Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7502
Peer-review started: December 20, 2021
First decision: April 8, 2022
Revised: April 17, 2022
Accepted: June 3, 2022
Article in press: June 3, 2022
Published online: July 26, 2022
Processing time: 202 Days and 20 Hours
Diffuse large B-cell lymphoma (DLBCL) is a common aggressive non-Hodgkin's lymphoma (NHL), accounting for 30%-40% of adult NHL. Primary testicular (PT) lymphoma is an uncommon extranodal disease representing approximately 1%-2% of lymphoma. Approximately 30%–40% of patients are refractory to frontline therapy or relapse after complete remission. Refractory DLBCL responds poorly to other lines of chemotherapy, and experiences short-term survival.
We present a 41-year-old male patient who was diagnosed with PT-DLBCL. Further disease progression was observed after multiline chemotherapy. Chimeric antigen receptor T cells (CAR-T) therapy salvaged the patient. Unfortunately, a new mass was observed in the right adrenal area after six months. The patient was administered programmed cell death protein-1 (PD-1) inhibitor therapy and maintained progression-free survival at more than 17 mo of follow-up.
Our findings support the potential benefit of CAR-T combined with PD-1 inhibitor therapies in this type of relapsed and refractory PT-DLBCL.
Core Tip: Primary testicular diffuse large B-cell lymphoma (DLBCL) is an uncommon extranodal disease of lymphomas. Refractory DLBCL responds poorly to other lines of chemotherapy, and is associated with short-term survival. Herein, we report one rare case of chimeric antigen receptor T cells (CAR-T) combined with programmed cell-death protein-1 (PD-1) inhibitor to treat refractory DLBCL in a 41-year-old male. Our findings support the potential benefit of CAR-T combined with PD-1 inhibitor therapies in this type of refractory DLBCL.
- Citation: Zhang CJ, Zhang JY, Li LJ, Xu NW. Refractory lymphoma treated with chimeric antigen receptor T cells combined with programmed cell death-1 inhibitor: A case report. World J Clin Cases 2022; 10(21): 7502-7508
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7502.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7502
Primary testicular lymphoma (PTL) is a rare lymphoma with a poor prognosis and poor response to conventional chemotherapy. PLT represents approximately 1%-2% of lymphomas[1]. Approximately 30%–40% of patients are refractory to frontline therapy or relapse after complete remission[2]. Chimeric antigen receptor T cells (CAR-T) have a significant effect on recurrent refractory lymphoma, with good effects on most clinical manifestations in the early stage but for a short duration. The use of programmed cell death protein-1 (PD-1) inhibitors improves tumor immunity in the microenvironment and the immune efficacy of CAR-T cells. It is unclear whether it has unique clinical and biological characteristics, and the therapeutic mechanism needs further study. Therefore, we report a case of primary testicular diffuse large B-cell lymphoma (PT-DLBCL) treated with a PD-1 inhibitor after CAR-T therapy. The clinicopathological characteristics and the mechanism of CRT-T combined with PD-1 inhibitor therapy are discussed based on relevant literature, which helps to improve clinical understanding.
A 41-year-old man with an 8-mo history of right testicular enlargement.
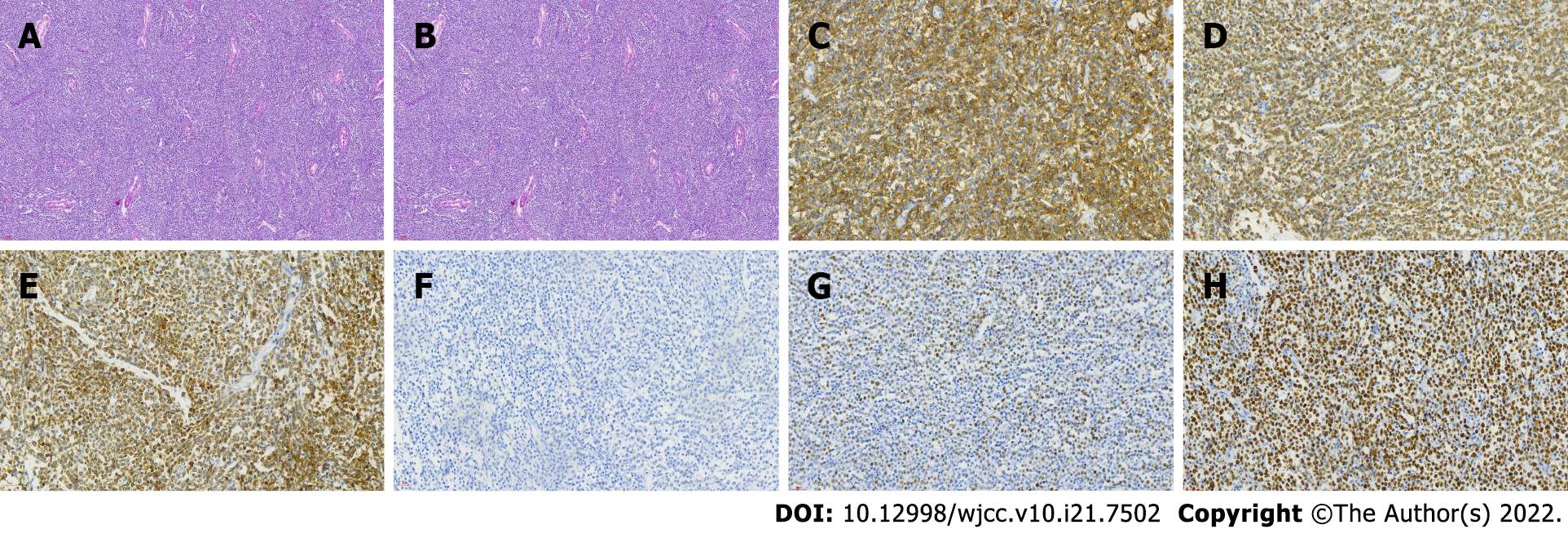
On June 29, 2018, he underwent right orchiectomy and right inguinal lymph node biopsy. He had no history of trauma, fever, or other complaints. Physical examination showed unilateral enlargement of the right testicle without any superficial lymph node enlargement. The patient received a right orchidectomy. The histopathological diagnosis of DLBCL (non-GCB) was rendered. Hematoxylin and eosin-stained sections showed diffuse proliferation of medium-sized round cells. Immunohistochemistry revealed that the neoplastic cells expressed CD19, CD20, CD79a, and CD21 and were negative for CD3, CD5, CD10, CyclinD1, and ALK. Ki-67 was positive in 80% of tumor cells. BCL-2 was positive in 80% of tumor cells. BCL-6 was partially positive. C-myc was positive in 60% of tumor cells (Figure 1). The patient was diagnosed with DLBCL (non-GCB) IV. On July 13, 2018, four cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) were given at the local hospital. A follow-up abdominal computed tomography (CT) scan showed that the lymph nodes continued to enlarge beside the right iliac vessels. On November 13, 2018, a positron emission tomography/CT (PET-CT) scan showed multiple enlarged lymph nodes (4.3 cm × 2.7 cm) beside the right iliac vessels (Figure 2A). Two cycles of R2-HyperCVADA (lenalidomide, rituximab, cyclophosphamide, doxor
His past medical history included diabetes and cervical spondylosis.
No personal or family history was available.
Physical examination showed no palpable lymph nodes, organomegaly, or cutaneous lesions.
The peripheral blood and biochemical parameters (liver and renal function and serum lactate dehydrogenase level) were within normal limits. Bone marrow (BM) smear and biopsy did not show evidence of involvement by lymphoma cells.
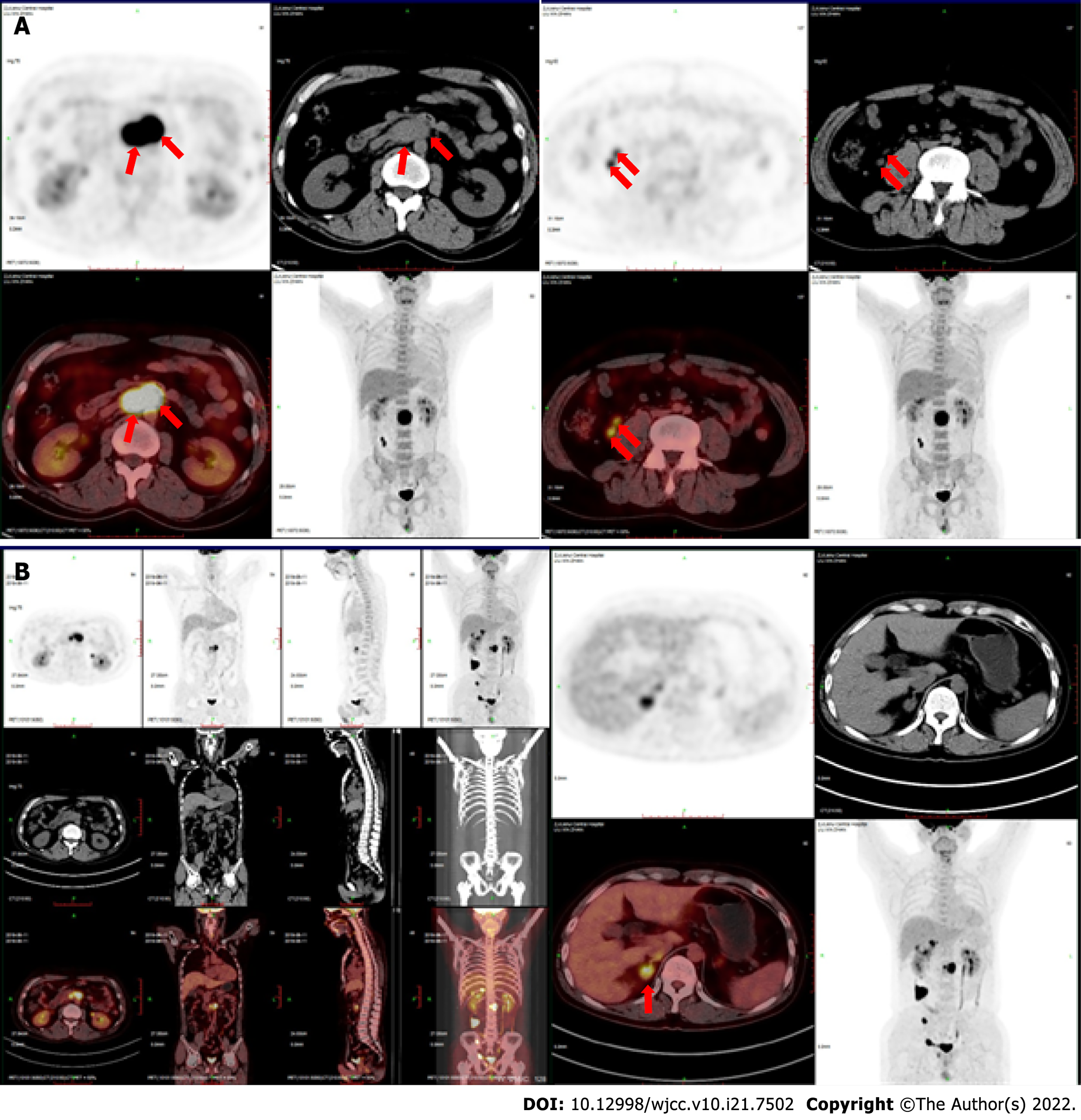
On November 13, 2018, a PET-CT scan showed multiple enlarged lymph nodes (4.3 cm × 2.7 cm) beside the right iliac vessels and a high standard uptake value (SUV) with a Deauville score of 18.4 (Figure 2A).
On July 15, 2019, a follow-up PET-CT scan showed that, in addition to the right iliac vessels, the lymph nodes continued to enlarge (4.3 cm × 3.8 cm). The SUV was high, with a Deauville score of 17. New viable lesions were found in the right adrenal gland, right seminal vesicle gland and surrounding prostate gland, and right groin (Figure 2B).
Based on the above findings, the final diagnosis was made as refractory PT-DLBCL, stage IVB.
On July 24, 2019, the patient was transferred to the First Affiliated Hospital of Zhejiang University and treated with CAR-T cells at a dose of 5 × 106/kg. Lymph node size significantly reduced after CAR-T therapy. Unfortunately, in June 2020, an abdominal CT scan showed a new mass in the right adrenal area with a size of approximately 2.8 cm × 1.3 cm. On June 11, 2020, PD-1 blockade therapy with sintilimab (100 mg once every 3 wk) commenced. The mass shrank soon after the sintilimab injection.
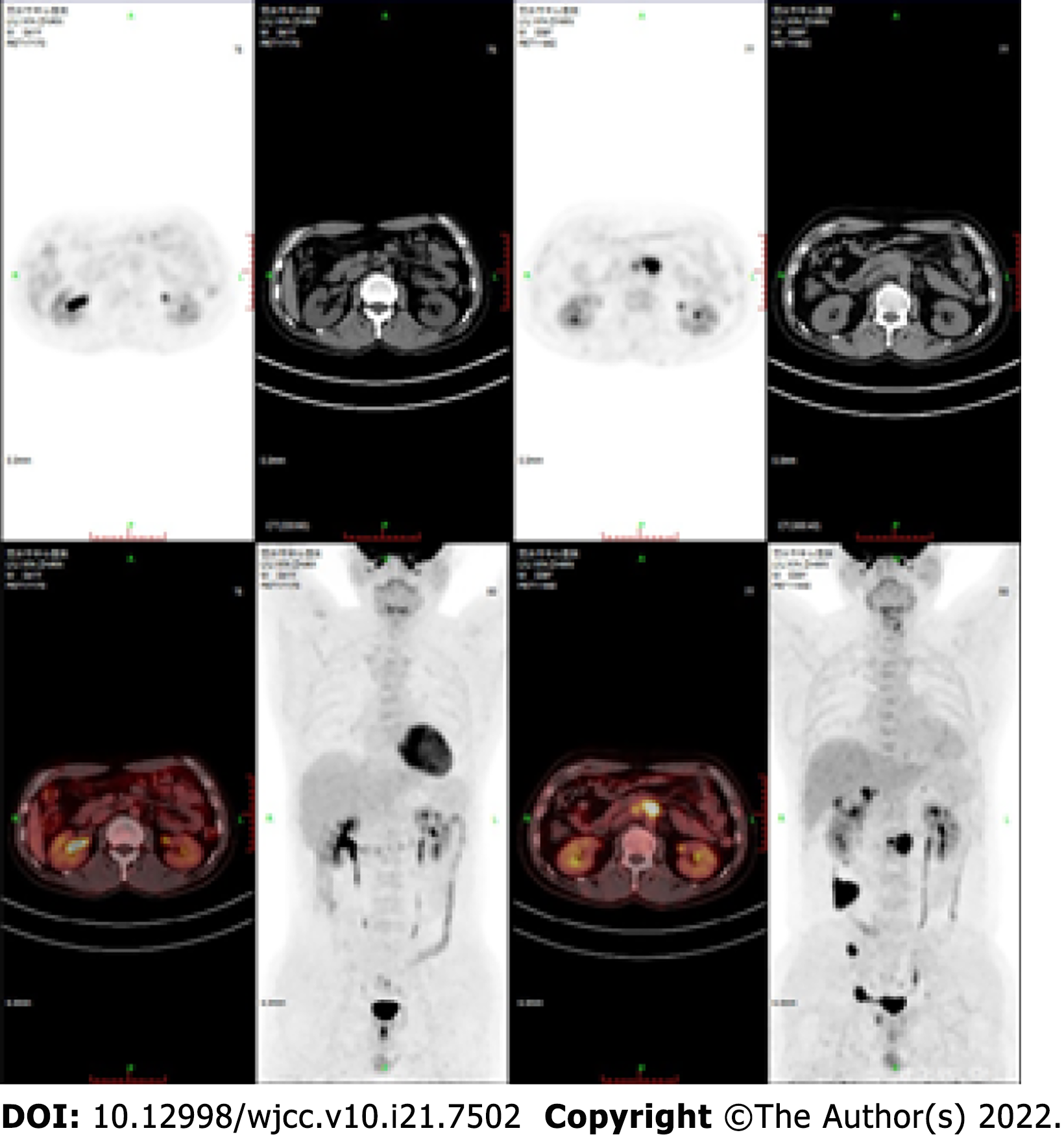
A year later, a PET-CT scan showed no viable lesions (Figure 3). CAR copies were 9574.28 cells/L on July 6, 2021. He has maintained complete remission until now.
PTL is a hematological malignancy with a low clinical incidence of 0.26 cases per 100000 person-years[3]. PTL accounts for approximately 1%-7% of testicular malignancies and approximately 1%-2% of all lymphomas[1]. PTL is the most common lymphoma among males over 60 years of age. The most common histopathological DLBCL in PTL, which accounts for approximately 80%-90% of all PTLs, is called PT-DLBCL[4]. The primary clinical manifestation of PT-DLBCL is a painless enlargement of testicular tissue with occasional fever and night sweats, and weight loss. PT-DLBCL is highly aggressive and has a poor prognosis, with a median survival of 1-2 years, especially in patients with late clinical stages (stages III-IV), usually presenting with systemic multivisceral involvement within 2 years[5].
The clinical characteristics of PT-DLBCL are low incidence, high aggressiveness and complex treatment, which may explain the lack of a standard treatment at present. The recommended conventional treatment is chemotherapy after orchiectomy, which cures 60%-70% of patients[6]. However, a small number of patients have a poor response to treatment, with frequent recurrence and poor prognosis. Prognostic factors regarding testicular lymphoma have been suggested in several large retrospective reports[3]. In 2003, the International Extranodal Lymphoma Study Group suggested that advanced age, advanced stage, a high IPI score, elevated lactate dehydrogenase, and the absence of surgery or radiation therapy were significantly associated with poor prognosis[7]. In 2012, Richie[8] found that first-line treatment with R-CHOP, IT-MTX and testicular radiotherapy could improve the prognosis, while non-GCB cell phenotypes had a worse prognosis than GCB cell phenotypes[8]. In 2018, Ollila and Olszewski[9] found that patients with B symptoms, intranodal lymphoma, and concurrent MYC, BCL-2, or BCL-6 rearrangements ("double hit" or "triple hit") generally have a poor prognosis[9]. In this patient, advanced age, a high IPI score, elevated lactate dehydrogenase, and the fact that lymphoma persisted after multiple first-line chemotherapies suggested a poor prognosis. Therefore, second-line treatment can be selected according to 2021 NCNN guidelines[10], and CAR-T treatment can be given in combination with therapy depending on the patient's family’s economic situation.
In recent years, there has been an increase in tumor immunotherapy use, especially CAR-T, which have been widely used to treat hematological tumors. CAR-T has an efficiency of approximately 80% and an OS rate of 52% at 18 mo in refractory large B-cell lymphoma[11]. The principle of CAR-T therapy is to genetically modify T lymphocytes to express a specific receptor (CAR) to target and bind specific antigens so that T cells can specifically recognize tumor cells and kill tumors[12]. In contrast to T cells under normal conditions, CAR-T cell recognition bypasses the antigen presentation phase. It thus is not restricted by MHC molecules, preventing cancer cells from escaping immune system recognition due to the downregulation of tumor MHC molecules[13]. CAR-T cell therapy can significantly improve the remission rate of relapsed refractory lymphomas, but some patients fail to achieve the desired outcome.
In the disease state, tumor cells upregulate the expression of immune checkpoints by immunosuppressive cells and bind to corresponding sites on T cells. This inhibits the killing activity of T cells and helps the cancer cells evade immune monitoring and attack from the body, thereby promoting their survival[14]. PD-1 is an immune checkpoint protein expressed on T cells. PD-1 binding to the receptor induces phosphorylation, which inhibits downstream activation of the T-cell receptor, limits T-cell proliferation activity and reduces its killing effect on tumor cells. In addition, the immunosuppressive effect of PD-1 Limits T cells. It affects the function of other lymphocyte subsets, such as promoting the proliferation and immunosuppression of regulatory T cells (Tregs) and inhibiting the activity of B cells and natural killer cells. Therefore, blocking the PD-1/programmed cell death ligand 1 (PD-L1) pathway increases the number of T cells and enhances cytokine secretion and reduces Treg cells and BM-derived suppressor cells to alter the inhibitory tumor microenvironment[15]. CAR-T cell therapy works by enhancing the antitumor capacity of T cells. The overexpression of immune checkpoints limits the lethality of T cells. Immune checkpoint inhibitors may enhance the efficacy of CAR-T cell therapy since the inhibition of immune checkpoint expression increases the antitumor ability of T cells. Studies such as that by Cherkassky proved that the inhibition of the PD-1 receptor could weaken the inhibition of the PD-1 pathway in CAR-T cells, thus enhancing the ability of CAR-T cells[16]. A study showed that PD-L1 expression was upregulated in hepatocellular carcinoma cells exposed to GPC3 CAR-T cells, and the antitumor activity of CAR-T cells could be enhanced by the knockdown of the PD-1 gene[17]. Chong et al[18] reported a patient with refractory and recurrent DLBCL. He received a PD-1 inhibitor 28 d after CAR-T cell treatment, after which the tumor cells shrank significantly. The patient was followed up for 12 mo, at which point sustained remission was achieved[18]. Wang et al[19] also reported a case of refractory follicular lymphoma (FL) treatment. After 6 cycles of chemotherapy, the patient was diagnosed with refractory FL, and the results were poor. The patient was treated with CD19 CAR-T cells in combination with a reduced dose of nivolumab. To date, the patient has maintained CR for 16 mo[19]. Zhang et al[20] showed a case of refractory DLBCL that developed disease progression after 12 wk of CAR-T cell treatment. Then, the patient was treated with a PD-1 inhibitor. To date, the patient has maintained CR[20]. Relevant literature reports were made by retrieving relevant literature at home and abroad (Table 1). In this article, the patient relapsed 6 mo after CAR-T cell therapy. PD-1 inhibitors were still effective, and CAR-T cells could still be detected in the patient 2 years later. These findings suggest that PD-1 inhibitors may affect the efficacy of CAR-T cell therapy in the tumor microenvironment of immune suppression.
CAR-T cells have been widely used to treat hematological malignancies, but their associated remission rates still need improvement. Immune checkpoint inhibitors can vastly alter the immunosuppressive microenvironment where CAR-T cells live, improving their proliferative activity and antitumor capacity and increasing the prognosis of relapsed refractory tumors. Even after the failure of CAR-T therapy, the choice of PD-1 inhibitor therapy may still be effective. Our center will continue to treat patients who have failed CAR-T therapy with PD-1 inhibitors to explore the therapeutic feasibility of this treatment option and to provide new treatment strategies for relapsed refractory lymphoma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Watanabe T, Japan A-Editor: Yao QG, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Menter T, Ernst M, Drachneris J, Dirnhofer S, Barghorn A, Went P, Tzankov A. Phenotype profiling of primary testicular diffuse large B-cell lymphomas. Hematol Oncol. 2014;32:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Pfreundschuh M, Kuhnt E, Trümper L, Osterborg A, Trneny M, Shepherd L, Gill DS, Walewski J, Pettengell R, Jaeger U, Zinzani PL, Shpilberg O, Kvaloy S, de Nully Brown P, Stahel R, Milpied N, López-Guillermo A, Poeschel V, Grass S, Loeffler M, Murawski N; MabThera International Trial (MInT) Group. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 578] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 3. | Vannata B, Zucca E. Primary extranodal B-cell lymphoma: current concepts and treatment strategies. Chin Clin Oncol. 2015;4:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 4. | Twa DDW, Mottok A, Savage KJ, Steidl C. The pathobiology of primary testicular diffuse large B-cell lymphoma: Implications for novel therapies. Blood Rev. 2018;32:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Wang C, Wang H, Wang Q, Shi B. Primary testicular lymphoma: experience with 13 cases and literature review. Int J Hematol. 2013;97:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Klapper W, Stoecklein H, Zeynalova S, Ott G, Kosari F, Rosenwald A, Loeffler M, Trümper L, Pfreundschuh M, Siebert R; German High-Grade Non-Hodgkin's Lymphoma Study Group. Structural aberrations affecting the MYC locus indicate a poor prognosis independent of clinical risk factors in diffuse large B-cell lymphomas treated within randomized trials of the German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL). Leukemia. 2008;22:2226-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Christie D, Gracias E, Gospodarowicz MM. Patterns of outcome and prognostic factors in primary bone lymphoma (osteolymphoma): A survey of 499 cases by the International Extranodal Lymphoma Study Group. Haematologica. 2007;92. |
| 8. | Richie JP. Re: First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Urol. 2012;188:115-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol. 2018;19:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 10. | Zelenetz AD, Gordon LI, Chang JE, Christian B, Abramson JS, Advani RH, Bartlett NL, Budde LE, Caimi PF, De Vos S, Dholaria B, Fakhri B, Fayad LE, Glenn MJ, Habermann TM, Hernandez-Ilizaliturri F, Hsi E, Hu B, Kaminski MS, Kelsey CR, Khan N, Krivacic S, LaCasce AS, Lim M, Narkhede M, Rabinovitch R, Ramakrishnan P, Reid E, Roberts KB, Saeed H, Smith SD, Svoboda J, Swinnen LJ, Tuscano J, Vose JM, Dwyer MA, Sundar H. NCCN Guidelines® Insights: B-Cell Lymphomas, Version 5.2021. J Natl Compr Canc Netw. 2021;19:1218-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 11. | Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377:2531-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3408] [Cited by in RCA: 4207] [Article Influence: 525.9] [Reference Citation Analysis (0)] |
| 12. | Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2553] [Cited by in RCA: 2786] [Article Influence: 199.0] [Reference Citation Analysis (0)] |
| 13. | Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168:724-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 790] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 14. | Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2010] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 15. | Zhu X, Lang J. Programmed death-1 pathway blockade produces a synergistic antitumor effect: combined application in ovarian cancer. J Gynecol Oncol. 2017;28:e64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130-3144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 795] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 17. | Liu G, Zhang Q, Li D, Zhang L, Gu Z, Liu J, Liu G, Yang M, Gu J, Cui X, Pan Y, Tian X. PD-1 silencing improves anti-tumor activities of human mesothelin-targeted CAR T cells. Hum Immunol. 2021;82:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129:1039-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 394] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 19. | Wang J, Deng Q, Jiang YY, Zhang R, Zhu HB, Meng JX, Li YM. CAR-T 19 combined with reduced-dose PD-1 blockade therapy for treatment of refractory follicular lymphoma: A case report. Oncol Lett. 2019;18:4415-4420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Zhang L, Zhang W, Zhou D. Long-term treatment response to PD-1 blockade therapy in a patient with DLBCL relapsed after anti-CD19 chimeric antigen receptor T cell treatment. Ann Hematol. 2021;100:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |