Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7545
Peer-review started: February 6, 2022
First decision: March 23, 2022
Revised: April 1, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: July 26, 2022
Processing time: 154 Days and 24 Hours
Patients with keloids who receive radiotherapy (RT) after surgery can develop refractory wounds that cannot be healed by the patient's own repair system. Such chronic wounds are uneven and complex due to persistent abscess and ulceration. Without external intervention, they can easily result in local tissue necrosis or, in severe cases, large area tissue resection, amputation, and even death.
This article describes the use of hydrogen to treat a 42-year-old female patient with a chronic wound on her left shoulder. The patient had a skin graft that involved implanting a dilator under the skin of her left shoulder, and then transferring excess skin from her shoulder onto scar tissue on her chest. The skin grafting was followed by two rounds of RT, after which the shoulder wound had difficulty healing. For six months, the patient was treated with 2 h of hydrogen inhalation (HI) therapy per day, in addition to application of sterile gauze on the wound and periodic debridement. We also performed one deep, large, sharp debridement to enlarge the wound area. The wound healed completely within 6 mo of beginning the HI treatment.
After HI therapy, the patient showed superior progress in reepithelialization and wound repair, with eventual wound closure in 6 mo, in comparison with the previous failures of hyperbaric oxygen and recombinant bovine basic fibroblast growth factor therapies. Our work showed that HI therapy could be a new strategy for wound healing that is cleaner, more convenient, and less expensive than other therapies, as well as easily accessible for further application in clinical wound care.
Core Tip: A keloid is a benign protuberance of scar tissue formed after a skin wound. Radiotherapy (RT) immediately after surgical resection can effectively reduce the recurrence rate of keloids, but can also disrupt the ordered sequence of cell interactions, leading to repeated inflammatory responses and inadequate healing, thus resulting in a chronic wound. We aimed to test the efficacy of hydrogen inhalation (HI) therapy in the treatment of a patient who developed a chronic wound after skin grafting and RT. The novel adjuvant therapy of HI resulted in effective reepithelialization and successful healing.
- Citation: Zhao PX, Luo RL, Dang Z, Wang YB, Zhang XJ, Liu ZY, Wen XH, Liu MY, Zhang MZ, Adzavon YM, Ma XM. Effect of hydrogen intervention on refractory wounds after radiotherapy: A case report. World J Clin Cases 2022; 10(21): 7545-7552
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7545.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7545
A keloid is a benign raised scar that sometimes results from postoperative radiotherapy (RT) immediately after surgical resection. Repeated inflammatory responses can cause delayed and inadequate wound healing. Current strategies for treating radiation-delayed wound healing typically include standard wound care and measures to optimize blood and oxygen supply[1]. Chronic wound healing is a complex process with a series of interrupted cascade events and the blocking of cellular interactions.
Cutaneous wounds always proceed through three independent but overlapping stages[2-5]. The inflammation stage starts immediately and is associated with the formation of blood clots and the recruitment of inflammatory cells. The second stage involves regeneration, characterized by reepithelialization of the wound, the creation of new epidermal cells, and the formation of granulation tissue. The last stage is the remodeling stage, in which the epidermis, dermis, and extracellular matrix (ECM) are rearranged. Defects in any of these stages will cause chronic wounds. Chronic wounds fail to heal despite the use of current therapies because they become stalled in the inflammation stage of wound healing[6]. In contrast, acute wounds progress through all three stages in a timely manner as they heal.
Human wounds add a substantial burden to worldwide clinical, social, and economic resources. A 2018 retrospective analysis of United States Medicare beneficiaries identified that about 8.2 million people had wounds with or without infections. Medicare cost estimates for acute and chronic wound treatment were $28.1–96.8 billion for the year 2014 alone[7]. Although various therapies have been developed, more efficient, cleaner, and more advanced treatments are still needed in dealing with both acute and chronic wounds.
