Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7609
Peer-review started: March 17, 2022
First decision: May 9, 2022
Revised: May 18, 2022
Accepted: June 15, 2022
Article in press: June 15, 2022
Published online: July 26, 2022
Processing time: 115 Days and 21.5 Hours
Gastrografin swallow, methylthioninium chloride test, and computed tomography (CT) are the main methods for postoperative anastomotic fistula detection. Correct selection and application of examinations and therapies are significant for the early diagnosis and treatment of small anastomotic fistulas after radical gastrectomy, which are conducive to postoperative recovery.
A 44-year-old woman underwent radical total gastrectomy for laparoscopic gastric cancer. The patient developed a fever after surgery. The methylthioninium chloride test and early CT suggested no anastomotic fistula, but gastrografin swallow and late CT showed the opposite result. The fistula was successfully closed using an endoscopic clip. The methylthioninium chloride test, gastrografin, and CT performed on different postoperative dates for small esophagojejunostomy fistulas are different. The size of the anastomotic fistula is an important factor for the success of endoscopic treatment.
The advantages and limitations of the diagnosis of different examinations of small esophagojejunostomy fistulas are noteworthy. The size of the leakage of the anastomosis is an important basis for selecting the repair method.
Core Tip: Gastrointestinal anastomotic fistula is one of the major complications after gastrointestinal anastomosis. The early diagnosis of small anastomotic fistulas and the choice of treatment are particularly important. We reported a case of a gastrointestinal anastomotic fistula that was not easily diagnosed at an early stage and discussed the advantages and limitations of the current main methods of examination in the context of medical imaging. In addition, we discussed the appropriate treatment for different anastomotic fistulas.
- Citation: Lu CY, Liu YL, Liu KJ, Xu S, Yao HL, Li L, Guo ZS. Differences in examination results of small anastomotic fistula after radical gastrectomy with afterward treatments: A case report . World J Clin Cases 2022; 10(21): 7609-7616
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7609.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7609
Esophagojejunal anastomotic fistula (EJF) is a major complication of radical gastrectomy. Mediastinal and lung infections are often seen in patients with EJF, which seriously endanger the health of patients[1]. The reported rates of the EJF after radical gastrectomy vary from 0% to 5.8%, with 0% to 5.8% after laparoscopic, 0% to 3.4% after robotic surgery, and 0% to 5.8% after open surgery[2-6]. The mortality rate was 26.32%[7]. Gastrografin swallow, methylthioninium chloride test, and computed tomography (CT) are currently the main means of postoperative anastomotic fistula detection at present. The treatment of anastomotic fistula mainly includes endoscopic clamping, stent placement, tissue sealant filling, and surgical re-operation if necessary[8]. Correct selection and application of examinations and therapies are significant for the early diagnosis and treatment of small anastomotic fistulas after radical gastrectomy, which are conducive to postoperative recovery.
Based on the diagnosis and treatment process of a patient with anastomotic fistula after radical gastrectomy for gastric cancer, we discuss the main diagnosis and treatment methods of anastomotic fistulas in depth, which is conducive to the timely diagnosis and accurate treatment of postoperative anastomotic fistula.
A 44-year-old woman presented to the Department of Gastrointestinal Surgery of our hospital complaining of upper epigastric pain.
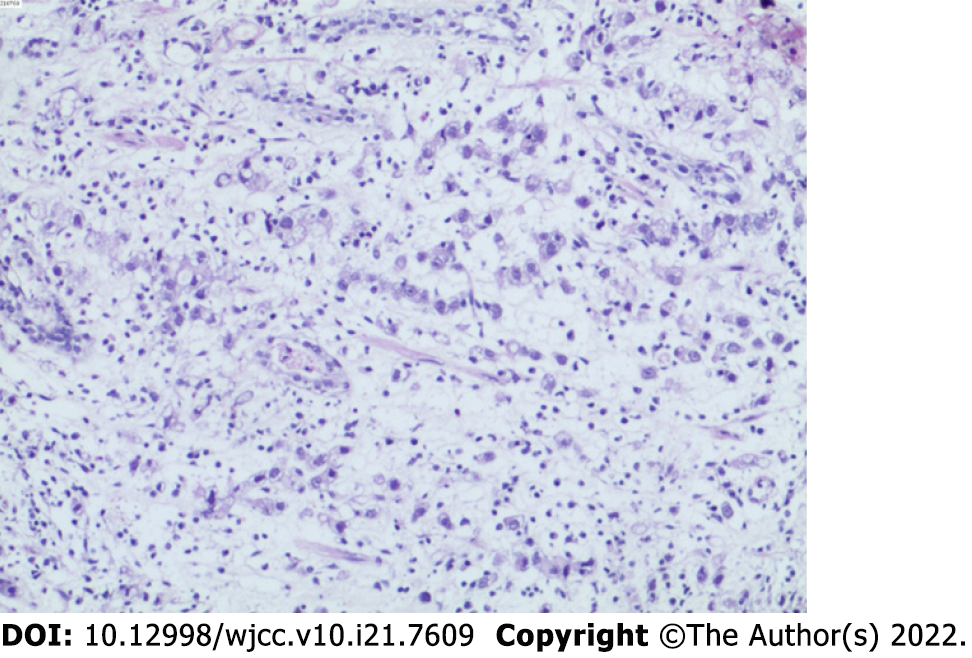
Patient’s symptoms started 2 months ago, usually between meals. Acid burps or acid belching can occasionally occur. Antacids usually resolve her symptoms. Gastroscopy and biopsy were performed 10 days ago, and gastroscopy revealed a stage A1 gastric ulcer with bleeding. In addition, biopsy showed abnormal cell proliferation and infiltration between the inherent glands of the gastric mucosa, which indicated a poorly differentiated adenocarcinoma (Figure 1). A contrast-enhanced abdomen CT scan showed local thickening of the gastric wall along the greater curvature of the gastric body, enlarged small lymph nodes around the stomach, and multiple enlarged lymph nodes enlarged in the mesentery.
The patient underwent laparoscopic radical gastrectomy (total gastrectomy Roux-en-Y anastomotic regional lymph node dissection) + jejunostomy + cholecystectomy + splenic hilar lymph node dissection. Side-to-side anastomosis between the oesophagus and jejunum was performed. She developed a persistent fever after surgery, which meant she started having a fever on t postoperative day 1 and was unable to reduce it to a normal temperature on her own.
The patient had a free previous medical history.
The patient had no personal or family history related to gastric cancer and anastomotic fistula.
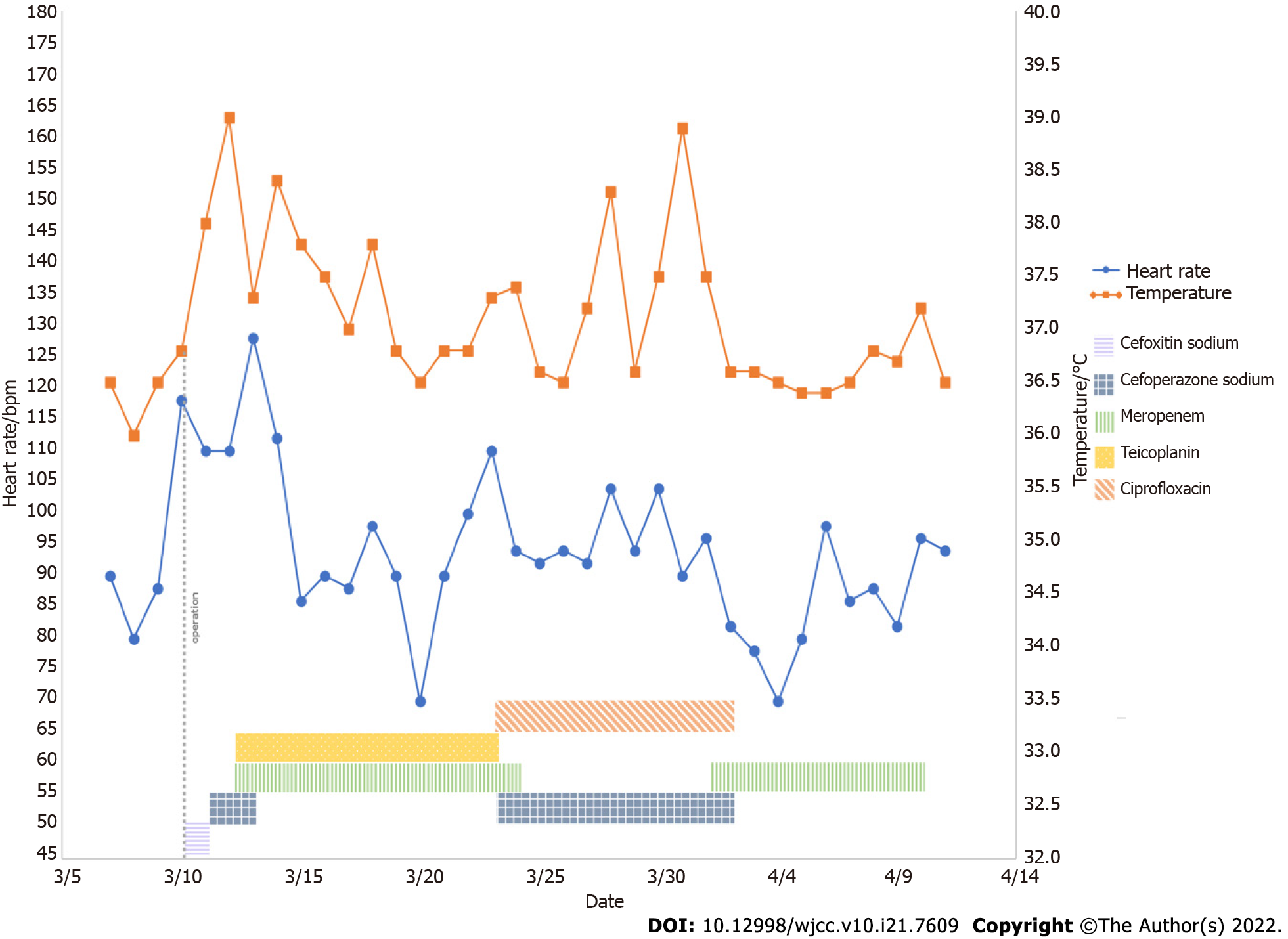
Physical examination revealed mild tenderness in the upper abdomen without rebound pain. She developed a persistent fever after surgery, which cannot be completely cured by physical cooling and the use of antibiotics (Figure 2).
A severe leukocytosis 25.69 × 109/L appeared on postoperative day 1, with predominant neutrophils (90.20%), which indicated a possible bacterial infection. A similar situation persisted until postoperative day 4, which was the third day after Cefoperazone Sodium and Sulbactam Sodium was given to the patient. Since then, blood analysis has consistently revealed a mild leukocytosis. Ciprofloxacin was applied on postoperative day 15, after which the infection indicators dropped to normal.
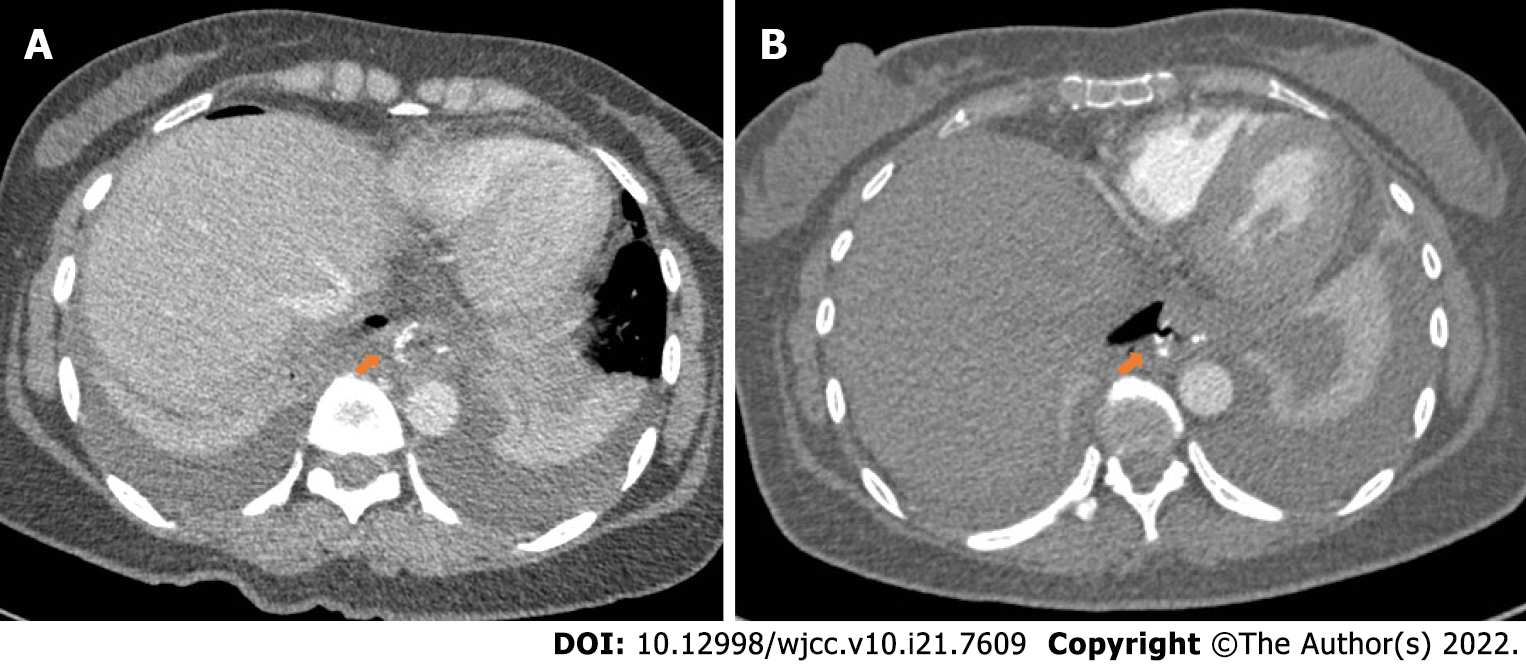
On postoperative day 4, CT showed dilatation of the esophagus, duodenum, and jejunum with effusion. A small amount of ascites was found around the spleen, and pleural effusion was observed. On postoperative day 6, after the patient received oral methylthioninium chloride, there was no blue fluid draining from the tube. The CT and oral methylthioninium chloride results suggest that the anastomosis appears to be well closed (Figure 3A). However, despite the use of various anti-inflammatory treatments over the following few days, the patient's symptoms were not relieved. Therefore, CT was performed again on the postoperative day 13, and showed that the anastomotic site at the lower end of the oesophagus was thickened and blurred, and the right parastinastinal cystoid air cavity was connected to the anastomotic site (Figure 3B).
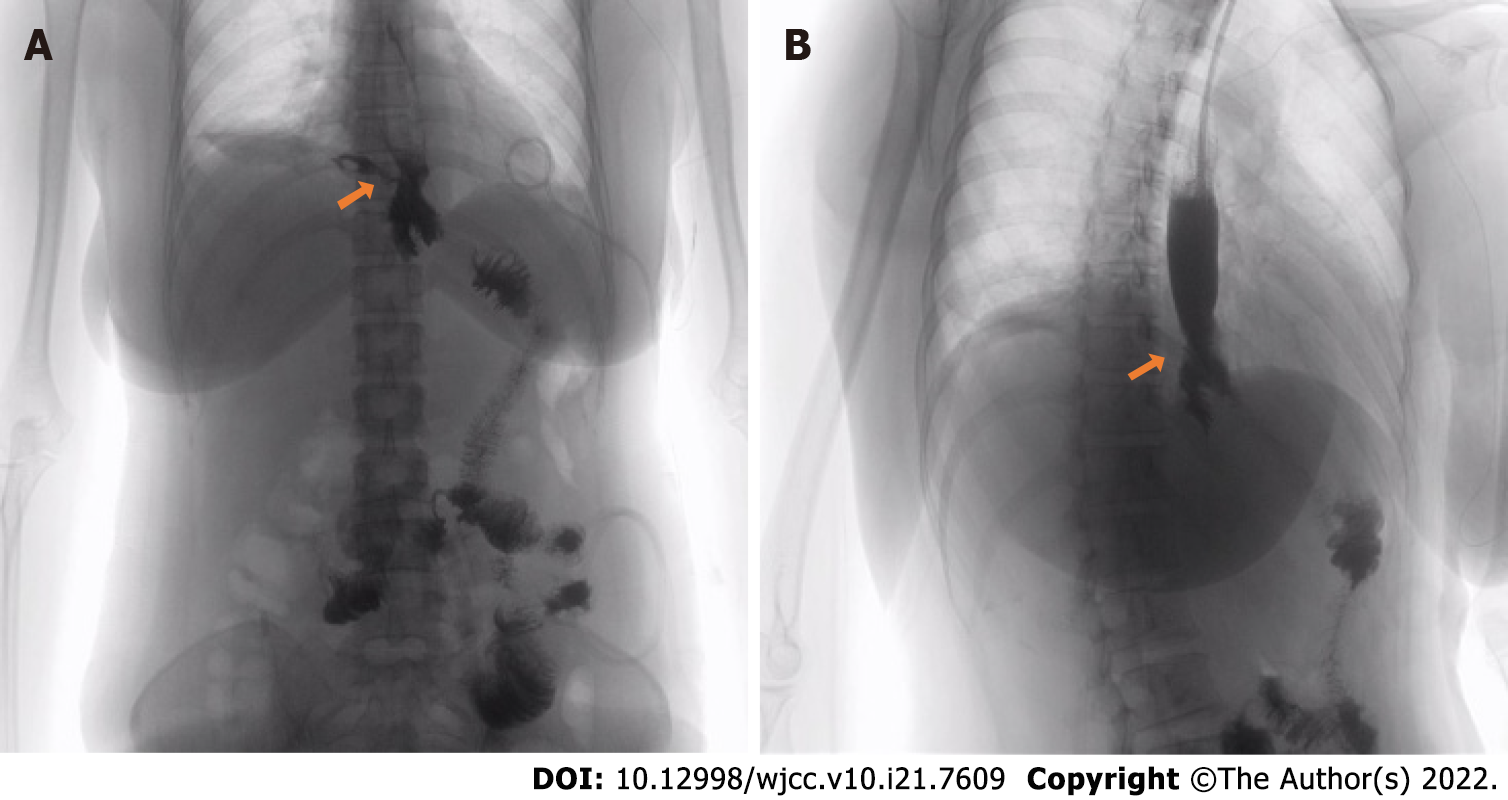
Gastrografin swallow on postoperative day 13 was used to confirm the diagnosis of anastomotic fistula (Figure 4A).
Sputum culture was performed on postoperative day 9. The Ralstonia mannitolilytica infection was reported on postoperative day 14, which was sensitive for Ciprofloxacin.
The possibility of anastomotic fistula was highly considered.
After ciprofloxacin had controlled the infection, endoscopic metal clip therapy was performed on postoperative day 23 to clamp the anastomotic fistula. And meglumine diatrizoate esophagogram showed no anastomotic fistula (Figure 4B).
After the application of antibiotics and clamping of the anastomotic fistula, the infection of the patient was gradually controlled on the postoperative day 24. Since then, anastomotic fistulas related signs did not appear again.
A 44-year-old woman developed a persistent fever after laparoscopic radical total gastrectomy for gastric cancer. The methylthioninium chloride test and early CT suggested no anastomotic fistula; however, gastrografin swallow and late CT showed the opposite result. The fistula was successfully closed using an endoscopic clip. The methylthioninium chloride test, gastrografin, and CT performed on different postoperative dates for small esophagojejunostomy fistulas are different. The size of the anastomotic fistula is an important factor for the success of endoscopic treatment.
The reported rates of the EJF after radical gastrectomy vary from 0% to 5.8%[2-6], and with a mortality rate of 26.32%[7], which is one of the main causes of postoperative sepsis[9]. EJF usually occurs near the suture line. Cardiovascular disease, age, smoking, malnutrition, operative hormones, local blood supply, and inflammatory reactions to suture materials are the risk factors for anastomotic fistulas[6]. However, the effect of tumour stage and the timing of lymph node dissection on the incidence of anastomotic fistula remains controversial[10,11]. Anastomotic fistulas can be classified based on the time of onset, clinical presentation, site of anastomotic fistula, radio-appearance, and mixed factors[12] (Table 1), which are critical for selecting diagnosis and treatment.
| Basis of classification | Classification | Definition |
| The time of anastomotic fistula | Early leaks | Early leaks appear 1 to 4 days after surgery |
| Intermediate leaks | Intermediate leaks appear 5 to 9 days after surgery | |
| Late leaks | Late leaks appear 10 or more days after surgery | |
| Clinical relevance and extent of dissemination | Type Ⅰ leaks | TypeⅠleaks are well localized, have no pleural or peritoneal spread, do not induce systemic clinical manifestations, and are usually readily treatable with medication |
| Type Ⅱ leaks | Type Ⅱ leak spread to the abdominal cavity or pleura, or the drainage tube, followed by severe systemic clinical manifestations | |
| Clinical and radiological findings | Type A leaks | Type A leaks have no clinical or radiological evidence |
| Type B leaks | Type B leaks can be detected by radiological studies but without any clinical finding | |
| Type C leaks | Type C leaks have both radiological and clinical evidence |
There is no gold standard for the diagnosing anastomotic fistulas. The most common manifestations of intraperitoneal complications of anastomotic fistula include signs of sepsis and laboratory signs (leukocytosis and elevated C-reactive protein levels) postoperative 7 to 10[13]. In this case, the persistent postoperative fever suggested a possible anastomotic fistula. To detect anastomotic fistula, air leak testing and transgastric methylthioninium chloride injection were used during the surgery, but Sethi et al[14] believed that intraoperative leak testing has no correlation with leak due to laparoscopic sleeve gastrectomy. Postoperative examination of the anastomotic fistula is important. Gastrografin swallow, methylthioninium chloride test, and CT are the main means of postoperative anastomotic fistula detection at present[15,16]. Many studies have shown that postoperative gastrografin swallow and methylthioninium chloride test were effective in confirming clinical evidence of anastomotic fistula[15]. The methylthioninium chloride test was performed on the postoperative day 6 and showed a negative result, while the result of gastrografin swallow on postoperative day 22 was positive. Although the sensitivity of gastrografin swallow was higher than that of the methylthioninium chloride test, it had a high false-negative rate[16]. Some studies found that CT had more advantages in diagnosing anastomotic fistulas than gastrografin swallow and methylthioninium chloride test[17]. Oral contrast agents may also be used to increase the sensitivity to CT[18]. Factors associated with the CT-based diagnosis of anastomotic fistula include mediastinal fluid, mediastinal air, wall discontinuity, and fistula. However, isolated mediastinal gas can be observed in patients without any leakage, which is a common finding after surgery and the diagnostic value of CT in different postoperative periods for anastomotic fistulas is also different[17]. In this case, CT was performed on postoperative days 4 and 14. Notably, the examination result indicated anastomotic leakage on postoperative day 14. Although some studies have discussed the CT-based diagnostic score for anastomotic fistula and the diagnostic value of radiographic image details, such as the differences in the number of bubbles around the anastomosis and the mediastinal space, CT cannot completely replace other diagnostic methods[17,19].
The treatment of anastomotic fistulas mainly includes endoscopic clamping, stent placement, and tissue sealant filling. The use of fibrin to promote the healing of anastomotic fistulas had a good clinical effect, while other researchers believed that it was only due to the mechanical sealing[20,21]. The actual therapeutic gold standard for postoperative oesophageal anastomotic fistulas is stent implantation[22]. Endoscopic clipping is another treatment for postoperative anastomotic fistula, which is suitable for anastomotic fistulas with small circumferences and ineffective conservative treatment. Two types of clips, through-the-scope clip (TTSC) and over-the-scope clip (OTSC), were used for endoscopic clipping. Anastomotic fistulas less than 10 mm in diameter are recommended to be clipped using a single TTSC[23]. Meanwhile, TTSCs and OTSCs have a high clipping success rate in anastomotic fistulas with a diameter of 10 to 20 mm[24,25]. Due to the limitation of the clip arm width and grasping force, TTSC is only applicable to anastomotic fistulas of less than 20 mm in diameter with healthy non-everted regular edges[26]. However, OTSCs have high clinical success rates for large anastomotic fistulas of up to 30 mm in diameter[24]. Anastomotic fistulas larger than 30mm in diameter are difficult to be clipped by endoscopy[27]. Endoscopic vacuum therapy (EVT) was introduced 10 years ago to treat anastomotic fistulas, and it has a higher closure rate than stent implantation[28]. Anastomotic fistula associated with fluid collection is a common indication for EVT, and EVT has a higher cure rate than stents for this type of anastomotic fistulas[27,29]. However, EVT requires frequent replacement of endoscopic devices, which means a higher risk for recurrent sedation. Hence, the correct choice of repair method is helpful in avoiding repeated surgeries. The European Society of Gastrointestinal Endoscopy (ESGE) recommends to consider endoscopic closure based on the type and size of the anastomotic fistula, the presence and characteristics of leaking fluid, the general situation of the patient, and the endoscopy expertise available at the center[27].
Early diagnosis of the small anastomotic fistulas is particularly important for the patient prognosis. Current studies suggest that the sensitivity of CT is higher than that of Gastrografin swallow and methylthioninium chloride tests; however, it cannot be used as the gold standard for the diagnosis of anastomotic fistula. Accurate diagnosis of anastomotic fistula should be combined with clinical manifestations and appropriate examination methods should be selected to avoid false results. The selection of anastomotic fistula repair should be conducted according to the ESGE recommendations. Endoscopic therapy, including stent placement, endoscopic clipping, and vacuum therapy, is preferred for patients with stable leakage and without peritonitis.
We thank the patient and her family who participated in this study. And we wish to thank the timely help given by Jiachen Ji, Ningyu Qin, Zhilan Yin and Ke Gong in taking part in revising and critically reviewing the article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Moshref L, Saudi Arabia; Tsoulfas G, Greece S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Gil-Rendo A, Hernández-Lizoain JL, Martínez-Regueira F, Sierra Martínez A, Rotellar Sastre F, Cervera Delgado M, Valentí Azcarate V, Pastor Idoate C, Alvarez-Cienfuegos J. Risk factors related to operative morbidity in patients undergoing gastrectomy for gastric cancer. Clin Transl Oncol. 2006;8:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Kostakis ID, Alexandrou A, Armeni E, Damaskos C, Kouraklis G, Diamantis T, Tsigris C. Comparison Between Minimally Invasive and Open Gastrectomy for Gastric Cancer in Europe: A Systematic Review and Meta-analysis. Scand J Surg. 2017;106:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Inokuchi M, Otsuki S, Fujimori Y, Sato Y, Nakagawa M, Kojima K. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J Gastroenterol. 2015;21:9656-9665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Hyodo M, Hosoya Y, Hirashima Y, Haruta H, Kurashina K, Saito S, Yokoyama T, Arai W, Zuiki T, Yasuda Y, Nagai H. Minimum leakage rate (0.5%) of stapled esophagojejunostomy with sacrifice of a small part of the jejunum after total gastrectomy in 390 consecutive patients. Dig Surg. 2007;24:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Haga Y, Wada Y, Takeuchi H, Ikejiri K, Ikenaga M. Prediction of anastomotic leak and its prognosis in digestive surgery. World J Surg. 2011;35:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Barchi LC, Ramos MFKP, Pereira MA, Dias AR, Ribeiro-Júnior U, Zilberstein B, Cecconello I. Esophagojejunal anastomotic fistula: a major issue after radical total gastrectomy. Updates Surg. 2019;71:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Aurello P, Magistri P, D'Angelo F, Valabrega S, Sirimarco D, Tierno SM, Nava AK, Ramacciato G. Treatment of esophagojejunal anastomosis leakage: a systematic review from the last two decades. Am Surg. 2015;81:450-453. [PubMed] |
| 8. | Cereatti F, Grassia R, Drago A, Conti CB, Donatelli G. Endoscopic management of gastrointestinal leaks and fistulae: What option do we have? World J Gastroenterol. 2020;26:4198-4217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (2)] |
| 9. | Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis 2014. 10: 713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 10. | Barchi LC, Charruf AZ, de Oliveira RJ, Jacob CE, Cecconello I, Zilberstein B. Management of postoperative complications of lymphadenectomy. Transl Gastroenterol Hepatol. 2016;1:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Barchi LC, Ramos MFKP, Dias AR, Yagi OK, Ribeiro-Júnior U, Zilberstein B, Cecconello I. TOTAL OMENTECTOMY IN GASTRIC CANCER SURGERY: IS IT ALWAYS NECESSARY? Arq Bras Cir Dig. 2019;32:e1425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Abou Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: review of its prevention and management. World J Gastroenterol. 2014;20:13904-13910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 141] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 13. | Tonolini M, Bracchi E. Early postoperative imaging after non-bariatric gastric resection: a primer for radiologists. Insights Imaging. 2017;8:393-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Sethi M, Zagzag J, Patel K, Magrath M, Somoza E, Parikh MS, Saunders JK, Ude-Welcome A, Schwack BF, Kurian MS, Fielding GA, Ren-Fielding CJ. Intraoperative leak testing has no correlation with leak after laparoscopic sleeve gastrectomy. Surg Endosc. 2016;30:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, Matter I, Alfici R, Mahajna A, Waksman I, Shimonov M, Assalia A. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 16. | Terterov D, Leung PH, Twells LK, Gregory DM, Smith C, Boone D, Pace D. The usefulness and costs of routine contrast studies after laparoscopic sleeve gastrectomy for detecting staple line leaks. Can J Surg. 2017;60:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Goense L, Stassen PMC, Wessels FJ, van Rossum PSN, Ruurda JP, van Leeuwen MS, van Hillegersberg R. Diagnostic performance of a CT-based scoring system for diagnosis of anastomotic leakage after esophagectomy: comparison with subjective CT assessment. Eur Radiol. 2017;27:4426-4434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Kim TH, Kim JH, Shin CI, Kim SH, Han JK, Choi BI. CT findings suggesting anastomotic leak and predicting the recovery period following gastric surgery. Eur Radiol. 2015;25:1958-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Shoji Y, Takeuchi H, Fukuda K, Nakamura R, Wada N, Kawakubo H, Kitagawa Y. Air Bubble Sign: A New Screening Method for Anastomotic Leakage After Esophagectomy for Esophageal Cancer. Ann Surg Oncol. 2018;25:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Kotzampassi K, Eleftheriadis E. Tissue sealants in endoscopic applications for anastomotic leakage during a 25-year period. Surgery. 2015;157:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Tashiro K, Takeno S, Kawano F, Kitamura E, Hamada R, Ikenoue M, Munakata S, Nanashima A, Nakamura K. Endoscopic filling with polyglycolic acid sheets and fibrin glue of persistent fistula after esophagectomy. Endoscopy. 2021;53:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Persson S, Rouvelas I, Irino T, Lundell L. Outcomes following the main treatment options in patients with a leaking esophagus: a systematic literature review. Dis Esophagus. 2017;30:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Chan SM, Auyeung KKY, Lam SF, Chiu PWY, Teoh AYB. Current status in endoscopic management of upper gastrointestinal perforations, leaks and fistulas. Dig Endosc. 2022;34:43-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, Weusten B, Seewald S, Costamagna G, Deprez P, Fockens P; CLIPPER Study Group. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol. 2012;10:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Magdeburg R, Collet P, Post S, Kaehler G. Endoclipping of iatrogenic colonic perforation to avoid surgery. Surg Endosc. 2008;22:1500-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | de Moura DTH, Sachdev AH, Thompson CC. Endoscopic Full-Thickness Defects and Closure Techniques. Curr Treat Options Gastroenterol. 2018;16:386-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Paspatis GA, Arvanitakis M, Dumonceau JM, Barthet M, Saunders B, Turino SY, Dhillon A, Fragaki M, Gonzalez JM, Repici A, van Wanrooij RLJ, van Hooft JE. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement - Update 2020. Endoscopy. 2020;52:792-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 28. | Scognamiglio P, Reeh M, Karstens K, Bellon E, Kantowski M, Schön G, Zapf A, Chon SH, Izbicki JR, Tachezy M. Endoscopic vacuum therapy vs stenting for postoperative esophago-enteric anastomotic leakage: systematic review and meta-analysis. Endoscopy. 2020;52:632-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Rausa E, Asti E, Aiolfi A, Bianco F, Bonitta G, Bonavina L. Comparison of endoscopic vacuum therapy vs endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |