Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7445
Peer-review started: September 25, 2021
First decision: March 23, 2022
Revised: March 31, 2022
Accepted: June 15, 2022
Article in press: June, 15, 2022
Published online: July 26, 2022
Processing time: 288 Days and 19.1 Hours
Upper limb venous thrombosis (ULVT) is rarer than lower-extremity deep venous thrombosis, and is related to Paget-Schroetter syndrome, central venous catheterization, and malignancy. There are few reports of pulmonary embolism (PE) from upper-extremity vein thrombosis due to surgery. Herein, we report two cases of PE that originated from upper limb venous thrombosis on the surgical side in two patients undergoing modified radical mastectomy for breast cancer. These cases challenge the traditional theory that PE originate only from the lower extremities.
We describe two female patients, aged 68 and 65 years, respectively, who had undergone modified radical mastectomy for breast cancer. They did not have a central venous catheter and did not undergo preoperative neoadjuvant chemotherapy. They were transferred to the intensive care unit due to sympto
ULVT as a source of PE after breast cancer surgery cannot be ignored.
Core Tip: We report two cases of pulmonary embolism (PE) that originated from upper limb venous thrombosis (ULVT) on the side undergoing modified radical mastectomy for breast cancer. Our findings should spur surgeons to pay more attention to upper limb venous thrombosis. These cases challenge the traditional theory that PE originates from the lower extremities. Although there is a lower incidence of clinical PE in patients with upper limb venous thrombosis, since PE is a serious complication that can be potentially life-threatening, ULVT as a source of PE after breast cancer surgery cannot be ignored.
- Citation: Duan Y, Wang GL, Guo X, Yang LL, Tian FG. Acute pulmonary embolism originating from upper limb venous thrombosis following breast cancer surgery: Two case reports. World J Clin Cases 2022; 10(21): 7445-7450
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7445.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7445
The incidence rate of female breast cancer is rising fast and has surpassed lung cancer as the leading cause of global cancer incidence in 2020[1]. Modified radical mastectomy (MRM) has become the standard surgical treatment for breast cancer. However, the frequency and potential morbidity of pulmonary embolism (PE) as a surgical complication in this patient population have not been clearly defined; specifically, the source of the thrombus is unknown. To the best of our knowledge, this is the first report of PE caused by upper limb venous thrombosis (ULVT) after MRM for breast cancer.
Case 1: A 68-year-old woman presented to the Breast Diagnosis and Treatment Center of Shanxi Provincial Cancer Hospital due to a right breast mass that was found in a physical examination a week ago.
Case 2: A 65-year-old Chinese woman was hospitalized in March 2021, mainly due to the discovery of a right breast mass.
Case 1: The body mass index (BMI) of this patient was 24.6 kg/m2, which suggested overweight. Classic clinical symptoms of venous thrombosis including pain and unilateral edema were not present. D-dimer concentration was normal. Therefore, the diagnosis of venous thrombosis was ruled out. She underwent MRM for invasive carcinoma of the right breast. She had not received neoadjuvant chemotherapy or a central venous catheter. However, more advanced age, overweight, hospitalization, and surgery were shown to be associated with an increased risk for venous thrombosis. The patient noticed swelling of the right upper limb, accompanied by mild dyspnoea on the first day after surgery, and her blood oxygen saturation dropped to 89% the following day (nasal cannula oxygen inhalation at 3 L/min). She was transferred to the intensive care unit.
Case 2: The patient had a tumour located at 10 o'clock on the upper outside quadrant of the right breast. The area of the mass was 2 cm × 3 cm, with an irregular shape and unclear boundary. It was 2 cm from the nipple. Ultrasound-guided needle biopsy confirmed invasive carcinoma. There were complaints from the patient of arm swelling or pain and no signs of venous thrombosis on admission. The patient underwent MRM of the right breast. Axillary dissection was performed with levels I and II dissection, and the operation lasted 60 min. Although the operation time was short, more advanced age, overweight (BMI 24 kg/m2), and hospitalization were independent risk factors of developing a subsequent venous thrombosis. After the operation, she developed hypoxemia and was transferred to the intensive care unit.
Case 1: The patient had no previous history of other diseases.
Case 2: The patient had a history of hypertension, and no other significant abnormalities were found.
Cases 1 and 2: Both patients denied the history of similar diseases in close relatives.
Case 1: After admission, the patient’s temperature was 36.0°C, respiratory rate was 25 breaths per minute, and she had tachycardia (heart rate 105 beats/min). Her initial haemodynamics were stable and her blood pressure was 117/91 mmHg. Mild swelling was found on the patient’s right upper limb, while there was no numbness. Her Wells score for PE was 9, which indicated a high probability of PE.
Case 2: On the first day after surgery, the patient had no discomfort. However, she developed hypoxaemia, and routine postoperative testing showed that her blood oxygen saturation had decreased on the second day after surgery.
Case 1: Arterial blood gas analysis showed that the partial pressure of oxygen was 56 mmHg and the partial pressure of carbon dioxide was 28 mmHg. The patient’s D-dimer concentration increased to 979 ng/mL (0-243 ng/mL), compared to only 145 ng/mL before surgery. Cardiac troponin and brain natriuretic peptide were normal. Electrocardiogram was also normal.
Case 2: The partial pressure of blood oxygen was only 46 mmHg without oxygen. In fact, the patient’s breathing rate was nearly 30 times per minute. Her haemodynamics were stable and ECG was normal. Her brain natriuretic peptide increased to 320 pg/mL (0-100 pg/mL). Moreover, D-dimer increased significantly from 178 ng/mL before surgery to 3286 ng/mL (0-243 ng/mL). Her Wells score indicated a high probability of PE.
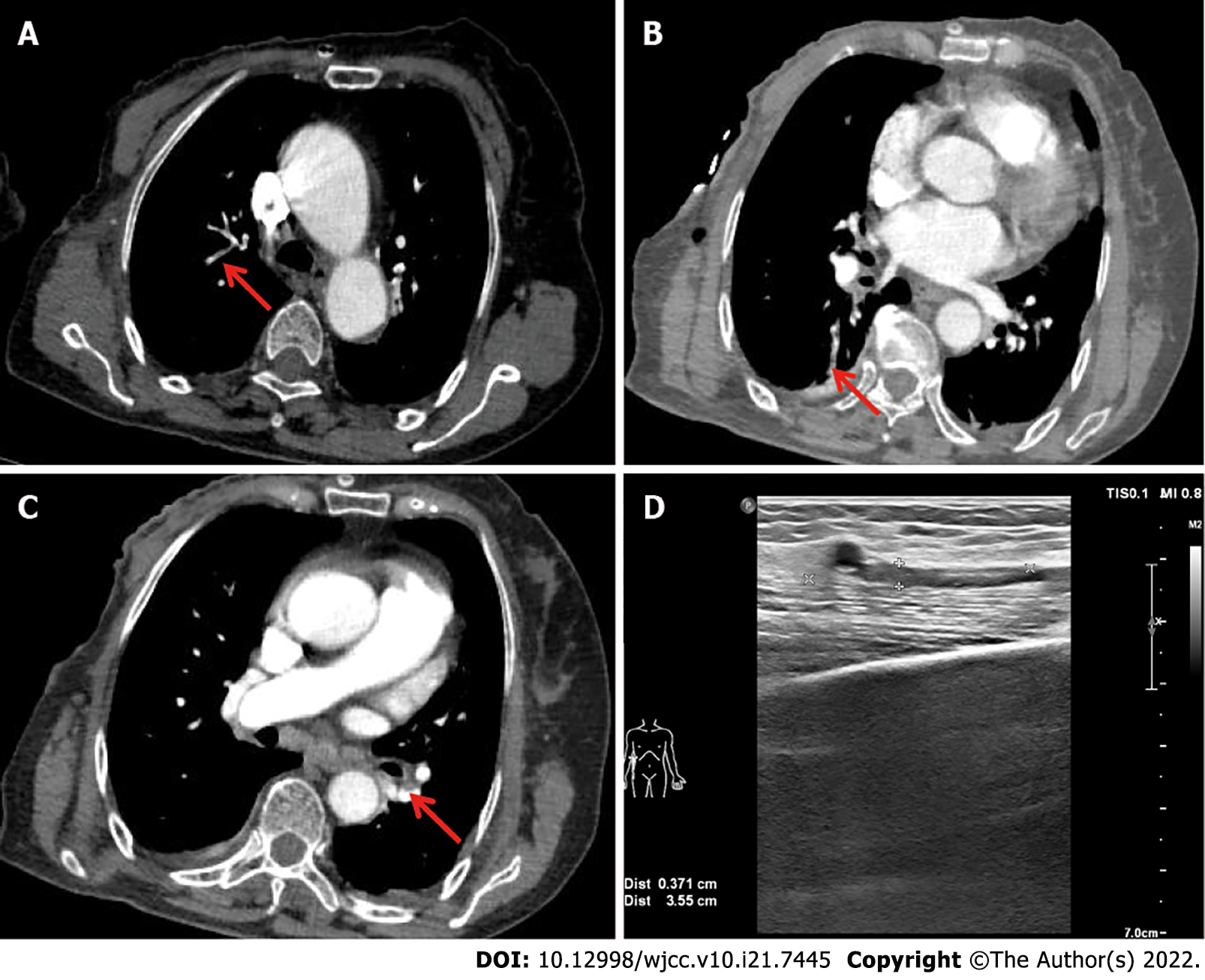
Case 1: The patient underwent computed tomography pulmonary angiography, and PE was confirmed. Multiple pulmonary embolisms were located in smaller subsegments of the pulmonary artery (Figure 1A-C). Four-limb Doppler ultrasonography was performed, which is very important for speculating on the source of the venous thrombosis event. A fresh thrombus was found in the brachial vein of the right upper limb, which was in the operative extremity (Figure 1D).
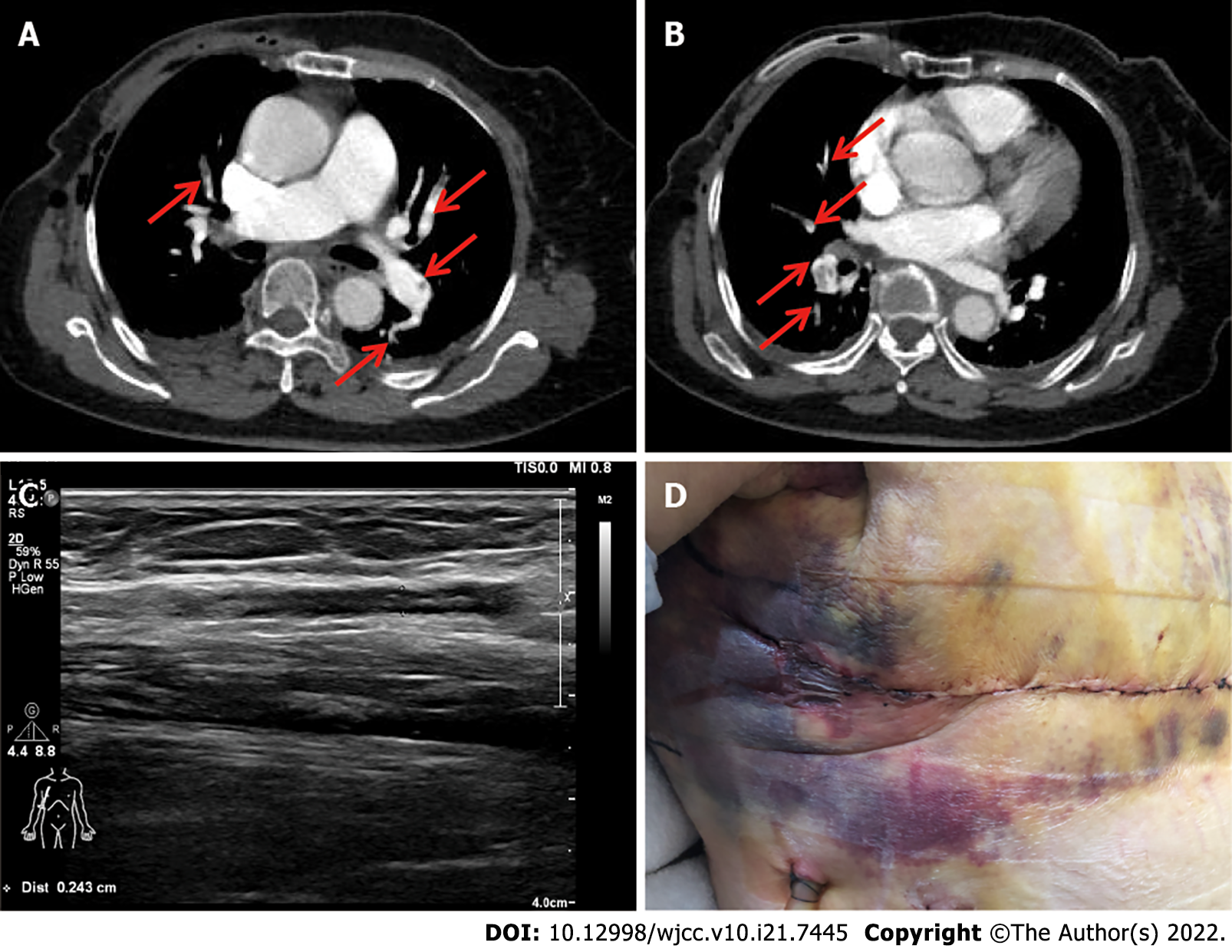
Case 2: A spiral CT scan showed bilateral massive PE located in both lower pulmonary arteries and branches, the superior branch of the right upper pulmonary artery, and the left pulmonary artery and some of its branches (Figure 2A and B). Four-limb surveillance Doppler ultrasound showed upper limb venous thrombosis in the operative extremity involving the vena basilica, the presumed source of the venous thromboembolism (VTE) event (Figure 2C).
Cases 1 and 2: PE.
Subsequently, this patient was treated with Enoxaparin (6150 IU subcutaneously twice-daily dose. However, she had to stop the Enoxaparin due to bleeding in the surgical area 2 d after treatment (Figure 2D). The patient developed a drop hemoglobin level from from 122 g/L to 88 g/L. Bleeding in the surgical field did not require invasive intervention and blood transfusion, nor did it affect the overall clinical outcome. After the bleeding had stopped, anticoagulant drugs were transitioned to Rivaroxaban (15 mg PO twice daily) which is direct oral anticoagulants.
Six days after restarting anticoagulation, the patient was discharged in stable condition. Rivaroxaban was given at a dose of 15 mg twice-daily for the initial three weeks followed by a 20 mg once-daily dosing thereafter. At 6 mo, the patient returns for outpatient follow-up. She has remained on anticoagulant treatment and denies bleeding episodes. The thrombosis of the vena basilica was ruled out using colour venous ultrasonography. Laboratory investigations demonstrated the concentration of D-dimer was normal. During anticoagulant therapy, this patient received six cycles of doxorubicin plus cyclophosphamide chemotherapy.
ULVT is an infrequent condition that is characterized as primary or secondary upper-extremity venous thrombosis. The prevalence of patients with ULVT is 3%-11%[2-5]. Paget-Schroetter syndrome and thoracic outlet syndrome are the main causes of primary ULVT. While central venous catheter placement is the commonest risk factor for secondary ULVT, accounting for 45%-62% of upper-extremity venous thromboses[3,6,7], malignancy is also a risk factor. A total of 29.2%-38% of patients with upper-extremity venous thromboses are diagnosed with malignant tumours[3,6]. Additionally, a small study reported that upper extremity deep vein thrombosis (DVT) was identified in 6% of patients who had undergone shoulder arthroplasty. These venous thromboses were found in the operative extremity[8]. However, the specific risk of ULVT related to MRM for breast cancer is therefore unclear.
MRM has become the standard operative treatment for breast cancer. Identifying and reducing thrombotic complications after surgery are very important. In particular, PE is a serious complication that can be potentially life-threatening. The prognosis related to upper-extremity deep venous thrombosis includes a risk of recurrent thromboembolic events, and limiting the risk of post-thrombotic syndrome and mortality is key to improving breast cancer outcomes. The findings from the RIETE registry reveal that 9.0% of patients with ULVT had clinically overt PE[3]. However, despite the lower incidence of clinical PE in patients with ULVT than those with lower-limb DVT, the 3-mo outcome was similar among groups in terms of the incidence of major or fatal bleeding, recurrent DVT, recurrent PE, or fatal PE, and those with ULVT had a higher mortality rate. Furthermore, malignant tumours are an important risk factor for poor prognosis in patients with ULVT. Cancer patients with ULVT have increased incidences of major bleeding, recurrent VTE, and death. These findings show that the occurrence of ULVT with cancer cannot be ignored.
We report two cases of PE originating from ULVT after MRM for breast cancer. Intimal injury, venous stasis, and hypercoagulability, which are Virchow's triad, have been postulated to explain the pathogenesis of VTE after MRM. First, malignant tumours appear to cause a prothrombotic or hypercoagulable state by changing the balance between the coagulation and fibrinolysis systems[9]. Second, during axillary lymph node dissection, direct manipulation, twisting, and stretching of the axillary vein increase the chance of intimal damage to the vessel. Third, MRM requires the patient to be in an abduction posture on the affected side of the upper extremity for an extended period of time, which may result in significant pooling of blood in the upper limb extremities. Surgical procedures have also been related to haematologic alterations and the systemic release of thrombogenic factors[10]. Fourth, postoperative compression bandaging and immobilization of the upper limb on the surgical side cause venous stasis because of the lack of muscular pumping.
The clinical symptoms of these two patients were tumescence, mild dyspnea, and hypoxaemia. Screening surveillance Doppler ultrasound and D-dimer test found that there was no thrombosis before surgery. These two cases of PE after MRM developed on the first day after mastectomy. As a result, surveillance colour flow Doppler ultrasound of the upper extremities and D-dimer testing should be performed in these patients on the second day. The second patient had subcutaneous haemorrhage after being treated with low-molecular-weight heparin, which was considered to be related to the larger wound area of her breast cancer surgery. Therefore, prophylactic anticoagulation and the dosage of anticoagulant drugs must be carefully considered due to bleeding, wound complications, and reoperation in these patients. More importantly, prevention rather than treatment is considered necessary in these patients undergoing breast cancer surgery. No objective clinical guidelines currently exist to help assess and manage upper extremity deep venous thrombosis after MRM for breast cancer. Functional exercise of the upper limbs on the operative extremity, pneumatic compression devices, or intraoperative heparin administration may be effective during perioperative DVT prophylaxis.
These two cases of pulmonary thrombosis caused by upper extremity thrombosis may change the strategy of perioperative thrombosis management for breast cancer and should spur surgeons to pay more attention to ULVT. This is the presumed source of symptomatic or fatal PE in patients undergoing MRM, which should stimulate further research in this area.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Peripheral vascular disease
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Le PH, Taiwan; Patel L, United States S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Wu YXJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, Oi M, Akao M, Kobayashi Y, Toyofuku M, Izumi T, Tada T, Chen PM, Murata K, Tsuyuki Y, Saga S, Nishimoto Y, Sasa T, Sakamoto J, Kinoshita M, Togi K, Mabuchi H, Takabayashi K, Yoshikawa Y, Shiomi H, Kato T, Makiyama T, Ono K, Kimura T; COMMAND VTE Registry Investigators. Deep vein thrombosis in upper extremities: Clinical characteristics, management strategies and long-term outcomes from the COMMAND VTE Registry. Thromb Res. 2019;177:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Muñoz FJ, Mismetti P, Poggio R, Valle R, Barrón M, Guil M, Monreal M; RIETE Investigators. Clinical outcome of patients with upper-extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 4. | Joffe HV, Kucher N, Tapson VF, Goldhaber SZ; Deep Vein Thrombosis (DVT) FREE Steering Committee. Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 263] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Lechner D, Wiener C, Weltermann A, Eischer L, Eichinger S, Kyrle PA. Comparison between idiopathic deep vein thrombosis of the upper and lower extremity regarding risk factors and recurrence. J Thromb Haemost. 2008;6:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Owens CA, Bui JT, Knuttinen MG, Gaba RC, Carrillo TC. Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radiol. 2010;21:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Spencer FA, Emery C, Lessard D, Goldberg RJ; Worcester Venous Thromboembolism Study. Upper extremity deep vein thrombosis: a community-based perspective. Am J Med. 2007;120:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Willis AA, Warren RF, Craig EV, Adler RS, Cordasco FA, Lyman S, Fealy S. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002;3:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 285] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 10. | Mansfield AO. Alteration in fibrinolysis associated with surgery and venous thrombosis. Br J Surg. 1972;59:754-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 1.5] [Reference Citation Analysis (0)] |