Since 2007[8], increased attention has been paid to the physiological functions and signal transduction pathways of hydrogen as a gaseous signaling molecule. Hydrogen is not only environmentally friendly, with no other metabolites in vivo except water, but also easy to obtain via simple electrolysis of water[8]. Its antioxidant[9], anti-inflammatory[10], and anti-apoptotic[11] effects have been discovered in studies using multiple animal models and, thereafter, in some clinical trials. It has been reported that hydrogen has beneficial effects on skin diseases such as pressure ulcers, burns, and psoriasis[12]. Hydrogen can reduce oxidative stress, alleviate inflammation in local wounds, and accelerate wound healing.
Our clinical study showed that hydrogen inhalation (HI) therapy improved the adhesion between skin and muscle tissues and promoted the reepithelialization process and wound healing speed. Therefore, we believe that HI therapy may be a clean, inexpensive, convenient, and readily available new wound-healing strategy that can be further applied to clinical wound care.
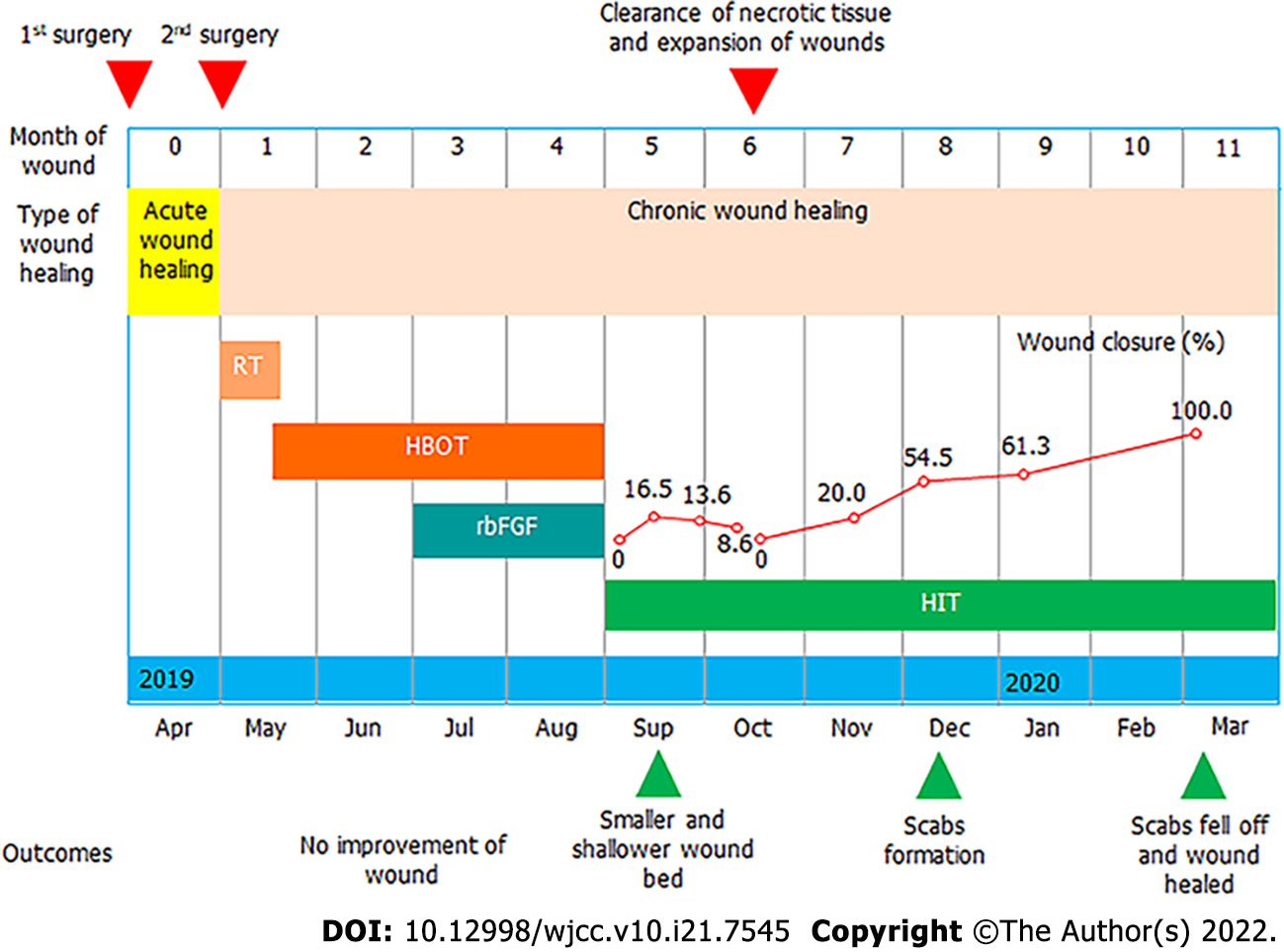
A 42-year-old woman with a chronic wound on the left shoulder was advised to try HI therapy (Figure 1). Because of her scar diathesis, we transplanted excess skin from the back of her left shoulder to scar tissue on her chest by implanting a dilator under the skin of her left shoulder on April 3, 2019. After skin grafting on April 25, 2019, two rounds of RT were performed. The first round was given 1 d after the first surgery, and the second occurred 1 wk after the first round; the dose was 900 centigrade. However, the defective shoulder skin became difficult to heal.
After 4 mo of other treatments in which no progress in healing was observed, the patient volunteered to take HI therapy for 6 mo. During the first month, we also performed mild sharp debridement weekly. On October 14, 2019, we performed a deep, large, sharp debridement, enlarging the wound area. Within 6 mo of the start of HI therapy, the wound tissue was completely healed.
No other positive history of past illness was known.
The patient declared no smoking or alcohol history, nor any other noteworthy family medical history.
A round chronic wound with a diameter of about 2 cm was found in the patient’s left shoulder.
Blood tests showed no obvious abnormality.
No imaging examinations were performed as the patient displayed good conditions except the wound on the patient’s left shoulder.
The final diagnosis of the case was chronic wound.
In the first surgery, the wound was sutured after implantation of the dilator under skin at the back of the patient’s shoulder. In the second surgery, we removed the dilator and transplanted the expanded shoulder skin to the chest.
Two rounds of RT followed surgery. The first round was given 1 d after the first surgery, and the second occurred 1 wk after the first round. The dose was 900 centigrade.
No progress in healing was observed during the first 4 mo of treatments, which included topical spray of recombinant bovine basic fibroblast growth factor (rbFGF) and hyperbaric oxygen (HBO) therapy, together with topical application of sterile gauze. Then, the patient volunteered to take HI therapy, along with topical application of sterile gauze for 6 mo. During the first month of HI therapy, we also performed mild sharp debridement weekly. On October 14, 2019, we performed a deep, large, sharp debridement, enlarging the wound area (Figure 1).
HBO therapy was provided with 82% oxygen at a pressure of 1.5 atm. Pressure was increased from 1.0 to 1.5 atm over the course of 15 min, after which it was sustained at the latter level for exactly 1 h. The decrease of oxygen pressure took another 15 min, after which the patient exited the chamber.
rbFGF therapy involved daily topical spray of rbFGF at 150 AU/cm2 three times per day.
HI therapy involved inhalation of a mix of hydrogen (66%) and oxygen (33%) gas at a flow rate of 3 L/min using KLE-H7 hydrogen generator (Kelieng Biomedical Co. Ltd., Shenzhen, China) for 2 h/d.
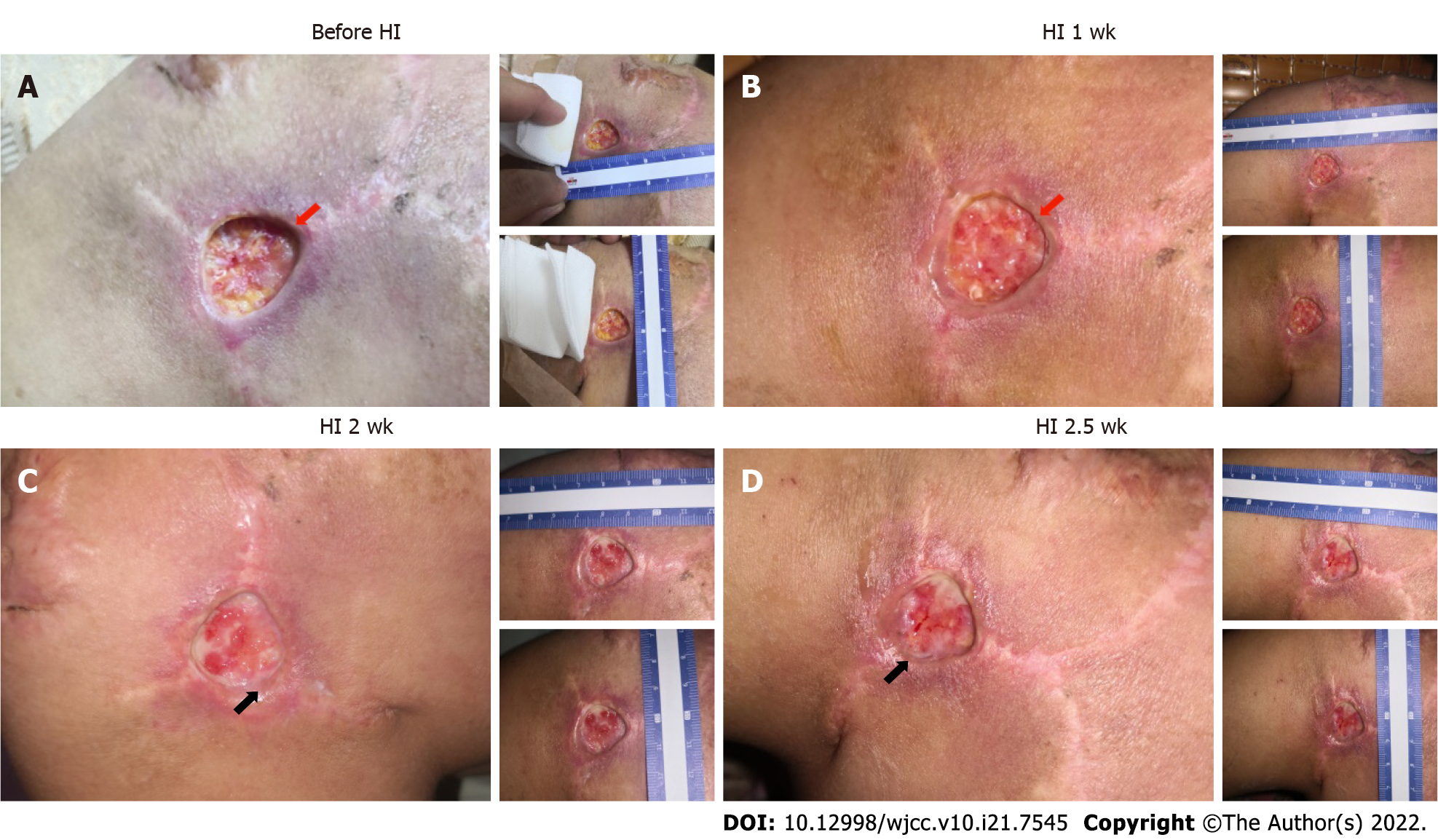
During the first 40 d of HI treatment before sharp debridement on October 14, 2019, the patient’s wound showed improvements, though we saw no significant wound closure resulting from the weekly mild sharp debridement. Due to the early subcutaneous implantation of the dilator, the skin and muscle layers at the wound were severely separated. One week after HI treatment, the wound bed became shallower, and adhesion between the skin and wound bed was tighter (Figure 2A and B). From week two onward, fresh granulation tissue was seen around the wound edge, which could have reflected the start of reepithelialization. In addition, more blood vessels were observed at week two and after two and a half weeks, which was another sign of improved healing (Figure 2C and D).
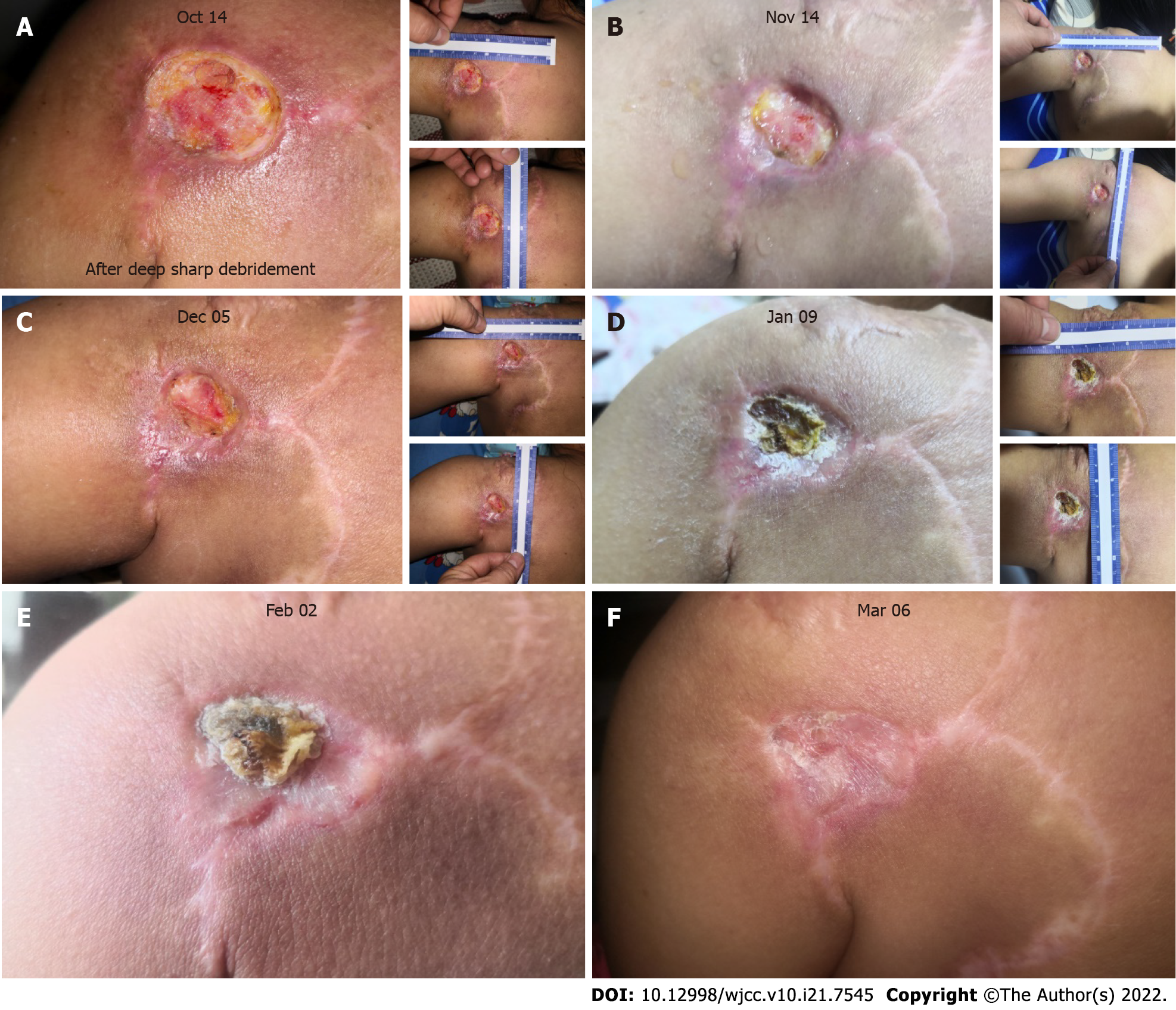
After the final sharp debridement on October 14, 2019, although the wound area was enlarged due to the removal of slough tissue (Figure 3A), wound closure changes became visible. Wound closure reached 20% in the following month and 54% in the subsequent month compared to the area on October 14, 2019 (Figure 3B and C); no infection was observed. From January of 2020 onward, a scab began to form, covering the wound bed (Figure 3D). On February 2, the skin around the scab was better remodeled, with thickening of the epidermis (Figure 3E). The scab fell off on March 6, and the wound closure process was finished (Figure 3F), although further remodeling was still ongoing.
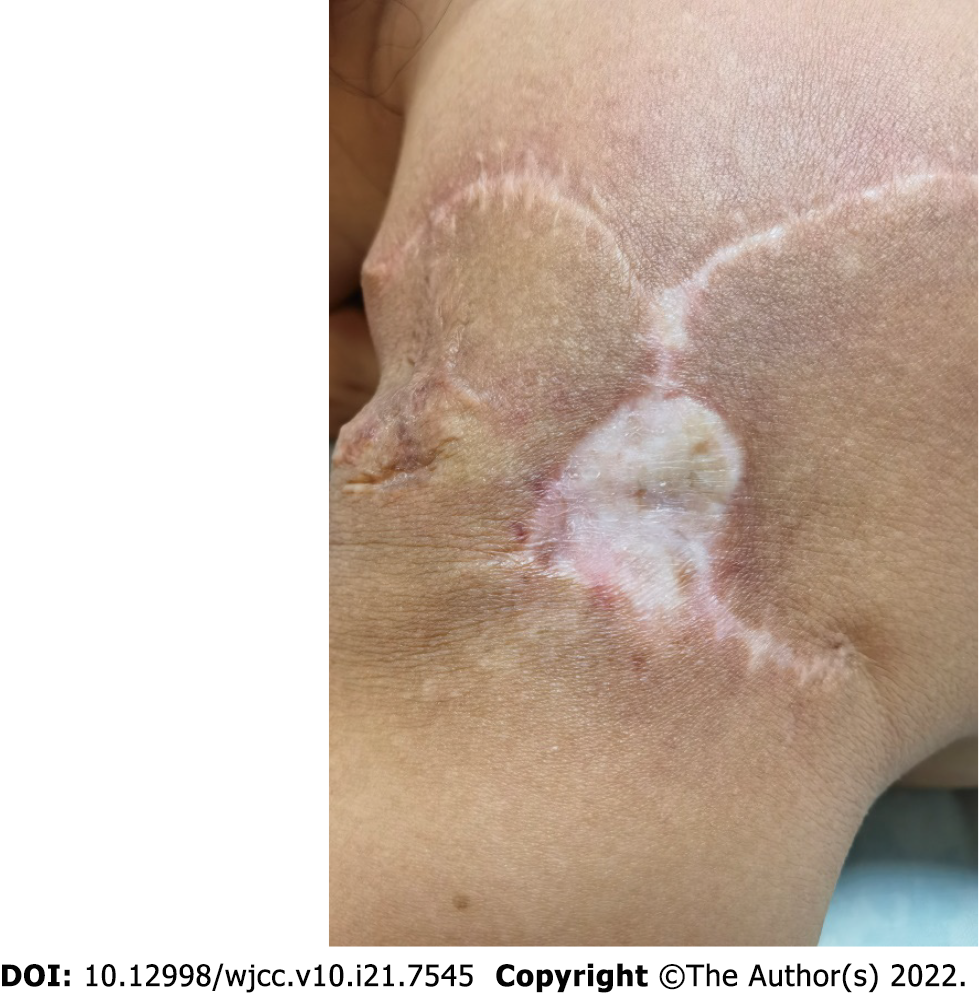
The operation went smoothly, and the patient recovered and was discharged. Examinations immediately after surgery and 6 mo later showed that the surgical incision healed well without obvious scarring (Figure 4). The patient was satisfied with the effectiveness of treatment.
Keloids are benign raised scars resulting from excessive tissue proliferation and excess collagen deposit during wound healing[13]. Surgical excision followed by immediate postoperative RT is one of the most effective treatments in reducing the recurrence rate of keloids[14,15]. However, RT can also disrupt the ordered sequence of cellular interactions, resulting in repetitive inflammatory responses and delayed and inadequate wound healing[16]. Current strategies for treating irradiated delayed wounds always include standard wound care, vacuum-assisted devices, supplementation for nutritional deficiencies, and measures to optimize blood and oxygen supply. HBO, growth factors, special dressings, and even injections of cells are used in clinical treatment[1].
It is hard to determine whether HBO or rbFGF combination treatments have a significant influence on the healing of irradiated chronic wounds because of the inability to compare with controls. These treatments also have some limitations. For example, HBO therapy is more effective for hypoxic wounds so it has the disadvantage of limited treatment scope[16,17]. Patients with complications such as untreated pneumothorax, multiple fractures, retinal detachment, internal bleeding, severe cold and rhinitis, high fever, high blood pressure, and early pregnancy and menstrual period are not suitable for the HBO therapy[18]. Due to their side effects, growth factors have seen little use in clinical practice until now. This involves the safety of recombinant products. It is necessary to establish a variety of quality control standards and toxicity inspection[19].
In our case study, at least within the first 4 mo, we saw very little progress in wound healing. Healing of chronic wounds is a complex process, with a disrupted cascade of events and blocked cellular interaction[20]. Our findings showed that HI treatment promoted better adhesion between skin and muscle tissues that had been separated by dilator implantation and flap surgery (Figure 2), and it improved the process of reepithelialization (growth of wound edge; Figure 2) and wound closure speed (Figure 3).
Most chronic wounds share common inflammatory disorders[21]. The skin is composed of cells (mainly fibroblasts, endothelial cells, and keratinocytes) and ECM[22]. The migration of fibroblasts and related cells is responsible for wound bed closure, and the deposition of ECM has many key functions, including providing structure, organization, and orientation to cells and tissues, as well as strengthening and binding tissues. The successful outcome for our patient after HI treatment could have been related to the suppression of inflammation, as well as to better ECM deposit and cell migration. Further experimental evidence is still needed, however.
Hydrogen plays an important role in many disease models due to its antioxidant, anti- apoptotic, and anti-inflammatory properties. Previous studies have proven that hydrogen therapy promotes the process of burn tissue recovery[9,23]. Molecular hydrogen has been used in a variety of novel therapeutic applications[9,10,24,25]. There are many ways to give hydrogen, such as intraperitoneal and intravenous injection of hydrogen-rich water, drinking hydrogen-rich water, and inhaling a mixture of hydrogen with the help of a ventilator[24,26,27].
Some studies have shown that one-time hydrogen preconditioning did not reduce the ischemia-reperfusion injury of a rat skin flap, but this may be related to the short treatment time or the short residence time of hydrogen[26]. Therefore, the selection of treatment time and administration mode of hydrogen also is very important. In previous studies, the hydrogen absorption concentration was below 5% in low concentration treatments and in high concentration treatments[28]. Administration times also differed, which may affect the treatment outcome. Further exploration is needed to find the most appropriate hydrogen delivery mode, dosage, and treatment time to achieve the best outcome.
Patients with keloids who undergo radiation after skin graft surgery can develop refractory, or chronic, wounds. Our case study found that HI was effective in healing a chronic shoulder skin wound, with better treatment results than HBO and growth factor therapy. The HI therapy accelerated the reepithelialization process and probably also the accumulation of ECM deposit. Therefore, HI therapy shows promise as a less expensive, cleaner, and more available option for future clinical wound care.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim YJ, Malaysia; Sandoval C, Chile A-Editor: Lin FY, China S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: review of the literature. Radiat Oncol. 2012;7:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 2. | Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 352] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 3. | Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3609] [Cited by in RCA: 4325] [Article Influence: 254.4] [Reference Citation Analysis (0)] |
| 4. | Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol. 2003;121:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol. 2012;23:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front Physiol. 2018;9:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 487] [Cited by in RCA: 930] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 7. | Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M, Cartwright D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health. 2018;21:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 739] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 8. | Shirahata S, Li Y, Hamasaki T, Gadek Z, Teruya K, Kabayama S, Otsubo K, Morisawa S, Ishii Y, Katakura Y. Redox regulation by reduced waters as active hydrogen donors and intracellular ROS scavengers for prevention of type 2 diabetes. In: Smith R (eds) Cell Technology for Cell Products. Springer, Dordrecht, 2007: 99-101. [DOI] [Full Text] |
| 9. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1690] [Article Influence: 93.9] [Reference Citation Analysis (1)] |
| 10. | Zhao L, Wang YB, Qin SR, Ma XM, Sun XJ, Wang ML, Zhong RG. Protective effect of hydrogen-rich saline on ischemia/reperfusion injury in rat skin flap. J Zhejiang Univ Sci B. 2013;14:382-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Liu YQ, Liu YF, Ma XM, Xiao YD, Wang YB, Zhang MZ, Cheng AX, Wang TT, Li JL, Zhao PX, Xie F, Zhang X. Hydrogen-rich saline attenuates skin ischemia/reperfusion induced apoptosis via regulating Bax/Bcl-2 ratio and ASK-1/JNK pathway. J Plast Reconstr Aesthet Surg. 2015;68:e147-e156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Ichihara M, Sobue S, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 13. | Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr. 2006;18:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Hou C, Peng Y, Qin C, Fan F, Liu J, Long J. Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Aβ clearance. Free Radic Res. 2018;52:1311-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Song G, Tian H, Qin S, Sun X, Yao S, Zong C, Luo Y, Liu J, Yu Y, Sang H, Wang X. Hydrogen decreases athero-susceptibility in apolipoprotein B-containing lipoproteins and aorta of apolipoprotein E knockout mice. Atherosclerosis. 2012;221:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Ogawa R, Akaishi S, Kuribayashi S, Miyashita T. Keloids and Hypertrophic Scars Can Now Be Cured Completely: Recent Progress in Our Understanding of the Pathogenesis of Keloids and Hypertrophic Scars and the Most Promising Current Therapeutic Strategy. J Nippon Med Sch. 2016;83:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Kim K, Son D, Kim J. Radiation Therapy Following Total Keloidectomy: A Retrospective Study over 11 Years. Arch Plast Surg. 2015;42:588-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, Vowden K, Vowden P. Health economic burden that different wound types impose on the UK's National Health Service. Int Wound J. 2017;14:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 19. | Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1002] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 20. | Jee JP, Pangeni R, Jha SK, Byun Y, Park JW. Preparation and in vivo evaluation of a topical hydrogel system incorporating highly skin-permeable growth factors, quercetin, and oxygen carriers for enhanced diabetic wound-healing therapy. Int J Nanomedicine. 2019;14:5449-5475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Kaur P, Gondil VS, Chhibber S. A novel wound dressing consisting of PVA-SA hybrid hydrogel membrane for topical delivery of bacteriophages and antibiotics. Int J Pharm. 2019;572:118779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Han G, Ceilley R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther. 2017;34:599-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 763] [Cited by in RCA: 1197] [Article Influence: 149.6] [Reference Citation Analysis (0)] |
| 23. | Guo SX, Jin YY, Fang Q, You CG, Wang XG, Hu XL, Han CM. Beneficial effects of hydrogen-rich saline on early burn-wound progression in rats. PLoS One. 2015;10:e0124897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Fang Y, Fu XJ, Gu C, Xu P, Wang Y, Yu WR, Sun Q, Sun XJ, Yao M. Hydrogen-rich saline protects against acute lung injury induced by extensive burn in rat model. J Burn Care Res. 2011;32:e82-e91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Liu C, Kurokawa R, Fujino M, Hirano S, Sato B, Li XK. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci Rep. 2014;4:5485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Hao Y, Dong X, Liu H, Wang Y. Preconditioning with one-time hydrogen gas does not attenuate skin flap ischemia-reperfusion injury in rat models. J Plast Reconstr Aesthet Surg. 2019;72:1661-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Tian R, Hou Z, Hao S, Wu W, Mao X, Tao X, Lu T, Liu B. Hydrogen-rich water attenuates brain damage and inflammation after traumatic brain injury in rats. Brain Res. 2016;1637:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Chen O, Cao Z, Li H, Ye Z, Zhang R, Zhang N, Huang J, Zhang T, Wang L, Han L, Liu W, Sun X. High-concentration hydrogen protects mouse heart against ischemia/reperfusion injury through activation of thePI3K/Akt1 pathway. Sci Rep. 2017;7:14871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |