P < 0.0001). In multivariate analysis, after adjusting for demographics, markers of liver disease severity, selective beta-blocker, lactulose and rifaximin use, NSBB use [Hazard ratio: 1.74 (95%CI: 1.29-2.34)] was independently associated with the first HE-related readmissions over a median follow-up of 3.8 years. These results warrant further multicenter clinical trials to explore the impact of NSBBs on HE and other portal hypertension complications.
NSBB use is patients with advanced cirrhosis is independently associated with increased HE-related readmissions, regardless of liver disease severity or biochemical abnormalities. This can be due to the role of NSBB use in decreasing the systemic perfusion pressure that can ultimately lead to a decrease in hepatic perfusion in advanced cirrhosis that will result in an increased blood ammonia level shunting systemically to the brain.
As this study was a retrospective study, future prospective cohort and randomized clinical trials are warranted to explore the impact of NSBBs on HE and other portal hypertension complications.
We thank Mr. Daniel Bizzarri for his help with data gathering.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases, No. 224919.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu CF, China; Zhang LL, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Ferenci P. Hepatic encephalopathy. Gastroenterol Rep (Oxf). 2017;5:138-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Fallahzadeh MA, Rahimi RS. Hepatic Encephalopathy and Nutrition Influences: A Narrative Review. Nutr Clin Pract. 2020;35:36-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10:1034-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Alsahhar JS, Rahimi RS. Updates on the pathophysiology and therapeutic targets for hepatic encephalopathy. Curr Opin Gastroenterol. 2019;35:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Bajaj JS, Reddy KR, Tandon P, Wong F, Kamath PS, Garcia-Tsao G, Maliakkal B, Biggins SW, Thuluvath PJ, Fallon MB, Subramanian RM, Vargas H, Thacker LR, O'Leary JG; North American Consortium for the Study of End-Stage Liver Disease. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. 2016;64:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 6. | Hirode G, Vittinghoff E, Wong RJ. Increasing Burden of Hepatic Encephalopathy Among Hospitalized Adults: An Analysis of the 2010-2014 National Inpatient Sample. Dig Dis Sci. 2019;64:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Tapper EB, Parikh ND, Sengupta N, Mellinger J, Ratz D, Lok AS, Su GL. A risk score to predict the development of hepatic encephalopathy in a population-based cohort of patients with cirrhosis. Hepatology. 2018;68:1498-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Qi XS, Bai M, Fan DM. Nonselective β-blockers may induce development of portal vein thrombosis in cirrhosis. World J Gastroenterol. 2014;20:11463-11466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 9. | Giannelli V, Lattanzi B, Thalheimer U, Merli M. Beta-blockers in liver cirrhosis. Ann Gastroenterol. 2014;27:20-26. [PubMed] |
| 10. | Kim SG, Larson JJ, Lee JS, Therneau TM, Kim WR. Beneficial and harmful effects of nonselective beta blockade on acute kidney injury in liver transplant candidates. Liver Transpl. 2017;23:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, Hagmann M, Blacky A, Ferlitsch A, Sieghart W, Trauner M, Peck-Radosavljevic M, Reiberger T. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 12. | Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, Moreau R, Lebrec D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 13. | Téllez L, Ibáñez-Samaniego L, Pérez Del Villar C, Yotti R, Martínez J, Carrión L, Rodríguez de Santiago E, Rivera M, González-Mansilla A, Pastor Ó, Bermejo J, Bañares R, Albillos A. Non-selective beta-blockers impair global circulatory homeostasis and renal function in cirrhotic patients with refractory ascites. J Hepatol. 2020;73:1404-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Weissenborn K. Hepatic Encephalopathy: Definition, Clinical Grading and Diagnostic Principles. Drugs. 2019;79:5-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 15. | Bajaj JS, Lauridsen M, Tapper EB, Duarte-Rojo A, Rahimi RS, Tandon P, Shawcross DL, Thabut D, Dhiman RK, Romero-Gomez M, Sharma BC, Montagnese S. Important Unresolved Questions in the Management of Hepatic Encephalopathy: An ISHEN Consensus. Am J Gastroenterol. 2020;115:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 16. | Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 579] [Article Influence: 82.7] [Reference Citation Analysis (1)] |
| 17. | Acharya C, Thacker L, Fagan A, White MB, Sterling RK, Stravitz RT, Sanyal AJ, Puri P, Heuman D, Fuchs M, John B, Lee H, Matherly S, Siddiqui MS, Bajaj JS. 422 - Beta Blockers Use in Cirrhosis is Independently Associated with Minimal Hepatic Encephalopathy. Gastroenterology. 2018;154:S-1089. [DOI] [Full Text] |
| 18. | Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 367] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 19. | Dunk AA, Moore J, Symon A, Dickie A, Sinclair TS, Mowat NA, Brunt PW. The effects of propranolol on hepatic encephalopathy in patients with cirrhosis and portal hypertension. Aliment Pharmacol Ther. 1988;2:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Rajini K, Solomon TR, Arumugham A, Ananthavadivelu M, Rangachari B, Kalyanasundaram M, Govindarajan R, Vaishnavi Priyaa C, Kavitha S. Influence of Beta Blockers on Complications of Cirrhosis. J Clin Exp Hepatol. 2016;6:S52-S53. [DOI] [Full Text] |
| 21. | Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev. 2012;CD004544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Rodrigues SG, Mendoza YP, Bosch J. Beta-blockers in cirrhosis: Evidence-based indications and limitations. JHEP Rep. 2020;2:100063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 23. | Krag A, Wiest R, Albillos A, Gluud LL. The window hypothesis: haemodynamic and non-haemodynamic effects of β-blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61:967-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 24. | Kockerling D, Nathwani R, Forlano R, Manousou P, Mullish BH, Dhar A. Current and future pharmacological therapies for managing cirrhosis and its complications. World J Gastroenterol. 2019;25:888-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 25. | Iwakiri Y. The molecules: mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension. J Clin Gastroenterol. 2007;41 Suppl 3:S288-S294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |