Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7474
Peer-review started: November 27, 2021
First decision: January 12, 2022
Revised: January 22, 2022
Accepted: June 3, 2022
Article in press: June 3, 2022
Published online: July 26, 2022
Processing time: 226 Days and 1.3 Hours
Endometrial cancer (EC) is one of the most common cancers of the female reproductive tract, and the incidence is increasing rapidly. Immunotherapy using programmed cell death-1 (PD-1) inhibitors is an emerging research topic and treatment strategy for refractory gynecological malignancies. However, clinical management of EC with checkpoint inhibitors requires improvement. Herein, we discuss a case of refractory proficient mismatch repair (pMMR)/miscrosatellite-stable (MSS) EC treated with a combination of PD-1 and angiogenesis inhibitors and offer a review of the pathophysiology and clinical outcomes based on previous studies.
A 62-year-old woman diagnosed with invasive or metastatic EC in 2015 was treated with six courses of chemotherapy and refused further radiotherapy. Four years later, she developed chest pain, and lung biopsy indicated thyroid transcription factor-1 (-), Napsin A (-), estrogen receptor (+), progesterone receptor (+), anaplastic lymphoma kinase (D5F3) (-), and receptor tyrosine kinase (D4D6) (-) metastatic EC. Genetic testing results showed low tumor mutation burden, pMMR, PD ligand 1 (-), MSS, and HLA-class 1 heterogeneous disease. The patient was started on toripalimab combined with nab-paclitaxel for seven cycles (every 3 wk), but this regimen was terminated because of an intolerable chemotherapy adverse event. The disease progressed in 2020, and the patient’s treatment was switched from nab-paclitaxel to anlotinib, while immunotherapy using toripalimab was continued. The patient achieved a major partial response with well-tolerated toxicities, and treatment is ongoing.
Molecular testing is advised for clinical classifications of EC owing to its high heterogeneity. In this case, the patient had pMMR/MSS EC and achieved a positive outcome with combination PD-1 inhibitor treatment. These results warrant further clinical exploration.
Core Tip: Endometrial cancer (EC) with proficient mismatch repair/miscrosatellite-stable (pMMR/MSS)-type hardly responds to immune checkpoint therapy. This case reported a satisfactory outcome with well-tolerated toxicities using toripalimab combined with anlotinib treatment in an EC patient with pMMR/ MSS-type. Although need further clinical evidence, programmed cell death-1 inhibitor combined anti-angiogenesis therapy may present an option for EC patients with pMMR/MSS-type after multi-line treatment.
- Citation: Zhai CY, Yin LX, Han WD. Programmed cell death-1 inhibitor combination treatment for recurrent proficient mismatch repair/ miscrosatellite-stable type endometrial cancer: A case report. World J Clin Cases 2022; 10(21): 7474-7482
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7474.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7474
Endometrial cancer (EC) is one of the most common gynecological malignancies in women, with 382069 newly confirmed cases reported in 2018 and an occurrence rate that increases annually by an estimated 1% to 2% worldwide[1]. The prognosis of advanced EC is poor. Even though the 5-year survival rate for stage I EC is as high as 95%, the 5-year survival rate for metastatic EC is approximately 17%[2]. Immunotherapies, such as programmed cell death-1 (PD-1) inhibitors, block PD-1 and PD ligand 1 (PD-L1) activities on the cell surface to prevent immune escape and are currently being used to treat refractory gynecological malignancies, but the clinical application of immunotherapy for EC patients remains exploratory. Data from the Keynote-158 and GARNET trials demonstrate that immunotherapy alone offers promising results for patients with mismatch repair-deficient (dMMR)/microsatellite instability-high (MSI-H) advanced EC, with overall response rates (ORRs) of 57.1% and 56%, respectively[3]. However, the objective response rate of patients with proficient mismatch repair/miscrosatellite-stable (pMMR/MSS) malignancies was only 10%[3]. This article presents a case study and preliminary discussion of PD-1 inhibitor immunotherapy combined with chemotherapy or anti-angiogenesis targeted therapy for a patient with pMMR/MSS recurrent EC. We further elaborate the molecular classification, choice of treatment regimen, and therapeutic efficacy.
A 67-year-old female presented to our hospital with complaint of 5 years and 7 mo after endometrial carcinoma hysterectomy and a dull chest pain for 1 wk.
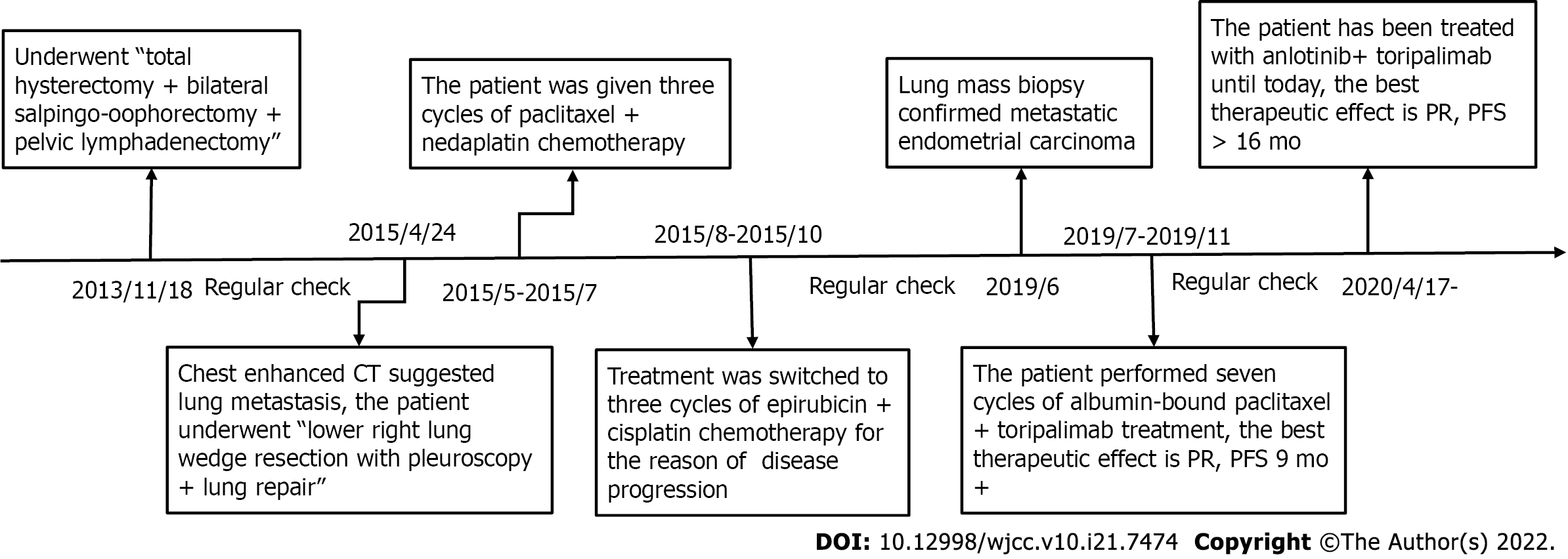
In November 2013, a female patient experienced “uterine cavity abnormality for 2 d” and went to an outpatient center, where she underwent total hysterectomy + bilateral salpingo-oophorectomy + pelvic lymphadenectomy on November 18, 2013. The postoperative pathology indicated Grade I endometrioid adenocarcinoma with squamous differentiation and superficial muscular infiltration, and all 16 Lymph nodes were negative. The patient underwent regular check-ups thereafter. On April 20, 2015, the patient was diagnosed with lung metastases by enhanced chest computed tomography (CT) scans, which suggested multiple nodules in both lungs. Four days later, the patient underwent lower right lung wedge resection with pleuroscopy + lung repair. Postoperative pathology showed adenocarcinoma infiltration or metastasis (0.6 cm × 0.5 cm). Three cycles of paclitaxel + nedaplatin chemotherapy were administered before the patient presented with disease progression, after which treatment was switched to three cycles of epirubicin + cisplatin chemotherapy. After chemotherapy, additional radiotherapy was recommended by the physician but refused by the patient. In June 2019, the patient visited our hospital due to a dull chest pain.
The patient had a ten-year history of hypertension and diabetes but had no history of trauma or surgery.
The patient’s height and weight were 156 cm and 58 kg, respectively, with a body mass index of 23.8 kg/m². No enlargement of lymph node was found in the superficial lymph nodes. Breath sounds were low in both lungs. Heart auscultation revealed a regular rhythm, normal heart sounds without murmur. Two surgical scars can be seen in the chest and abdomen. The abdomen was soft without tenderness, and the liver and spleen were not palpable under the ribs. There was no percussion pain in the liver area. Shifting dullness was negative, and bowel sounds were normal.
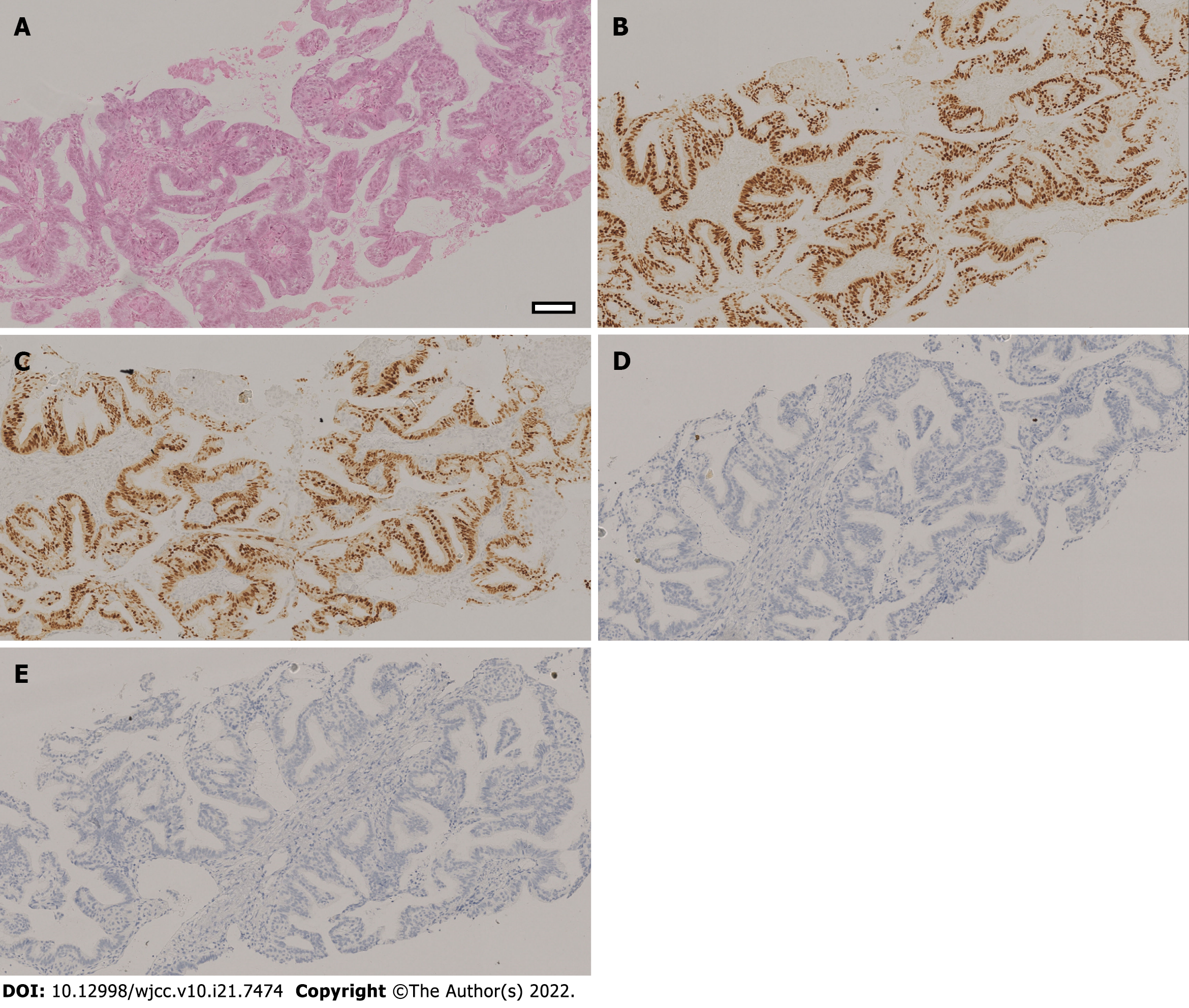
The patient underwent a tumor marker exam to find that the levels of carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), carbohydrate antigen 19-9 (CA19-9) were all within normal limits. None of the other laboratory values were considered clinically significant. Lung biopsy confirmed thyroid transcription factor-1 (TTF-1) (-), Napsin A (-), estrogen receptor (ER) (+), progesterone receptor (PR) (+), anaplastic lymphoma kinase (ALK, D5F3) (-), and receptor tyrosine kinase (ROS1, D4D6) (-) metastatic endometrial carcinoma (Figure 1). Genomic sequencing results demonstrated polymerase-epsilon (POLE) (-) tumor pathology, mouse double minute 4 (MDM4) amplification, v-Akt murine thymoma viral oncogene homolog 1 (AKT1) missense mutation, CTNNB1 missense mutation, TP53 (-), 2.09 Muts/Mb [tumor mutation burden-low (TMB-L)], pMMR, PD-L1 (-), MSS, and heterozygous HLA-1 (Table 1).
| Tests | Results |
| Next generation sequencing | |
| PD-L1 expression | Negative |
| Mismatch repair | pMMR |
| Tumor mutation burden | 2.09 Muts/Mb |
| MicroSatelite Instability | MSS |
| Human leukocyte antigen typing | Heterogeneous |
| Tumor neoantigens | 6 |
| POLE | Negative |
| TP53 | Negative |
| Predictive/prognostic biomarkers | |
| MDM4 | 4 amplifications |
| AKT1 | 83.05% p. Glu17 Lys mutation |
| CTNNB1 | 37.06% p. Ser37Ala mutation |
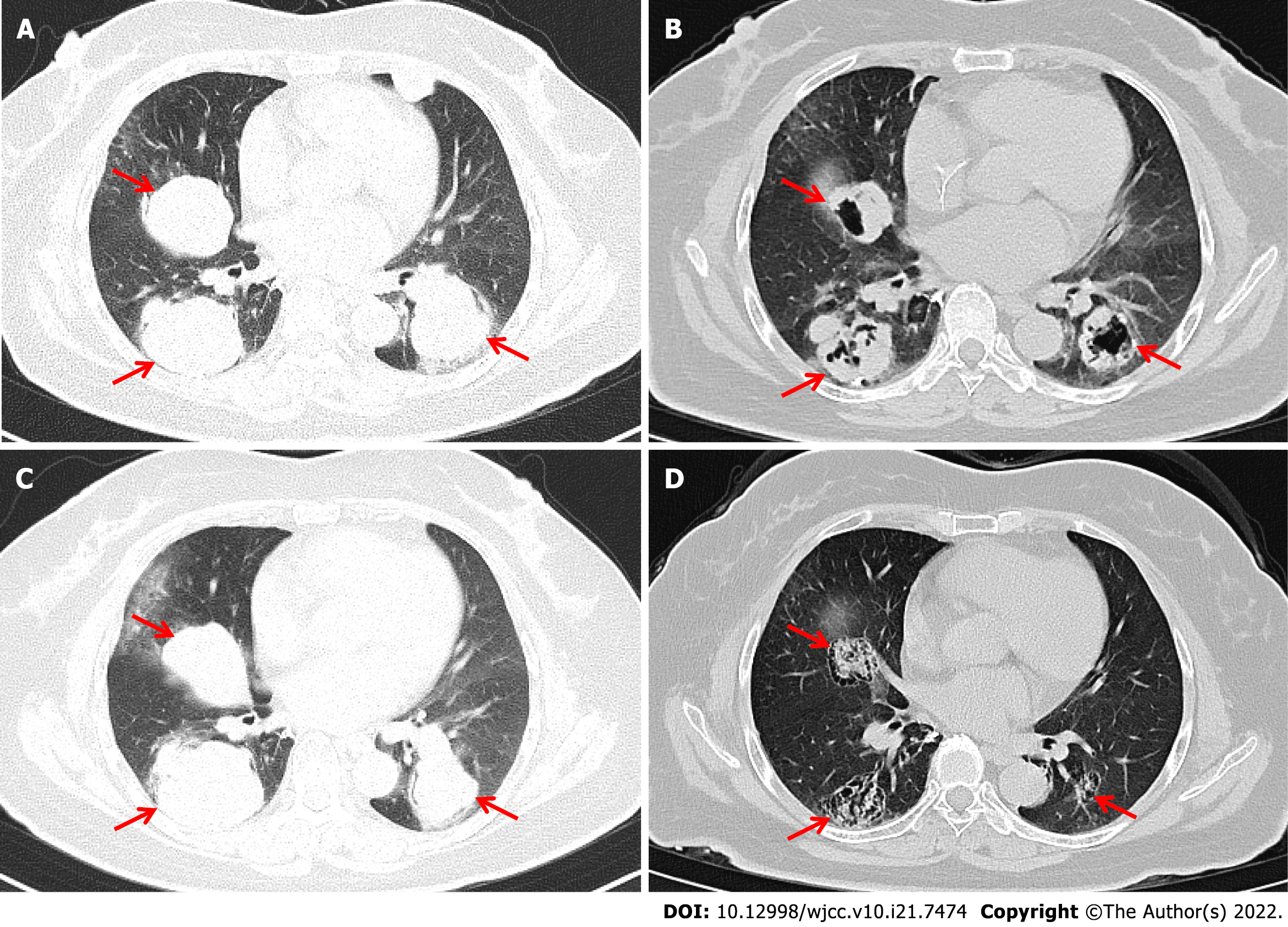
Enhanced chest CT showed multiple nodules and mass shadows in both lungs after the right lung operation, suggesting metastasis (Figure 2A).
The patient was diagnosed with endometrial cancer with lung metastases (Stage IV; MSS).
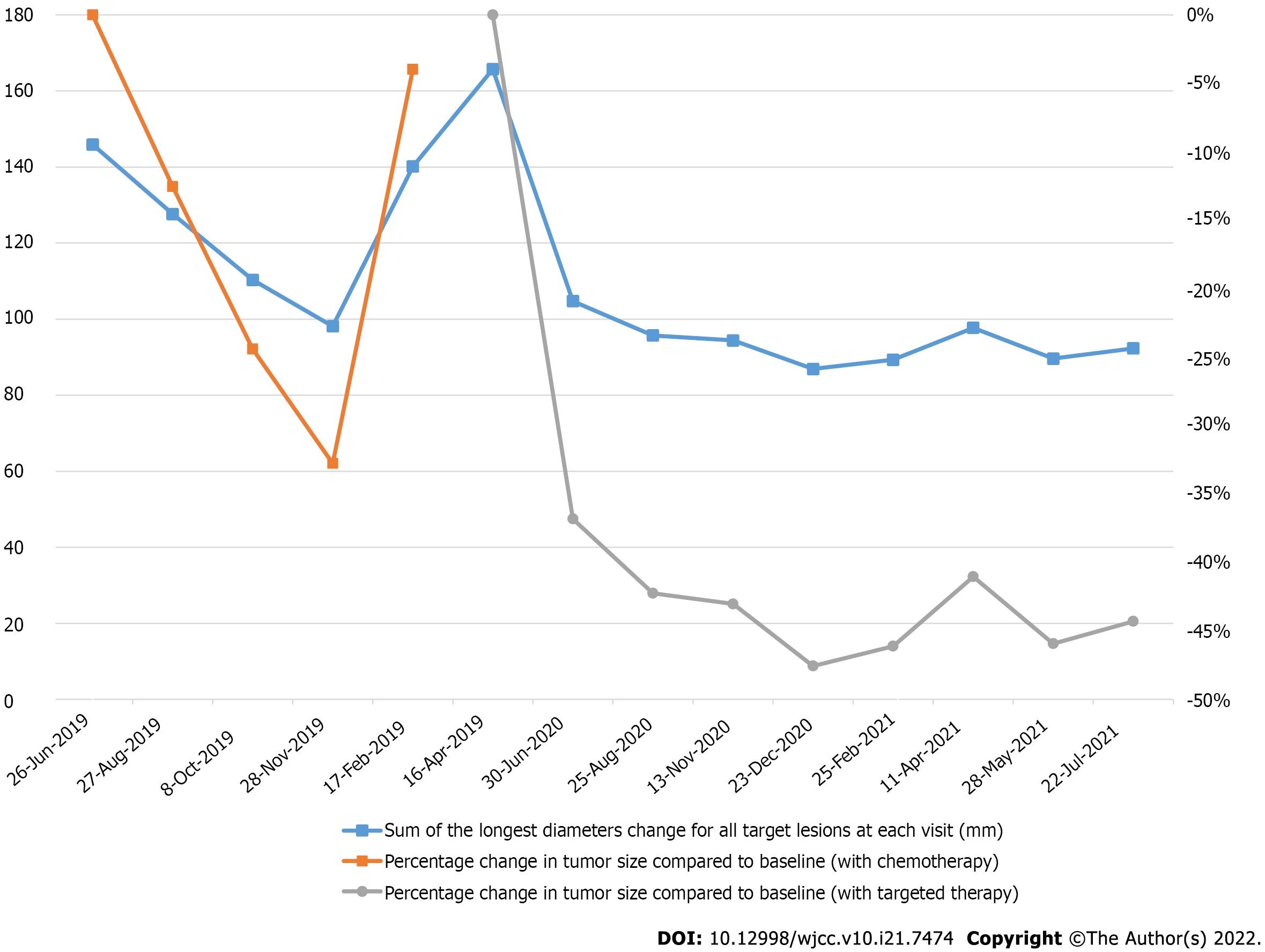
The patient was given seven cycles of albumin-bound paclitaxel + toripalimab; specifically, 240 mg toripalimab via intravenous drip infusion every 3 wk + 200 mg albumin-bound paclitaxel via intravenous drip infusion on Days 1 and 8 between July 5 and November 29, 2019. On June 26, 2019, baseline chest CT results showed that the sizes of the three target lung lesions were 47.4 mm, 50.1 mm, and 48.4 mm. On November 28, 2019, these tumors had shrunk to 28.9 mm, 37.2 mm, and 32.0 mm, respectively, after 6 cycles of treatments. The sum of the maximum diameter of the target lesions was reduced from 145.9 mm to 98.1 mm with a reduction rate of 32.8%, which meets the RECIST definition for partial response (PR). Unfortunately, treatment was discontinued because of the neurotoxicity of albumin-bound paclitaxel, and the patient refused to continue using a PD-1 inhibitor for immunotherapy. Progression-free survival (PFS1) lasted 9.7 mo, and the patient had regular follow-ups afterward. On April 16, 2020, a follow-up chest CT check suggested disease progression. According to the results of Keynote-146, pembrolizumab combined with lenvatinib has an ORR rate of 36.8% in patients with pMMR/MSS advanced EC. Accordingly, we believed that the patient might benefit from China’s own PD-1 checkpoint inhibitor combined with an antiangiogenic medication. The patient was administered anlotinib, 12 mg orally for two weeks, stopped for one week combined with toripalimab, 240 mg intravenous drip every 3 wk starting on April 17, 2020, until present (August 28, 2021). The patient’s diagnosis and treatment timeline are shown in Figure 2.
On April 16, 2020, chest CT results showed that the sizes of the target lung lesions were 57.6 mm, 56.9 mm, and 51.2 mm and had rapidly reduced to 37.4 mm, 39.5 mm, and 27.8 mm, respectively, on June 30, 2020. After 2 cycles of treatment, the sum of the maximum diameter of the target lesions decreased from 165.7 mm to 104.7 mm with a reduction rate of 36.8%, which meets PR. Regular examination showed that massive necrosis was clearly observed (Figure 3) and the therapeutic effect was long-lasting (Figure 4). The treatment is ongoing, and PFS2 has lasted more than 16 mo. During treatment, the patient developed anlotinib-related common terminology criteria for adverse events (CTCAE) grade 1 gingival bleeding, CTCAE grade 2 joint dull pain, and perineal skin ulceration, which were improved after short-term withdrawal and management of symptoms.
EC exhibits high heterogeneity in its molecular, biological, and pathological aspects. Endocrine therapy and chemotherapy are the primary treatment strategies for advanced, recurrent, and metastatic EC. However, the ORRs are not high, and the median PFS remains at approximately 1 year[4]. Traditional pathological classifications divide EC into types I and II. Type I EC is estrogen-dependent and accounts for 60% to 70% of all EC cases[5]. Type I primarily includes endometrioid adenocarcinoma and some rare types, such as the one we reported, in which the patient had endometrioid adenocarcinoma with squamous cells. This type of EC has a fairly good prognosis. Type II EC is hormone-independent and highly invasive and usually has a poor prognosis. However, the traditional pathological classification has certain limitations. For example, certain high-grade (G3) endometrioid and serous carcinomas are hard to distinguish morphologically. Traditional classifications have certain shortcomings when used as clinical risk predictions: Estrogen levels are positively correlated with the mortality risk of type I and II EC; the prognosis of patients with the same type could be very different, while molecular tests of the two types can show overlapping results. In 2013, The Cancer Genome Atlas of the United States proposed a molecular classification of EC based on whole-genome sequencing analysis. EC is divided into four types: Hypermutation of DNA polymerase E (POLE), MSI, low copy number/MSS, and high copy number[6]. In this case, genetic testing results based on a high-throughput sequencing platform indicated POLE (-), CTNNB1 missense mutations, TP53 (-), TMB-L, and MSS; thus, the patient belonged to the low copy number/MSS category.
Immunotherapy is currently being used to treat refractory gynecological malignant cancers. In 2019, pembrolizumab was included in the NCCN Clinical Practice Guidelines to treat patients with MSI-H/dMMR-type recurrent or metastatic endometrial cancer who failed previous treatments[7]. However, MSI-H/dMMR-type endometrial cancer only accounts for 25% to 30% of the cases, while the rest are pMMR/MSS-type endometrial cancer, which hardly responds to immune checkpoint inhibitor monotherapy[7]. In this case, the patient had endometrial carcinoma with TMB-L, PD-L1 (-), and MSS, suggesting limited effects of immunotherapy. MDM4 amplification also indicated high potential for drug resistance and disease hyperprogression[8]. However, with heterozygous HLA-1, we decided to administer a PD-1 inhibitor. After sufficient communication with the patient and her family, we decided to use albumin-bound paclitaxel combined with toripalimab. Toripalimab is a recombinant humanized anti-PD-1 monoclonal antibody developed by Shanghai Junshi Bioscience Co., Ltd. (Shanghai, China). It binds to PD-1 on the surface of T cells and blocks its binding to the ligands PD-L1 and PD-L2 on tumor cells, therefore reversing the immunosuppression of the PD-1 signaling pathway and activating T cell functions to inhibit tumor growth[9]. After the patient was treated with chemotherapy combined with PD-1 monoclonal antibody, satisfactory results were achieved, and the patient achieved PR. However, we had to discontinue treatment due to peripheral neurotoxicity caused by chemotherapy.
Vascular endothelial growth factor receptor (VEGFR) activation leads to angiogenesis, which plays a key role in EC growth and metastasis[10]. Patients with high VEGFR expression often have poor prognosis[10]. Moreover, blocking the vascular endothelial growth factor (VEGF) pathway combined with anti-PD-1 monoclonal antibody therapy could have synergistic effects[11]. With the success of Keynote-146, the FDA approved lenvatinib combined with pembrolizumab as a second-line treatment of EC with systemic treatment failure, with no effective surgery or radiotherapy, and not the MSI-H/dMMR-type[12] of EC. Anlotinib is a small molecule multitarget tyrosine kinase inhibitor that can inhibit kinases such as VEGFR, PDGFR, FGFR, and c-Kit and thus has antiangiogenesis and tumor growth inhibition activities[13]. Studies have shown that anlotinib can inhibit PD-L1 expression by vascular endothelial cells, improve the immune component of the tumor microenvironment, and induce and enhance antitumor CD8+ T lymphocyte infiltration, all of which provide a better tumor microenvironment for immunotherapy[13]. Considering the severe adverse reactions of chemotherapy, the patient was switched to anlotinib + toripalimab. This combination also reduces the possibility of tumor hyperprogression from using immunotherapy alone. Furthermore, anlotinib compensates for the slow onset of the immune checkpoint inhibitor, and the CT scan showed PR after two treatment cycles. PR status remained, and PFS has reached more than 16 mo to date. This successful result also demonstrates the feasibility of using China’s own checkpoint inhibitors with antiangiogenesis for treating patients with pMMR/MSS EC. Compared with imported medications, the significant economic advantage of domestic drugs can also reduce some of the financial burden on patients.
Although the combination of pembrolizumab and lenvatinib has remarkable therapeutic effects, this combination has a fairly high incidence of adverse events, with 97% experiencing treatment-related adverse events (TRAEs), among which 66.9% are grade 3 and above[14]. It is necessary to pay close attention to adverse events in clinical applications so that responses can be made for the best treatment results. In this case, the patient only experienced CTCAE grade 1 gingival bleeding, CTCAE grade 2 joint pain, and perineal skin ulceration, which were improved after short-term drug withdrawal and management of symptoms. In our previous study of advanced lung cancer, five patients were treated with anlotinib and toripalimab[15]. Three cases of CTCAE grade 1 pneumonitis and one case of CTCAE grade 2 asthenia and low appetite were reported. All adverse events were controlled through dose adjustment, medication suspension, and supportive treatment. Our previous study and this case both suggest that the combination of anlotinib with toripalimab presents safety advantages compared with other antitumor treatments.
In summary, EC is highly heterogeneous and requires molecular classification in clinical applications. For patients with pMMR/MSS EC, this case demonstrated satisfactory results using China’s own PD-1 inhibitor, toripalimab, and combined anti-angiogenesis treatment. Thus, these important findings deserve further clinical exploration.
In summary, EC is highly heterogeneous and requires molecular classification in clinical applications. For patients with pMMR/MSS EC, this case demonstrated satisfactory results using China’s own PD-1 inhibitor, toripalimab, and combined anti-angiogenesis treatment. Thus, these important findings deserve further clinical exploration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozden F, Turkey; Tanabe H, Japan A-Editor: Lin FY, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Vergote I, Powell MA, Teneriello MG, Miller DS, Garcia AA, Mikheeva ON, Bidzinski M, Cebotaru CL, Dutcus CE, Ren M, Kadowaki T, Funahashi Y, Penson RT. Second-line lenvatinib in patients with recurrent endometrial cancer. Gynecol Oncol. 2020;156:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Rousset-Rouviere S, Rochigneux P, Chrétien AS, Fattori S, Gorvel L, Provansal M, Lambaudie E, Olive D, Sabatier R. Endometrial Carcinoma: Immune Microenvironment and Emerging Treatments in Immuno-Oncology. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Miller DS, Filiaci VL, Mannel RS, Cohn DE, Matsumoto T, Tewari KS, DiSilvestro P, Pearl ML, Argenta PA, Powell MA, Zweizig SL, Warshal DP, Hanjani P, Carney ME, Huang H, Cella D, Zaino R, Fleming GF. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J Clin Oncol. 2020;38:3841-3850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 5. | Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15:e268-e278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 450] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 6. | Uppendahl L, Mullany SA, Winterhoff B. Molecular characterization of endometrial cancer and therapeutic implications. Curr Opin Obstet Gynecol. 2017;29:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Arora S, Balasubramaniam S, Zhang W, Zhang L, Sridhara R, Spillman D, Mathai JP, Scott B, Golding SJ, Coory M, Pazdur R, Beaver JA. FDA Approval Summary: Pembrolizumab plus Lenvatinib for Endometrial Carcinoma, a Collaborative International Review under Project Orbis. Clin Cancer Res. 2020;26:5062-5067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Depreeuw J, Stelloo E, Osse EM, Creutzberg CL, Nout RA, Moisse M, Garcia-Dios DA, Dewaele M, Willekens K, Marine JC, Matias-Guiu X, Amant F, Lambrechts D, Bosse T. Amplification of 1q32.1 Refines the Molecular Classification of Endometrial Carcinoma. Clin Cancer Res. 2017;23:7232-7241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Keam SJ. Toripalimab: First Global Approval. Drugs. 2019;79:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Umemoto M, Sakamoto T, Sato S, Mizunuma H, Smith SK. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer. 2003;88:237-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Zhu N, Weng S, Wang J, Chen J, Yu L, Fang X, Yuan Y. Preclinical rationale and clinical efficacy of antiangiogenic therapy and immune checkpoint blockade combination therapy in urogenital tumors. J Cancer Res Clin Oncol. 2019;145:3021-3036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Ackroyd SA, Huang ES, Kurnit KC, Lee NK. Pembrolizumab and lenvatinib versus carboplatin and paclitaxel as first-line therapy for advanced or recurrent endometrial cancer: A Markov analysis. Gynecol Oncol. 2021;162:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Liu S, Qin T, Liu Z, Wang J, Jia Y, Feng Y, Gao Y, Li K. anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 2020;11:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 14. | Taylor MH, Lee CH, Makker V, Rasco D, Dutcus CE, Wu J, Stepan DE, Shumaker RC, Motzer RJ. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J Clin Oncol. 2020;38:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 317] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 15. | Zhai C, Zhang X, Ren L, You L, Pan Q, Pan H, Han W. The Efficacy and Safety of Anlotinib Combined With PD-1 Antibody for Third-Line or Further-Line Treatment of Patients With Advanced Non-Small-Cell Lung Cancer. Front Oncol. 2020;10:619010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |