Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8076
Peer-review started: March 20, 2022
First decision: May 29, 2022
Revised: June 14, 2022
Accepted: July 11, 2022
Article in press: July 11, 202
Published online: August 16, 2022
Processing time: 133 Days and 10.2 Hours
Gastrointestinal (GI) involvement has been reported in approximately 50% of patients with coronavirus disease 2019 (COVID-19), which is due to the pathogenic role of inflammation and the intestinal function of the angiotensin-converting enzyme 2 and its receptor. Accumulating adult data has pointed out that gut dysbiosis might occur in these patients with a potential impact on the severity of the disease, however the role of gut microbiota in susceptibility and severity of COVID-19 disease in children is still poorly known. During the last decades, the crosstalk between gut and lung has been largely recognized resulting in the concept of “gut-lung axis” as a central player in modulating the deve
Core Tip: Growing evidence has shown that severe acute respiratory syndrome coronavirus 2 exerted a role upon the respiratory system. Due to the release of inflammatory cytokines, it might play a "pleiotropic" effect by modulating also the course of several diseases. In particular, recent adult data supported a bidirectional relationship between gut microbiota changes and coronavirus disease 2019 infection. However, similar evidence in the childhood population is less defined. We aimed to provide a comprehensive pediatric overview in this intriguing field.
- Citation: Valentino MS, Esposito C, Colosimo S, Caprio AM, Puzone S, Guarino S, Marzuillo P, Miraglia del Giudice E, Di Sessa A. Gut microbiota and COVID-19: An intriguing pediatric perspective. World J Clin Cases 2022; 10(23): 8076-8087
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8076.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8076
Since its first description in China, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread worldwide being declared a pandemic by the World Health Organization in March 2020[1]. Accumulating data has showed a different course (including severity, hospitalization and mortality) of the coronavirus disease 2019 (COVID-19) infection across different ages. In fact, a more severe form of the disease with increasing age has been reported, while a milder course of the infection and a relatively lower rate of death has been observed in children and young adults[2-5]. Of note, these findings have been supported by additional studies demonstrating remarkably low rates of vertical virus transmission (as from mother to offspring) and self-limited symptoms in most cases of horizontal transmission (as transmitted among individuals of the same generation)[6,7]. Nevertheless, the pandemic had a significant impact upon the respiratory tract by affecting both cardiovascular and gastrointestinal (GI) systems in children and adults[8,9]. In particular, different clinical GI features related to COVID-19 have been reported in the affected subjects ranging from vomiting, diarrhea and liver injury to gut microbial impairments. Noteworthy, is a potential role for gut dysbiosis induced by COVID-19 in modulating the course of the disease which has been recently suggested[10,11].
Also, lifestyle changes caused by the COVID-19 pandemic are supposed to modify microbiota composition[10].
Recent intriguing findings suggested a potential interaction between SARS-CoV-2 and microbiome[11]. As its role in immune response regulation, some authors focused on modifications of microbiome composition during COVID-19 infection, by supposing potential different patterns in adults and children and a possible link with disease severity[10,11]. On these observations, we aim to summarize the most recent evidence regarding the tangled relationship between gut microbiota and COVID-19 in children.
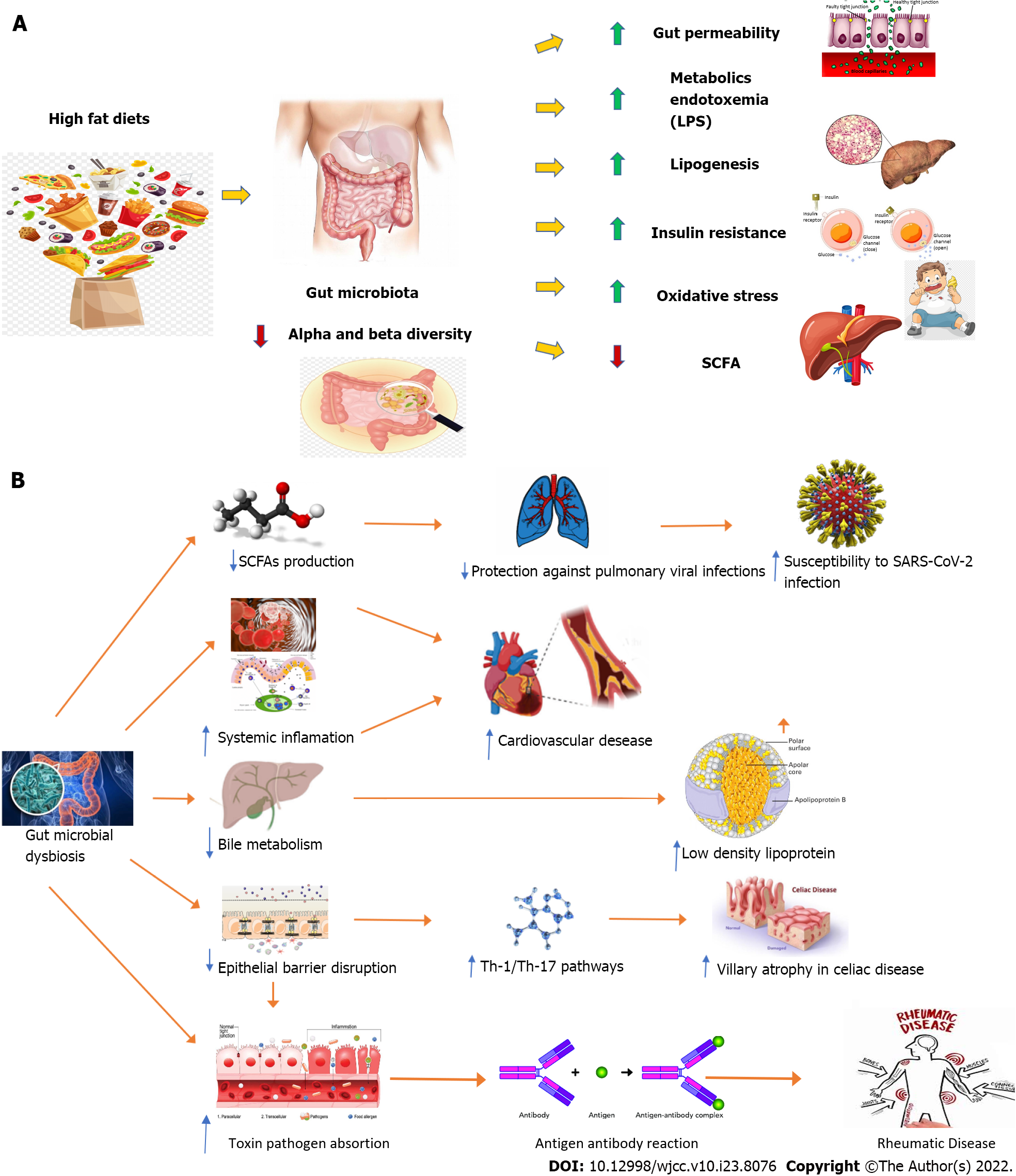
Microbiota refers to all the commensal microorganisms (more than 100 trillion) hosted by the human body, mainly located in the GI tract but also in the respiratory and skin systems. Robust evidence has supported its pivotal role in the development of innate and acquired immune system[12,13] and numerous factors such as delivery mode, nutrition, lifestyle and living environment have been found to influence both its composition and diversity in children[13]. Remarkably, gut microbiota abnormalities have been linked to a wide spectrum of non-communicable diseases[14] including metabolic derangements[15] (e.g., obesity, metabolic syndrome, type 2 diabetes and non-alcoholic fatty liver disease), cardiovascular disease[16], rheumatic disease[17] and celiac disease[18] both in adults and children[15-18] (Figure 1), although no specific microbiome signature has been currently demonstrated[18,19]. Noteworthy, evidence has supported a bidirectional influence of SARS-CoV-2 on the host microbiome through the well-known immune dysregulation driven by the virus[19,20]. As recently reported in adult and pediatric studies[20-22], both the interaction with the host microbiome and immunity dysregulation have been implied in the persistence of symptoms related to COVID-19 infection (also known as long COVID-19 syndrome) as potential pathogenic contributors[20,21].
The concept of “gut-lung axis” refers to the crosstalk between the gut and respiratory tract immune systems classically mediated by microbiota, microbiota metabolites, microbial dysbiosis and common mucosal immunity[23] (Table 1). Indeed, bidirectional interactions between the gut microbiota[24-28] and the respiratory mucosa[29-31] have been supposed to be involved in the response to SARS-CoV-2. Changes in the taxonomic composition and decreased diversity and function of the gut microbiota, known as dysbiosis, might affect the lung immunity status[23,30,31]. Conversely, the respiratory tract has its own microbiota and lung inflammation may lead to intestinal dysbiosis[23].
| Gut microbiome | Lung microbiome |
| Changes in the diversity of the intestinal microbiota have been found: (1) Decrease in the relative abundance of beneficial microbes (such as Agathobacter, Fusicatenibacter, Roseburia and Ruminococcaceae UCG−013); and (2) Oredominance of opportunistic genera (such as Actinomyces, Rothia, Streptococcus) and Veillonella[24] | Changes in the diversity of the lung microbiota have been found: (1) Prevalence of Acinetobacter, Brevundimonas, Burkholderia, Chryseobacterium, Sphingobium species and Enterobacteriaceae members; and (2) Among mycetes, prevalence of Cryptococcus, followed by Aspergillus, Alternaria, Dipodascus, Mortierella, Naganishia, Diutina, Candida, Cladosporium, Issatchenkia, and Wallemia[29] |
| COVID-19 severity: (1) Was positively associated to the relative abundance of Coprobacillus, Clostridium ramosum, and Clostridium hathewayi; and (2) Was inversely associated to the abundance of Faecalibacterium prausnitzii (which favors an anti-inflammatory microenvironment)[25] | The bronchoalveolar lavage fluid of COVID-19 patients characterized by relative abundance of: (1) Lactic acid bacteria such as Lactobacillus fermentum, Lactobacillus reuteri, Lactobacillus delbrueckii, and Lactobacillus salivarius; (2) Some pathogens such as Klebsiella oxytoca, Enterobacter cloacae (positively correlated with COVID-19 severity), and Bacillus cereus; (3) Some nosocomial infection pathogens such as Enterobacter kobei, Enterobacter cloacae, and Ralstonia pickettii; and (4) Several gut bacteria like Faecalibacterium prausnitzii, Enterococcus faecium, and Citrobacter freundii, and commensal bacteria residing in the mouth and respiratory tracts such as Rothia mucilaginosa[30] |
| Viral load in feces of COVID-19 patients inversely correlated to the relative abundance of Bacteroides dorei, B. massiliensis, B. ovatus, and B. thetaiotaomicron (that downregulate the ACE-2 expression in mouse intestine)[25] | Bacterial and fungal DNA burden in BAL specimens of patients with COVID-19-induced ARDS significantly higher than in negative experimental controls, with relative abundance of Staphylococcus, Streptococcus, and Enterococcus spp[31] |
| SARS-CoV-2 infectivity: (1) Was positively related to relative abundance of Collinsella aerofaciens, C. tanakaei, Morganella morganii, and Streptococcus infantis; and (2) Was inversely related to prevalence of Alistipes onderdonkii, Bacteroides stercoris, Lachnospiraceae bacterium and Parabacteroides merdae[26] | |
| Increased abundance of opportunistic fungi (including Candida albicans, C. auris, Aspergillus flavus and A. niger) in feces of COVID-19 patients was found when compared to controls[27] | |
| In patients with MIS-C a predominance of Eubacterium dolichum, Eggerthella lenta, Bacillus thermoamylovorans, Prevotella tannerae, and Bacteroides coprophilus and a decrease of Faecalibacterium prausnitzii were reported. In COVID-19 group an increase of Bifidobacterium adolescents and Dorea formicigenerasus was found[28] |
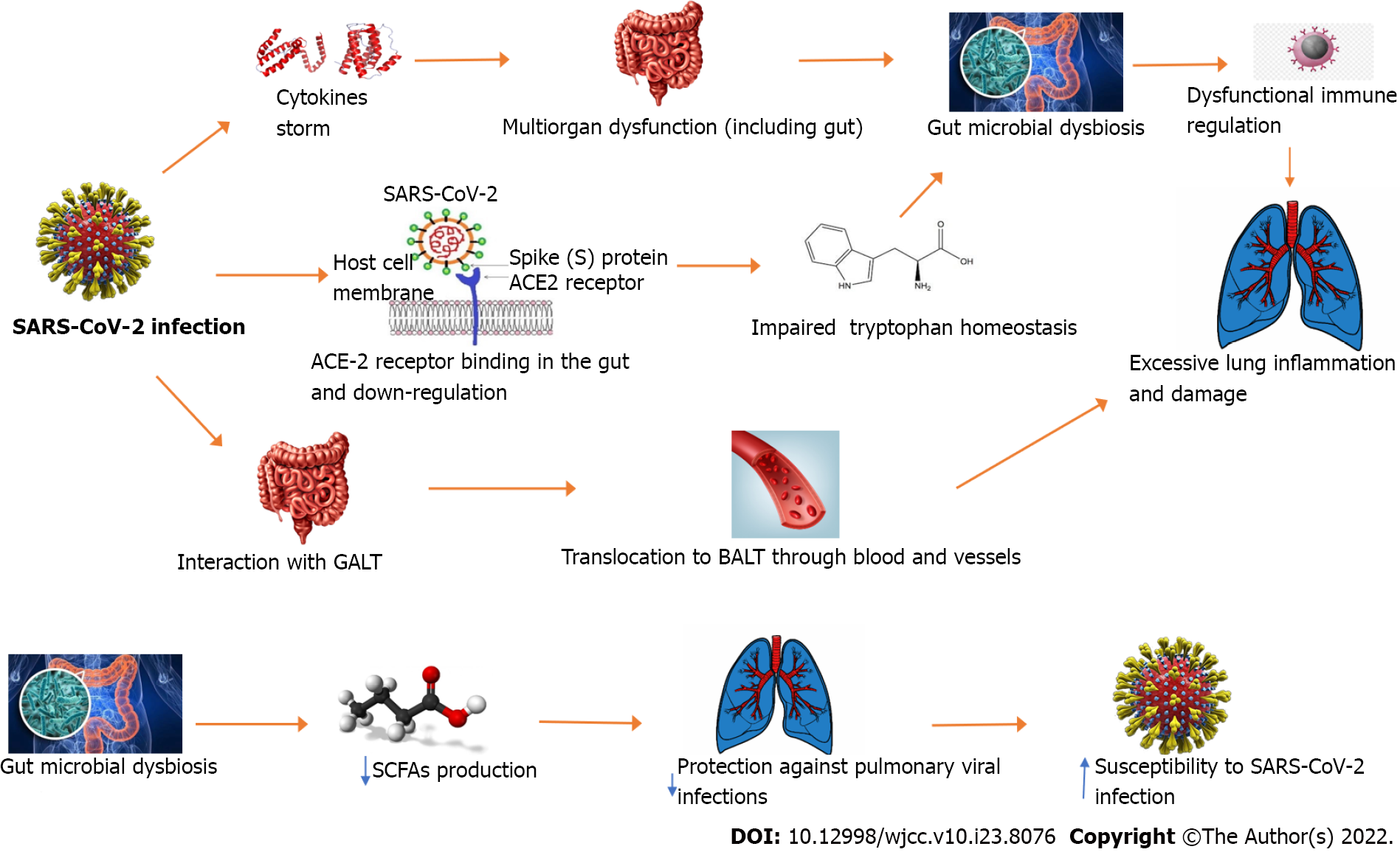
Since the common coexistence of GI and respiratory disorders in COVID-19 infection[9,32] and the potential detection of SARS-CoV-2 RNA in both oral and rectal swabs[33,34], Zhou et al[35] suggested a possible involvement of the axis in COVID-19 pathogenesis (Figure 2). As a consequence, the COVID-19 infection might act as a trigger for cytokine storm leading to multiorgan dysfunction including the gut. Therefore, this process might lead to gut microbiota composition changes with a dysfunctional immune modulation potentially influencing a more aggressive course of the disease[35]. From a pathogenic point of view, the sensitized immune cells switch from gut-associated lymphoid tissue to bronchus-associated lymphoid tissue and may enhance the lung immune response leading to a considerable increase of inflammation and subsequent organ injury. In addition, the potential role of the angiotensin-converting enzyme 2 (ACE-2) receptor (expressed both in respiratory and GI tracts) as a main route for SARS-CoV-2 invasion, its involvement in gut tryptophan homeostasis and its downregulation virus-mediation might contribute to gut dysbiosis[35].
On the other hand, this might result in a decreased production of some metabolites such as short-chain fatty acids (SCFAs) including butyrate, propionate and acetate[30,33]. In murine studies[36], the depletion of these metabolites has been related to an increased susceptibility to pulmonary viral infections[36].
Although the respiratory system is the main target of COVID-19 infection, the GI tract has been found to be largely involved in the disease[37]. Indeed, it has been demonstrated that SARS-CoV-2 can infect and replicate in human small intestine enterocytes[38] and virus RNA can be detected in fecal samples[33,34]. Given the well-known role of the GI tract as the largest human immunological organ and of its resident microbiota in modulating host immune responses[39], changes in fecal microbiomes of hospitalized patients with SARS-CoV-2 infection and their potential link with severity and fecal shedding of virus were explored[25]. Authors performed metagenomic sequencing analyses of fecal samples from 15 patients with COVID-19 from February through March 2020 and compared microbiome data with those from 6 subjects with community- acquired pneumonia and 15 healthy individuals, by assessing gut microbiome profiles according to disease severity and changes in fecal shedding of SARS-CoV-2[25]. Patients with COVID-19 had significant alterations in fecal microbiomes than the controls. The Covid-19 patients’ fecal samples were characterized by an overall enrichment of opportunistic pathogens and a depletion of beneficial commensals, even after SARS-CoV-2 clearance (determined from throat swabs) and resolution of respiratory symptoms. The baseline abundance of Coprobacillus, Clostridium ramosum and Clostridium hathewayi correlated with COVID-19 severity. An inverse correlation between Faecalibacterium prausnitzii (an anti-inflammatory bacterium) and disease severity was reported[25]. During hospitalization, different Bacteroides species (including B. dorei, B. thetaiotaomicron, B. massiliensis, and B. ovatus) determining downregulation of ACE-2 and ACE-2 receptor expression in murine gut were found to be associated with SARS-CoV-2 load in fecal samples of affected patients[25].
Similarly, Yeoh et al[37] obtained blood, stool and patient records from 100 patients with laboratory-confirmed SARS-CoV-2 infection. Serial stool samples were collected from 27 of the 100 patients up to 30 d after SARS-CoV-2 clearance. Gut microbiome composition was characterized by shotgun sequencing total DNA extracted from stools. Moreover, inflammatory cytokines and blood marker levels were assessed. Gut microbiome composition was significantly altered in patients with COVID-19 compared to non-COVID-19 individuals. In particular, several gut commensals with a well-known immunomodulatory potential such as Faecalibacterium prausnitzii, Eubacterium rectale and Bifidobacteria were underrepresented in patients and remained low in samples collected up to 30 d after disease resolution. Also, the altered composition in COVID-19 hospitalized patients was correlated with plasma concentrations of several cytokines, chemokines and inflammation markers suggesting that the gut microbiota might play a role in modulating host immune response and potentially influence disease course. Specifically, the depletion of several bacterial species in the COVID-19 cohort was linked to increased concentrations of tumor necrosis factor-α, CXCL10, CCL2 and interleukin-10 indicating that these depleted taxa may have a role in preventing overaggressive inflammation[37].
Unlike adults, pediatric evidence in this field is still limited as the common asymptomatic course of the disease at this stage (Table 2). Nashed et al[40] performed a case-control study by comparing microbiomes of 595 affected children aged 0-24 mo. Findings revealed that in affected patients, a decreased abundance of Bifidobacterium bifidum and Akkermansia muciniphila, both commonly exerting a protective effect against inflammation[41,42]. Of note, reduced levels of anti-inflammatory taxa were also detectable in asymptomatic infected infants, as described in symptomatic adults[37].
| Ref. | Study design and methods | Population (n) | Main findings |
| Romano-Keeler et al[44] | Observational cohort study | Twenty-one COVID-19 positive mothers delivering between March and August 2020 with a mean age of 26 (17-42) yr | Delayed cord clamping and skin-to-skin avoided; infants admitted to the NICU with maternal breast milk restricted. Discharge arranged with COVID-19 negative family members. All 21 infants COVID-19 negative at 24 and 48 h. Changes in perinatal care might negatively affect gut microbiome pattern early in life |
| Nashed et al[40] | Case-control study | 595 children aged 0-24 mo | Significantly different abundant species between SARS-CoV-2 positive infants and controls were found. A decreased abundance of Bifidobacterium bifidum and Akkermansia muciniphila in positive samples (both linked to protection against inflammation) was found |
| Xu et al[43] | Case-control study | (1) 9 children diagnosed with COVID-19 aged 7-139 mo; and (2) 14 age-matched healthy control children | Altered microbiome in COVID-19 children, with increased abundance of opportunistic pathogenic and environmental bacteria such as Pseudomonas, Herbaspirillum, and Burkholderia both in the upper respiratory tract and the gut was found. Dysbiosis up to 25-28 d in different subjects was reported |
In another case-control study[43], nine COVID-19 children aged between 7 and 139 mo were studied for 25-28 d after symptom onset and their microbiome composition was compared to that of 14 age-matched healthy control children. Microbiome patterns were significantly different between the two tested groups in the various human body tracts. Particularly, the microbiome composition in throat and nasal swabs had significantly lower richness in COVID-19 children than healthy controls. At the phylum level, Bacteroidetes and Firmicutes were predominant in the gut of COVID-19 patients, while Proteobacteria were enriched in the gut of healthy controls. On the contrary, higher Bacteroidetes and Firmicutes concentrations were found in in the upper respiratory tract of healthy controls, while in the same site of COVID-19 patients, Proteobacteria levels were predominant. Compared to COVID-19 patients, both gut and upper respiratory tracts of healthy controls were found to be mainly colonized by resident commensals, while some opportunistic pathogenic and environmental bacteria such as Pseudomonas, Herbaspirillum, and Burkholderia were significantly predominant in the gut and the upper respiratory tract of the affected subjects. Notably, data supported the persistence (up to 25-52 d after the onset of symptoms) of dysbiosis in COVID-19 children mainly in the upper respiratory tract. However, dynamic microbiome changes were divergent between the upper respiratory tract and the gut, by showing a nearly-full gut microbiome restoration at 50-55 d after the onset of symptoms. Based on these findings, it could be supposed that the “gut-lung axis” is still not established during childhood[28,43].
To sum up, current evidence suggests that COVID-19 infection might affect both gut and upper respiratory tract microbiomes in children resulting in a persistent dysbiosis as a potential risk factor for short and long-term adverse health outcomes.
The impact of COVID-19 infection has been explored from the earliest ages[1,6] (Table 3). In a single-center observational cohort study, Romano-Keeler et al[44] examined 21 deliveries of COVID-19 positive mothers between March and August 2020. A higher rate of Caesarean section emerged in the study population compared to institutional (29% in 2019) and national rates (31.9% in 2018)[44]. To prevent the virus transmission, mother-infant contact was minimized, delayed cord clamping and skin-to-skin were avoided and infants were admitted to the Neonatal Intensive Care Unit (NICU). No COVID-19 infection was detected in all the enrolled infants at 24 and 48 h and their average hospitalization time was 9 d. As these measures may decrease virus transmission, a potential impact on the neonatal microbiome has been described[44]. Compared to the colonization of lactobacillus after a vaginal delivery, the C-section delivery represents a well-known risk factor for early life intestinal dysbiosis due to colonization of the newborn with potentially skin or hospital pathogenic organisms[6,45]. Indeed, there are several evidences linking C-section delivery to an increased incidence of atopic disorders[46-48] and autoi
| Ref. | Study design and methods | Population (n) | Main findings |
| Romano-Keeler et al[44] | Observational cohort study | Twenty-one COVID-19 positive mothers delivering between March and August 2020 with a mean age of 26 (17-42) yr | Delayed cord clamping and skin-to-skin avoided; infants admitted to the NICU with maternal breast milk restricted. Discharge arranged with COVID-19 negative family members. All infants COVID-19 negative at 24 and 48 h. Changes in perinatal care might negatively affect gut microbiome pattern early in life |
| Salvatori et al[57] | Case report | Two maternal–infant dyads with a positive nasopharyngeal swab for SARS-CoV-2 both in the mother and in the child | SARS-CoV-2 was not detected by RT-PCR in breast milk samples of both mothers |
| Gómez-Torres et al[68] | Prospective case-control study | (1) 37 women with full-term pregnancies and mild SARS-CoV-2 infection; and (2) 63 healthy controls | No difference nor in Alpha-neither in Beta-diversity between breast milk samples collected from the two groups; Staphylococcus and Streptococcus were the most abundant genera and the only ones detected in all the samples. Disease state (symptomatic or asymptomatic infection) did not affect the metataxonomic profile |
Furthermore, the infant feeding pattern has been found to play a crucial role in the microbiota composition in the 1st year of life[10,50]. Of note, breastfeeding exerts an important influence on the gut microbiome compared to formula feeding. Jost et al[51] examined mother-infant fecal samples and maternal breast milk collected from seven mothers-newborn dyads. Authors identified a shared gut microbiota composition including obligate anaerobic genera such as Bifidobacterium, Bacteroides, Parabacteroides, and members of the Clostridia (Blautia, Clostridium, Collinsella and Veillonella). Notably, a viable strain of Bifidobacterium breve was shown to be shared among all three ecosystems within one dyad. Furthermore, pyrosequencing revealed that several butyrate-producing members of Clostridia (e.g., Coprococcus, Faecalibacterium, Roseburia, and Subdoligranulum) were shared between maternal feces and breast milk. Of note, this latter as a feeding mode has been previously linked to a reduced risk of type 1[52] and type 2 diabetes development[53]. In addition, further evidence pointed out the association between breastfeeding and a lower risk for multiple sclerosis in two case-control studies[54,55]. Since there is no evidence about the presence of SARS-CoV-2 in the breast milk of infected mothers and its transmission through breastfeeding[56,57], this feeding pattern has been recommended even for suspicious or infected new mothers[10,58].
Owing to the pandemic, the higher attention to hygiene resulting in an increased use of detergents as a further preventive measure has been experienced also at a very early age.
Gerasimidis et al[59] investigated the effect of food additives, artificial sweeteners and domestic hygiene products on the gut microbiome and fiber fermentation capacity. The use of dishwashing detergent was associated with an altered microbiota pattern including a decreased concentration of Firmicutes. As previously reported, metabolites of Firmicutes (e.g., Faecalibacterium and Subdoligranulum) as butyric acid-producing bacteria and other SCFAs have been linked to a reduced incidence of atopic disorders[60], multiple sclerosis[61] and type 1 diabetes[62,63].
Also, COVID-19 related social habits such as sedentariness and increased domestic contacts with pets should be considered. Geography and ethnicity are well-known critical determinants of microbial composition, including differences in the incidence of obesity, gastric cancer and chronic liver diseases[6,64-66], while it has been observed that living with pets increases the richness and diversity of infant gut microbiota. Azad et al[67] found that infants living with pets have significant over-representation of Clostridiaceae, Veillonella, Peptostreptococcaceae and Coprococcus, while Bifidobacteriaceae are under-represented. Moreover, interaction with pets within the 1st year of life has been associated with a decreased prevalence of allergic diseases[10,68].
Since numerous studies have shown the essential role of a healthy microbiota, the changes and the subsequent dysbiosis caused by the COVID-19 pandemic might increase the incidence of many disorders later in life such as allergic, metabolic and autoimmune diseases[6,10]. However, the exact impact of this condition on newborns cannot be currently established. Given the paucity of data in this field, more epidemiological studies are needed to better clarify this relationship and its implications.
Antibiotics use in COVID-19 infection represents a relevant issue[69]. In particular, azithromycin (a well-known antibiotic with anti-inflammatory and immunoregulatory effects) has been early administered in routine COVID-19 care, although there was no high-quality evidence[70,71].
A recent review reported conflicting results on azithromycin in COVID-19 infection and its widespread use outside of clinical trials was not endorsed[71]. Authors also recommended a careful monitoring of drug–drug interactions and subsequent cardiac adverse events (i.e., with hydroxychloroquine)[71].
Regarding the potential side effects of azithromycin, an interesting randomized clinical trial[72] evaluated the impact of azithromycin administration on the prevalence of GI carriage of macrolide-resistant bacteria in communities within the MORDOR Malawi study[73]. Significant changes in the antimicrobial resistance profile and gut microbiome after four biannual rounds of azithromycin with an increased carriage of macrolide resistance was demonstrated[72]. After treatment, the putative human enteropathogen E. albertii and several opportunistic Acinetobacter pathogens were found to be significantly increased. Taken together, these findings highlighted not only the need to consider and set the number of treatments and administration schedules but also with regard to their costs in antimicrobial resistance[72]. Given also the lack of consensus on clinical benefits of azithromycin in COVID-19 infection[74], more focused scientific efforts are required.
During the past years, several studies have examined the impact of the microbiota on innate and adaptive immunity[1], by demonstrating over time a dynamic equilibrium between microbes and the host[75]. In addition to defective production of immunoglobulin A, dysbiosis has been associated with an abnormal development of lymphoid tissues and intestinal T cells[75-79].
Nevertheless, microbiota also plays a role in the relationships between host and viral infections[75-79]. Indeed, microbiota composition has been found to influence vaccine responses both in adults and children[80]. Pediatric data found a positive association between Actinobacteria phylum and humoral and cellular responses both to oral and parenteral vaccines[81], while an inverse correlation of the phylum Proteobacteria with the responses to the same vaccines and of Bacteroidetes with humoral responses to oral vaccines have been reported[81,82]. Moreover, both in children and adults a prevalence of the phylum Firmicutes has been associated to higher humoral and cellular responses to oral vaccines[1,82,83].
Regarding SARS-CoV-2 vaccines, in a prospective observational study on adults receiving either the inactivated vaccine (CoronaVac; Sinovac) or the mRNA vaccine (BNT162b2; BioNTech; Comirnaty), Ng et al[84] found that Bifidobacterium was found to be persistently higher in subjects with high neutralizing antibodies to CoronaVac vaccine, while neutralizing antibodies in BNT162b2 vaccines showed a positive correlation with the total abundance of bacteria with flagella and fimbriae including Roseburia faecis. In individuals with fewer adverse events following either of the vaccines, a higher prevalence of Prevotella copri and two Megamonas species were detected indicating that these bacteria may play an anti-inflammatory role in host immune response[67].
Given the potential influence of microbiota composition on vaccine responses, especially in children, and its changes in different age groups[58], a similar role in viral infection through the modulation of immune function (both innate and adaptive immune responses) and composition could be supposed.
In the context of COVID-19 infection, there are no pediatric studies evaluating this tangled relationship. Further studies are needed to clarify the potential influence of the microbiota age-related differences on the disease severity and COVID-19 vaccine response in the pediatric population[1].
The occurrence of gut dysbiosis as a disruptor of the gut-lung axis homeostasis and its potential correlation with disease severity has been largely described in COVID-19 adult patients while there is a paucity of similar data in childhood. As observed in adults, changes in gut microbiota composition seem to negatively affect the course of the infection in very young children. Given also the higher risk of autoimmune and autoinflammatory diseases development in children with COVID-19, a deeper dissection of the role of gut microbiota might provide insightful therapeutic perspectives in this field.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Dhar D, India; Gassler N, Germany; Poddighe D, Kazakhstan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Buonsenso D, Sali M, Pata D, De Rose C, Sanguinetti M, Valentini P, Delogu G. Children and COVID-19: Microbiological and immunological insights. Pediatr Pulmonol. 2020;55:2547-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. 2020;39:355-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 714] [Cited by in RCA: 695] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 3. | Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr. 2020;174:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 777] [Article Influence: 155.4] [Reference Citation Analysis (0)] |
| 4. | Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1701] [Cited by in RCA: 1489] [Article Influence: 297.8] [Reference Citation Analysis (1)] |
| 5. | Zimmermann P, Curtis N. Why is COVID-19 Less severe in children? Arch Dis Child. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 295] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 6. | Romano-Keeler J, Zhang J, Sun J. COVID-19 and the neonatal microbiome: will the pandemic cost infants their microbes? Gut Microbes. 2021;13:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Lamouroux A, Attie-Bitach T, Martinovic J, Leruez-Ville M, Ville Y. Evidence for and against vertical transmission for severe acute respiratory syndrome coronavirus 2. Am J Obstet Gynecol. 2020;223:91.e1-91.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 8. | Liu F, Liu F, Wang L. COVID-19 and cardiovascular diseases. J Mol Cell Biol. 2021;13:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Di Sessa A, Lanzaro F, Zarrilli S, Picone V, Guarino S, Miraglia Del Giudice E, Marzuillo P. COVID-19 and pediatric fatty liver disease: Is there interplay? World J Gastroenterol. 2021;27:3064-3072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Ebrahimi S, Khatami S, Mesdaghi M. The Effect of COVID-19 Pandemic on the Infants' Microbiota and the Probability of Development of Allergic and Autoimmune Diseases. Int Arch Allergy Immunol. 2022;183:435-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Venegas-Borsellino C, Sankararaman S, Roche K, Burns J, Landis RM. Impact of COVID-19 on the Intestinal Microbiome. Curr Nutr Rep. 2021;10:300-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4762] [Cited by in RCA: 5460] [Article Influence: 420.0] [Reference Citation Analysis (0)] |
| 13. | Fieten KB, Totté JEE, Levin E, Reyman M, Meijer Y, Knulst A, Schuren F, Pasmans SGMA. Fecal Microbiome and Food Allergy in Pediatric Atopic Dermatitis: A Cross-Sectional Pilot Study. Int Arch Allergy Immunol. 2018;175:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Fromentin S, Forslund SK, Chechi K, Aron-Wisnewsky J, Chakaroun R, Nielsen T, Tremaroli V, Ji B, Prifti E, Myridakis A, Chilloux J, Andrikopoulos P, Fan Y, Olanipekun MT, Alves R, Adiouch S, Bar N, Talmor-Barkan Y, Belda E, Caesar R, Coelho LP, Falony G, Fellahi S, Galan P, Galleron N, Helft G, Hoyles L, Isnard R, Le Chatelier E, Julienne H, Olsson L, Pedersen HK, Pons N, Quinquis B, Rouault C, Roume H, Salem JE, Schmidt TSB, Vieira-Silva S, Li P, Zimmermann-Kogadeeva M, Lewinter C, Søndertoft NB, Hansen TH, Gauguier D, Gøtze JP, Køber L, Kornowski R, Vestergaard H, Hansen T, Zucker JD, Hercberg S, Letunic I, Bäckhed F, Oppert JM, Nielsen J, Raes J, Bork P, Stumvoll M, Segal E, Clément K, Dumas ME, Ehrlich SD, Pedersen O. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat Med. 2022;28:303-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 145] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 15. | Yuan X, Chen R, McCormick KL, Zhang Y, Lin X, Yang X. The role of the gut microbiota on the metabolic status of obese children. Microb Cell Fact. 2021;20:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Masenga SK, Hamooya B, Hangoma J, Hayumbu V, Ertuglu LA, Ishimwe J, Rahman S, Saleem M, Laffer CL, Elijovich F, Kirabo A. Recent advances in modulation of cardiovascular diseases by the gut microbiota. J Hum Hypertens. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 17. | Bao Y, Dong C, Ji J, Gu Z. Dysregulation of gut microbiome is linked to disease activity of rheumatic diseases. Clin Rheumatol. 2020;39:2523-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Abdukhakimova D, Dossybayeva K, Poddighe D. Fecal and Duodenal Microbiota in Pediatric Celiac Disease. Front Pediatr. 2021;9:652208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 19. | Mundula T, Russo E, Curini L, Giudici F, Piccioni A, Franceschi F, Amedei A. Chronic systemic low-grade inflammation and modern lifestyle: the dark role of gut microbiota on related diseases with a focus on pandemic COVID-19. Curr Med Chem. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front Microbiol. 2021;12:698169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 587] [Cited by in RCA: 536] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 21. | Izquierdo-Pujol J, Moron-Lopez S, Dalmau J, Gonzalez-Aumatell A, Carreras-Abad C, Mendez M, Rodrigo C, Martinez-Picado J. Post COVID-19 Condition in Children and Adolescents: An Emerging Problem. Front Pediatr. 2022;10:894204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 22. | Trapani G, Verlato G, Bertino E, Maiocco G, Vesentini R, Spadavecchia A, Dessì A, Fanos V. Long COVID-19 in children: an Italian cohort study. Ital J Pediatr. 2022;48:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | de Oliveira GLV, Oliveira CNS, Pinzan CF, de Salis LVV, Cardoso CRB. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front Immunol. 2021;12:635471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 24. | Gu S, Chen Y, Wu Z, Gao H, Lv L, Guo F, Zhang X, Luo R, Huang C, Lu H, Zheng B, Zhang J, Yan R, Zhang H, Jiang H, Xu Q, Guo J, Gong Y, Tang L, Li L. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin Infect Dis. 2020;71:2669-2678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 569] [Article Influence: 142.3] [Reference Citation Analysis (0)] |
| 25. | Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944-955.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 739] [Cited by in RCA: 1063] [Article Influence: 212.6] [Reference Citation Analysis (0)] |
| 26. | Zuo T, Liu Q, Zhang F, Lui GC, Tso EY, Yeoh YK, Chen Z, Boon SS, Chan FK, Chan PK, Ng SC. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 262] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 27. | Zuo T, Zhan H, Zhang F, Liu Q, Tso EYK, Lui GCY, Chen N, Li A, Lu W, Chan FKL, Chan PKS, Ng SC. Alterations in Fecal Fungal Microbiome of Patients With COVID-19 During Time of Hospitalization until Discharge. Gastroenterology. 2020;159:1302-1310.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 250] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 28. | Suskun C, Kilic O, Yilmaz Ciftdogan D, Guven S, Karbuz A, Ozkaya Parlakay A, Kara Y, Kacmaz E, Sahin A, Boga A, Kizmaz Isancli D, Gulhan B, Kanik-Yuksek S, Kiral E, Bozan G, Arslanoglu MO, Kizil MC, Dinleyici M, Us T, Varis A, Kaya M, Vandenplas Y, Dinleyici EC. Intestinal microbiota composition of children with infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and multisystem inflammatory syndrome (MIS-C). Eur J Pediatr. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 29. | Fan J, Li X, Gao Y, Zhou J, Wang S, Huang B, Wu J, Cao Q, Chen Y, Wang Z, Luo D, Zhou T, Li R, Shang Y, Nie X. The lung tissue microbiota features of 20 deceased patients with COVID-19. J Infect. 2020;81:e64-e67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 30. | Han Y, Jia Z, Shi J, Wang W, He K. The active lung microbiota landscape of COVID-19 patients through the metatranscriptome data analysis. Bioimpacts. 2022;12:139-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Kullberg RFJ, de Brabander J, Boers LS, Biemond JJ, Nossent EJ, Heunks LMA, Vlaar APJ, Bonta PI, van der Poll T, Duitman J, Bos LDJ, Wiersinga WJ; ArtDECO consortium and the Amsterdam UMC COVID-19 Biobank Study Group. Lung Microbiota of Critically Ill COVID-19 Patients are Associated with Non-Resolving Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Sansotta N, Norsa L, D'Antiga L. Gastrointestinal coronavirus disease 2019 manifestations in childhood. Curr Opin Clin Nutr Metab Care. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 992] [Cited by in RCA: 1052] [Article Influence: 210.4] [Reference Citation Analysis (0)] |
| 34. | Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4682] [Cited by in RCA: 4809] [Article Influence: 961.8] [Reference Citation Analysis (0)] |
| 35. | Zhou D, Wang Q, Liu H. Coronavirus disease 2019 and the gut-lung axis. Int J Infect Dis. 2021;113:300-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354-5359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 1137] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 37. | Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS, Chow KM, Ng SSS, Li TC, Ng RW, Yip TC, Wong GL, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 995] [Cited by in RCA: 863] [Article Influence: 215.8] [Reference Citation Analysis (0)] |
| 38. | Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1245] [Cited by in RCA: 1310] [Article Influence: 262.0] [Reference Citation Analysis (0)] |
| 39. | Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Ter Horst R, Jansen T, Jacobs L, Bonder MJ, Kurilshikov A, Fu J, Joosten LAB, Zhernakova A, Huttenhower C, Wijmenga C, Netea MG, Xavier RJ. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell. 2016;167:1125-1136.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 722] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 40. | Nashed L, Mani J, Hazrati S, Stern DB, Subramanian P, Mattei L, Bittinger K, Hu W, Levy S, Maxwell GL, Hourigan SK. Gut microbiota changes are detected in asymptomatic very young children with SARS-CoV-2 infection. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Al-Sadi R, Dharmaprakash V, Nighot P, Guo S, Nighot M, Do T, Ma TY. Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 42. | Earley H, Lennon G, Balfe Á, Coffey JC, Winter DC, O'Connell PR. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci Rep. 2019;9:15683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 43. | Xu R, Liu P, Zhang T, Wu Q, Zeng M, Ma Y, Jin X, Xu J, Zhang Z, Zhang C. Progressive deterioration of the upper respiratory tract and the gut microbiomes in children during the early infection stages of COVID-19. J Genet Genomics. 2021;48:803-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 44. | Romano-Keeler J, Fiszbein D, Zhang J, Horowitz J, Hayani K, Buhimschi I, Lopez C, Kadhem Z, Berman J, Rasamimari P, Raghavan A, Pillers DM, Sun J. Center-Based Experiences Implementing Strategies to Reduce Risk of Horizontal Transmission of SARS-Cov-2: Potential for Compromise of Neonatal Microbiome Assemblage. medRxiv. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971-11975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2922] [Cited by in RCA: 3166] [Article Influence: 211.1] [Reference Citation Analysis (0)] |
| 46. | Renz-Polster H, David MR, Buist AS, Vollmer WM, O'Connor EA, Frazier EA, Wall MA. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005;35:1466-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 47. | Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38:629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 458] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 48. | van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, Reijmerink NE, Dompeling E, van den Brandt PA, Ferreira I, Mommers M, Thijs C. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948-55.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 49. | Sevelsted A, Stokholm J, Bønnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135:e92-e98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 332] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 50. | Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1477] [Cited by in RCA: 1976] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 51. | Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol. 2014;16:2891-2904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 391] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 52. | Cardwell CR, Stene LC, Ludvigsson J, Rosenbauer J, Cinek O, Svensson J, Perez-Bravo F, Memon A, Gimeno SG, Wadsworth EJ, Strotmeyer ES, Goldacre MJ, Radon K, Chuang LM, Parslow RC, Chetwynd A, Karavanaki K, Brigis G, Pozzilli P, Urbonaite B, Schober E, Devoti G, Sipetic S, Joner G, Ionescu-Tirgoviste C, de Beaufort CE, Harrild K, Benson V, Savilahti E, Ponsonby AL, Salem M, Rabiei S, Patterson CC. Breast-feeding and childhood-onset type 1 diabetes: a pooled analysis of individual participant data from 43 observational studies. Diabetes Care. 2012;35:2215-2225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 53. | Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? Am J Clin Nutr. 2006;84:1043-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 288] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 54. | Conradi S, Malzahn U, Paul F, Quill S, Harms L, Then Bergh F, Ditzenbach A, Georgi T, Heuschmann P, Rosche B. Breastfeeding is associated with lower risk for multiple sclerosis. Mult Scler. 2013;19:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 55. | Ragnedda G, Leoni S, Parpinel M, Casetta I, Riise T, Myhr KM, Wolfson C, Pugliatti M. Reduced duration of breastfeeding is associated with a higher risk of multiple sclerosis in both Italian and Norwegian adult males: the EnvIMS study. J Neurol. 2015;262:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | . Breastfeeding and COVID-19. Bull Acad Natl Med. 2020;204:e140-e141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | Salvatori G, De Rose DU, Concato C, Alario D, Olivini N, Dotta A, Campana A. Managing COVID-19-Positive Maternal-Infant Dyads: An Italian Experience. Breastfeed Med. 2020;15:347-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 58. | . WHO Frequently Asked Questions : Breastfeeding and COVID-19 For health care workers. J Hum Lact. 2020;36:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Gerasimidis K, Bryden K, Chen X, Papachristou E, Verney A, Roig M, Hansen R, Nichols B, Papadopoulou R, Parrett A. The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity. Eur J Nutr. 2020;59:3213-3230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 60. | Roduit C, Frei R, Ferstl R, Loeliger S, Westermann P, Rhyner C, Schiavi E, Barcik W, Rodriguez-Perez N, Wawrzyniak M, Chassard C, Lacroix C, Schmausser-Hechfellner E, Depner M, von Mutius E, Braun-Fahrländer C, Karvonen AM, Kirjavainen PV, Pekkanen J, Dalphin JC, Riedler J, Akdis C, Lauener R, O'Mahony L; PASTURE/EFRAIM study group. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019;74:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 61. | Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim SW, Morita H, Hattori M, Yamamura T. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One. 2015;10:e0137429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 568] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 62. | Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6:e25792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 590] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 63. | Endesfelder D, Engel M, Davis-Richardson AG, Ardissone AN, Achenbach P, Hummel S, Winkler C, Atkinson M, Schatz D, Triplett E, Ziegler AG, zu Castell W. Towards a functional hypothesis relating anti-islet cell autoimmunity to the dietary impact on microbial communities and butyrate production. Microbiome. 2016;4:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 64. | Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018;16:e2006842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 65. | Prideaux L, Kang S, Wagner J, Buckley M, Mahar JE, De Cruz P, Wen Z, Chen L, Xia B, van Langenberg DR, Lockett T, Ng SC, Sung JJ, Desmond P, McSweeney C, Morrison M, Kirkwood CD, Kamm MA. Impact of ethnicity, geography, and disease on the microbiota in health and inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2906-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 66. | Sheh A, Fox JG. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes. 2013;4:505-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 67. | Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Sears MR, Becker AB, Scott JA, Kozyrskyj AL. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 68. | Gómez-Torres N, Sánchez-García L, Castro I, Arroyo R, Cabañas F, González-Sánchez R, López-Azorín M, Moral-Pumarega MT, Escuder-Vieco D, Cabañes-Alonso E, Rodríguez JM, Alba C, Pellicer A. Metataxonomic Analysis of Milk Samples From SARS-CoV-2-Positive and SARS-CoV-2-Negative Women. Front Nutr. 2022;9:853576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Tamanai-Shacoori Z, Le Gall-David S, Moussouni F, Sweidan A, Polard E, Bousarghin L, Jolivet-Gougeon A. SARS-CoV-2 and Prevotella spp.: friend or foe? J Med Microbiol. 2022;71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012;68:479-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 71. | Gyselinck I, Janssens W, Verhamme P, Vos R. Rationale for azithromycin in COVID-19: an overview of existing evidence. BMJ Open Respir Res. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 72. | Pickering H, Hart JD, Burr S, Stabler R, Maleta K, Kalua K, Bailey RL, Holland MJ. Impact of azithromycin mass drug administration on the antibiotic-resistant gut microbiome in children: a randomized, controlled trial. Gut Pathog. 2022;14:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Keenan JD, Bailey RL, West SK, Arzika AM, Hart J, Weaver J, Kalua K, Mrango Z, Ray KJ, Cook C, Lebas E, O'Brien KS, Emerson PM, Porco TC, Lietman TM; MORDOR Study Group. Azithromycin to Reduce Childhood Mortality in Sub-Saharan Africa. N Engl J Med. 2018;378:1583-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 270] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 74. | Poddighe D, Aljofan M. Clinical evidences on the antiviral properties of macrolide antibiotics in the COVID-19 era and beyond. Antivir Chem Chemother. 2020;28:2040206620961712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 75. | Tomkovich S, Jobin C. Microbiota and host immune responses: a love-hate relationship. Immunology. 2016;147:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 76. | Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA, Kasper DL. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 902] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 77. | Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 805] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 78. | Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3080] [Article Influence: 236.9] [Reference Citation Analysis (0)] |
| 79. | Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3232] [Article Influence: 248.6] [Reference Citation Analysis (0)] |
| 80. | Zimmermann P, Curtis N. The influence of the intestinal microbiome on vaccine responses. Vaccine. 2018;36:4433-4439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 81. | Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, Stephensen CB. Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134:e362-e372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 82. | Harris VC, Armah G, Fuentes S, Korpela KE, Parashar U, Victor JC, Tate J, de Weerth C, Giaquinto C, Wiersinga WJ, Lewis KD, de Vos WM. Significant Correlation Between the Infant Gut Microbiome and Rotavirus Vaccine Response in Rural Ghana. J Infect Dis. 2017;215:34-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 83. | Eloe-Fadrosh EA, McArthur MA, Seekatz AM, Drabek EF, Rasko DA, Sztein MB, Fraser CM. Impact of oral typhoid vaccination on the human gut microbiota and correlations with s. Typhi-specific immunological responses. PLoS One. 2013;8:e62026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 84. | Ng SC, Peng Y, Zhang L, Mok CK, Zhao S, Li A, Ching JY, Liu Y, Yan S, Chan DLS, Zhu J, Chen C, Fung AC, Wong KK, Hui DS, Chan FK, Tun HM. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut. 2022;71:1106-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |