Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7422
Peer-review started: September 29, 2021
First decision: November 11, 2021
Revised: November 24, 2021
Accepted: June 16, 2022
Article in press: June 16, 2022
Published online: July 26, 2022
Processing time: 284 Days and 18.8 Hours
We report a case of essential thrombocythemia (ET) in a 44-year-old male who exhibited non-ST-segment-elevation myocardial infarction (NSTEMI) as the first manifestation without known cardiovascular risk factors (CVRFs). For the first time, we reported a left main trifurcation lesion in NSTEMI caused by ET, in
A 44-year-old male patient went to a local hospital for treatment for intermittent chest pain occurring over 8 h. The examination at the local hospital revealed elevated cTnI and significantly elevated platelet. Then, he was diagnosed with acute myocardial infarction and transferred to our hospital for emergency interventional treatment by ambulance. During the operation, thrombus aspiration, the single-stent crossover technique, final kissing balloon technique and POT were performed. Dual antiplatelet therapy comprising aspirin and ticagrelor was used after PCI. Evidence of mutated JAK2 V617F and bone marrow biopsy shown the onset of ET. Together with JAK2 (exon 14) V617F mutation, ET was diagnosed according to the World Health Organization (WHO) diagnostic criteria, and the patient was placed on hydroxyurea. During the one-year postoperative period, repeated examinations showed a slight increase in PLTs, but the patient no longer had chest tightness, chest pain or bleeding or developed new thromboembolisms.
Routine physical examinations and screenings are conducive to the early detection of ET, and the risk for thrombosis should be assessed. Then, active antiplatelet therapy and myelosuppression therapy should be used for high-risk ET patients.
Core Tip: The emergency interventional treatment plan for acute myocardial infarction (AMI) caused by essential thrombocythemia is generally the same as that for AMI, and if conditions permit, intravenous ultrasound can provide imaging guidance for stent implantation. Taking aspirin to prevent the number of platelet (PLT) aggregation is very important, and conventional anticoagulation therapy is not recom
- Citation: Wang ZM, Chen WH, Wu YM, Wang LQ, Ye FL, Yin RL. Essential thrombocythemia with non-ST-segment elevation myocardial infarction as the first manifestation: A case report. World J Clin Cases 2022; 10(21): 7422-7428
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7422.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7422
Essential thrombocythemia is a group of relatively chronic myeloproliferative diseases. It is characterized by the abnormal proliferation of megakaryocytes in the bone marrow and a significant increase in peripheral blood platelet counts. The main clinical manifestations are increased incidence of thromboembolic and bleeding events[1]. Research shows that thrombotic complications are the main factors affecting mortality in essential thrombocythemia (ET) patients[2], and the incidence of major haemorrhagic complications was very low in comparison with that of thrombotic episodes. These complications are most common in cases of ischaemia caused by arterial thrombosis, followed by venous thrombosis and microcirculation disorders[3]. Early recognition of ET and the International Prognostic Score for Essential Thrombocythemia (IPSET) can be used as some of the most important methods to prevent and treat thrombotic events caused by ET[4].
Chest pain for 8 h.
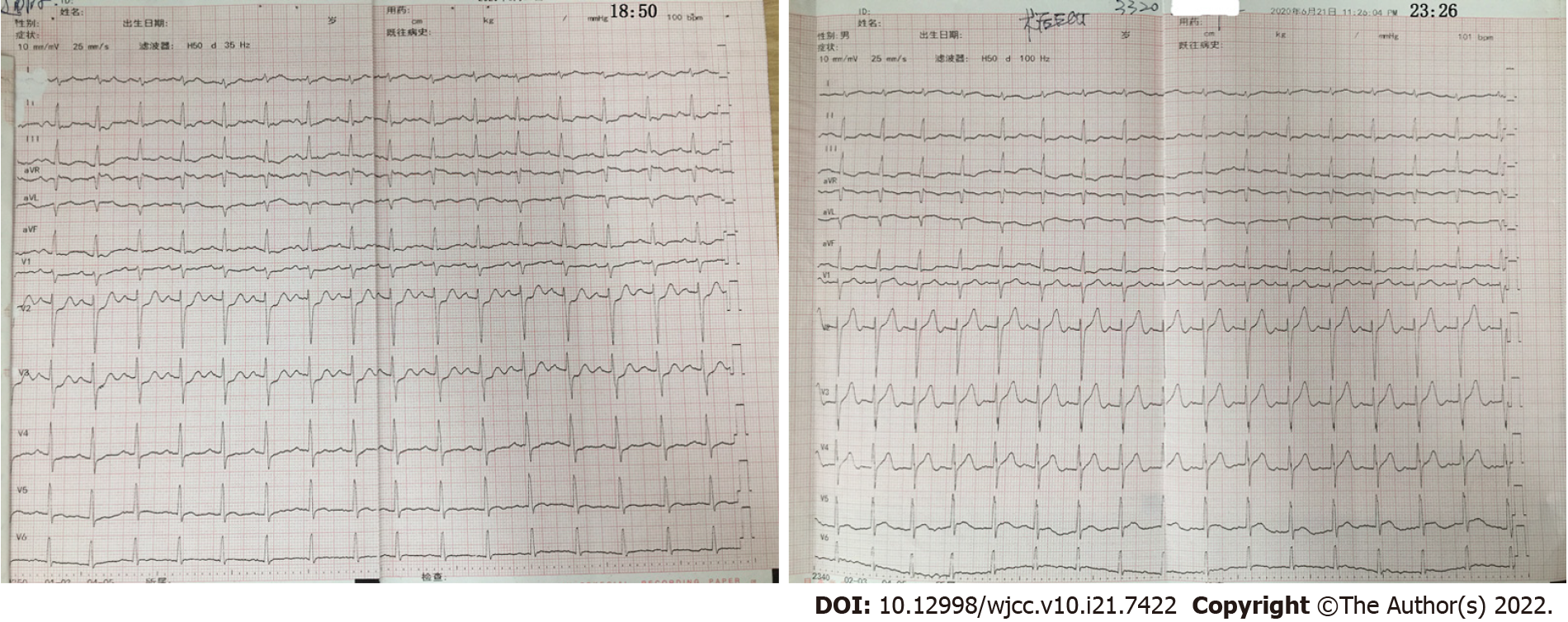
A 44-year-old male patient was admitted to our hospital with complaints of intermittent chest pain for 9 h. Starting at 11:45 am on June 21, 2021, chest pain and sweating appeared in a resting state with no obvious inducement and was located in the middle and lower part of the sternum, spreading to the shoulder and back. The whole process lasted for more than 10 minutes, and the chest pain was relieved by rest; however, during this period, the chest pain recurred intermittently. Later, he went to a local hospital for treatment. Electrocardiogram (ECG) revealed sinus rhythm, horizontal ST segment elevation of 0.05 mV in lead avR, upsloping ST segment depression of 0.1-0.25 mV in leads V1-V4, and a cTnI of 2.44 ng/mL, so he was diagnosed with acute myocardial infarction(AMI). Then, he was transferred to our hospital for emergency interventional treatment by 120 first aid.
The patient had no history of hypertension, diabetes, or hyperlipidaemia. However, he had a history of thrombocytosis, without a systematic diagnosis or previous treatment.
He denied any relevant personal medical history or a family history.
The physical examination revealed a patient in pain with clear consciousness, coarse breath sounds in both lungs, no rales. His heart rate (HR) of 88 times/min with no obvious murmur. The blood pressure (BP) was 140/98 mmHg. The abdomen was soft, with no tenderness and no rebound pain.
His initial peripheral blood panel findings were as follows: cTnI, 2.44 ng/mL; PLT, 736 x 109/L; and normal white blood cell (WBC) count, haemoglobin (Hb), D-dimer, prothrombin and partial thromboplastin time.
ECG revealed sinus rhythm, horizontal ST segment elevation of 0.05 mV in lead avR, and upsloping ST segment depression of 0.1-0.25 mV in leads V1-V4 (Figure 1).
The final diagnosis in the presented case was non-ST-segment-elevation myocardial infarction and ET.
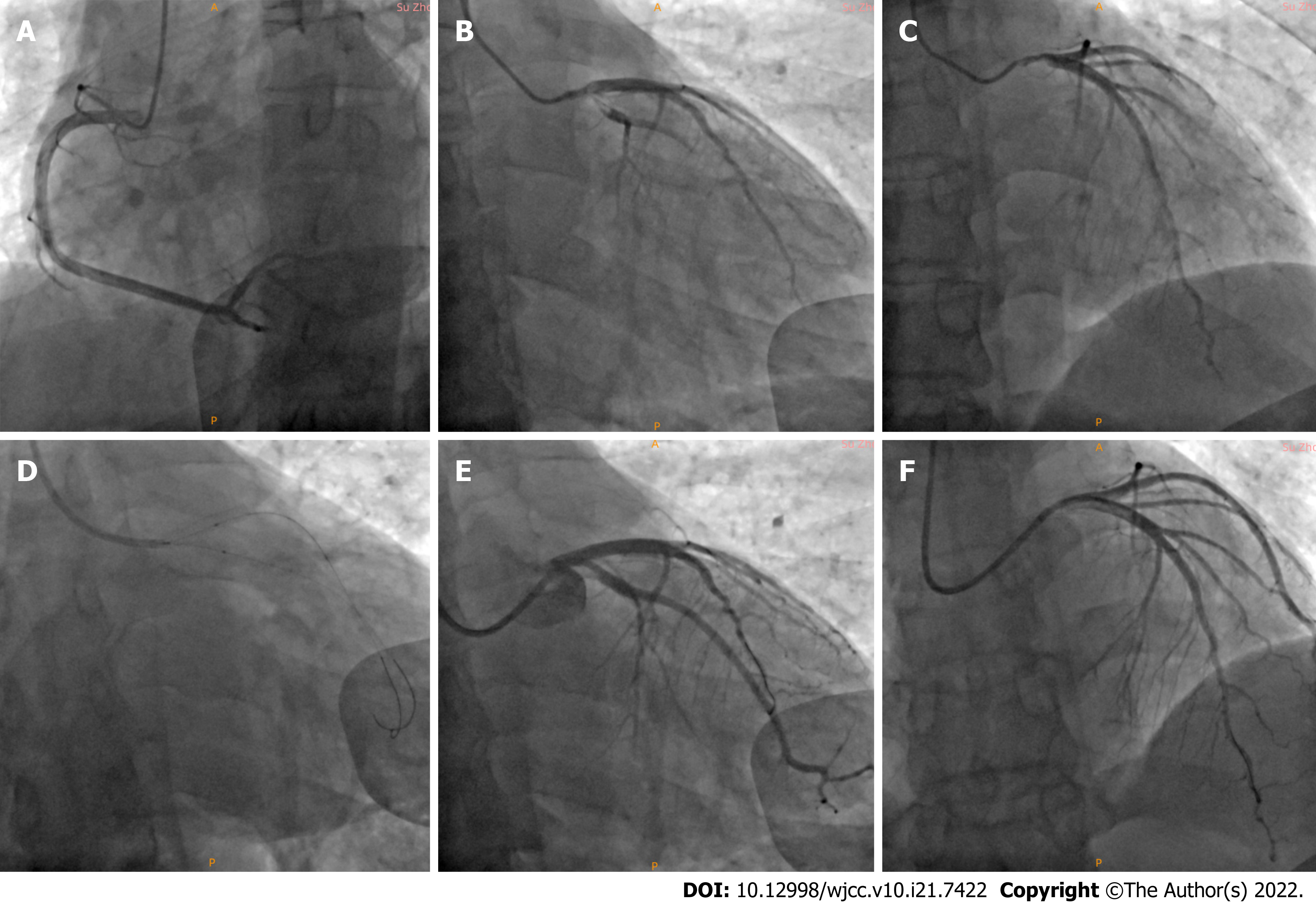
Emergency coronary angiography (CAG) was performed via a right radial artery approach and showed a left main (LM) bifurcation lesion with a Medina classification of 1%, 0%, 1%, 60% diffuse stenosis in the LM that extended to the ostial left anterior descending (LAD), 30% stenosis in the ostial LAD, thrombosis of the ostial LAD and proximal left circumflex (LCX), and 90% stenosis in the proximal LCX. The proximal LCX branched into a larger first obtuse marginal (OM1) artery, with a slow blood flow speed in OM1 and thrombolysis in myocardial infarction (TIMI) flow grade of 2. There was no obvious stenosis of the right coronary artery (Figure 2).
The process of interventional therapy could be described was not straightforward. After engaging the LM artery with a 6-Fr extra backup (EBU) 3.5 guide catheter (Launcher; Medtronic, United States), we advanced two guide wires (Runthrough NS, Terumo, Tokyo, Japan) to the distal LAD and LCX. After predilatation of the proximal LCX using a 2.0 mm × 20 mm semi-compliant balloon (Sprinter Legend, Medtronic, United States), the thrombus load of the ostial LCX was still very heavy. Tirofiban (10 mL) was injected twice through the guide catheter to try to reduce the thrombus load but did not achieve the expected results. Then, intracoronary thrombus aspiration was conducted in the LCX and LAD, but no thrombus was extracted. We performed balloon dilation again; this time choosing 2 new 2.5 mm × 20 mm semi-compliant balloons (Sprinter Legend, Medtronic, United States) to perform the KBT in the LCX and LM. Repeat angiography revealed that the proximal LAD stenosis significantly increased to 70% to 80%, probably from plaque displacement resulting in an increase in the stenosis of the ostial and proximal LAD. At the same time, the previously relieved chest pain worsened again. Considering continuous stenosis lesions from the LM to the ostial LAD and an obvious thrombotic lesion in the ostial LCX, we adjusted the initial treatment strategy to a revascularization strategy from the LAD to the unprotected LM using the single-stent crossover technique[5] to avoid the implantation of more stents and reduce the risk of acute stent thrombosis in the area of the stent. Ostial LM/LAD crossover stent implantation was conducted with a 3.75 mm × 24 mm drug-eluting stent (DES) (Endeavor Resolute, Medtronic, United States) at 12 atm. Repeat CAG showed no residual stenosis in the LAD and a TIMI grade of 3, but the ostial LCX was affected. Then, we rewired another guide wire that passed through the stent mesh to the LCX and used a 1.5 mm × 15 mm semi-compliant balloon (Sprinter Legend, Medtronic, United States) to fully predilate the stent mesh. Then, 3.75 mm × 15 mm and 3.5 mm × 15 mm non-compliant balloons (NC Sprinter, Medtronic, United States) were placed at the LM and ostial LCX, respectively, followed by the final kissing balloon technique. Finally, the LM proximal optimization technique (POT) was performed. Repeat angiography showed 60% residual stenosis in the proximal LCX and a TIMI flow grade of 3 (Figure 2).
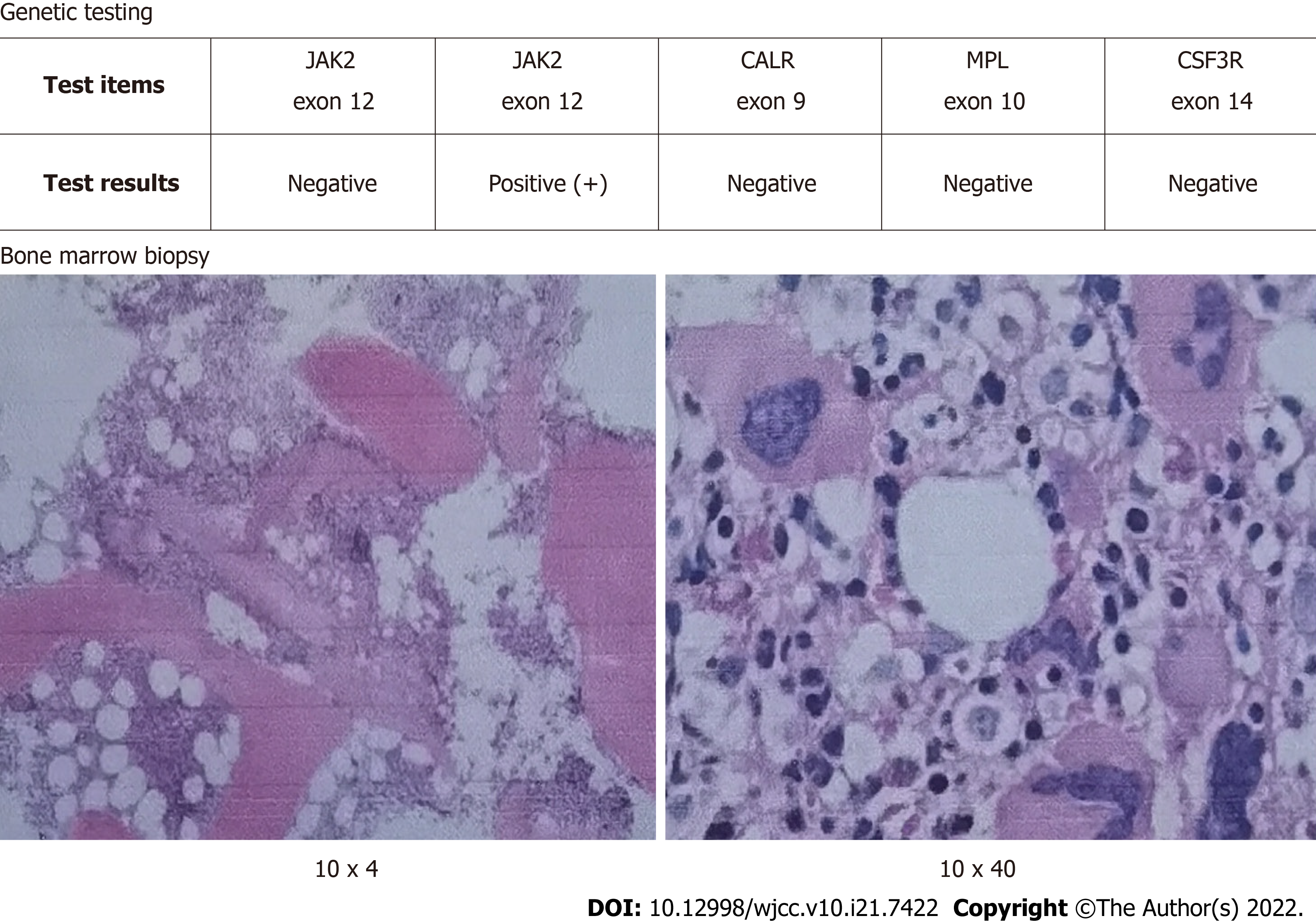
After percutaneous coronary intervention, triple antiplatelet therapy (with aspirin, ticagrelor and tirofiban) was given, an intravenous drip infusion of tirofiban was administered for 48 h (4 mL/h), followed by subcutaneous enoxaparin for 7 d (1 mg/kg every 12 h). On the second day, blood testing showed that the PLT count still increased significantly to as high as 696 × 109/L. The subsequent bone marrow biopsy showed that bone marrow hyperplasia was significantly active, the granulocyte/ nucleated red blood cell ratio was normal, and megakaryocytes were common. With positive JAK-2 V617F mutation (Figure 3), ET was diagnosed. Finally, we used hydroxyurea to inhibit bone marrow proliferation to control the number of the number of platelets.
After percutaneous coronary intervention (PCI), the patient's chest pain improved significantly, and ECG showed that the ST segment, which had been elevated in lead avR and depressed in leads V1-V4, was restored to the equipotential line. Echocardiography showed that the patient's ejection fraction was 64%. There was no chest pain and no signs of bleeding after PCI. During the recent telephone follow-up (November 12, 2021), we learned that the patient's platelet count increased again after stopped taking hydroxyurea. At present, the patient takes medication regularly, including aspirin and hydroxyurea.
ET is a myeloproliferative neoplasm (MPN) that mainly manifests as a PLT count ≥ 450 × 109/L, the abnormal proliferation of megakaryocytes in the bone marrow, with the presence of JAK2, CALR or MPL driver mutations, and other causes of thrombocytosis excluded[6]. The annual incidence is 0.2 to 2.5 cases/100000, and the incidence varies from 0.2 to 2.5:100000 people per year, with a prevalence of 38 to 57 cases per 100000 people[7]. The main complications of ET are thromboembolism and a small number of bleeding events; a history of thrombosis, age > 60 years, and JAK2/MPL mutations are considered the three major risk factors for thrombosis[1,8,9]. Thromboembolic events in important target organs are usually fatal; therefore, early discovery, risk stratification to predict thrombosis and early treatment are very important for ET[4].
By searching PubMed, we found that AMI due to ET is infrequent, with fewer than 35 case reports published in the literature. There were significantly more thrombotic events in the left coronary artery than in the right coronary artery, but these events happen in the LCX rarely. In our study, for the first time, we reported a case of an LM trifurcation lesion in NSTEMI caused by ET, including continuous stenosis lesions from the LM to the ostial LAD and an obvious thrombotic lesion in the ostial and proximal LCX. For the trifurcation lesion in this case, the LAD stenosis was especially aggravated after predilation, and obvious thrombosis was observed in the LCX. An acutely increased thrombus load, acute stent occlusion and no reflow risk can be caused by implantation of a DES in the LCX, especially when double stents (DK-Crush, DK-Culotte, TAP) are used. The single-stent crossover technique was performed to reduce the risk of no reflow and in-stent restenosis and which seemed to be a favourable option. In addition, for suitable lesions, a bioabsorbable stent is a relatively good choice, which can significantly shorten the time of antiplatelet therapy and reduce the risk of bleeding while avoiding long-term restenosis of the DES.
Intracoronary injections of tirofiban can improve thrombus load[10-13]; if there are no significant obstructive atherosclerotic plaques after the thrombus is aspirated and the TIMI flow grade is 3, it can be considered that there is no need to implant a DES in the emergency period. Usually, these AMI patients may be younger patients with no or low cardiovascular risk factors[14]. AMI caused by ET usually has a heavy thrombus load. Therefore, all operations during thrombus aspiration must be standardized. A sufficient negative pressure must be maintained in the aspiration catheter to actively prevent the thrombus from dislodging into other coronary arteries or peripheral blood vessels. Finally, low-dose aspirin to prevent platelet aggregation and the use of hydroxyurea can effectively reduce the occurrence of new and recurring vascular embolisms caused by ET.
Even if acute myocardial infarction is caused by ET, emergency interventional treatment is still necessary and should be carried out as soon as possible. Thrombus aspiration can be routinely applied, and platelet glycoprotein GPⅡb/Ⅲa complexes (GPⅡb/Ⅲa) can be routinely used in the coronary arteries. If the coronary flow is still unsatisfactory after thrombus aspiration, stent placement is needed and an important guarantee for establishing early revascularization and preventing restenosis in the short term. Achieving and maintain a TIMI flow grade of 3 is the purpose of the operation. Intravascular ultrasound (IVUS) can be used to distinguish between a simple thrombus or a plaque rupture and then provide imaging guidance for stent implantation. The fewest stents as possible should be implanted, and absorbable stents may be the first choice for patients with no known CVRFs. Routine physical examinations and screenings are conducive to the early detection of ET, and the risk of thrombosis should be assessed. Liquid biopsy may play a greater role in the future[15]. Then, patients with high-risk ET should be given active antiplatelet therapy (aspirin) and myelosuppressive therapy (hydroxyurea).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anatomy and morphology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barosi G, Italy; Gaman MA, Romania; Marickar F, India S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Tefferi A, Pardanani A. Essential Thrombocythemia. N Engl J Med. 2019;381:2135-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Maleknia M, Shahrabi S, Ghanavat M, Vosoughi T, Saki N. Essential thrombocythemia: a hemostatic view of thrombogenic risk factors and prognosis. Mol Biol Rep. 2020;47:4767-4778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Bellucci S. [Vascular complications of essential thrombocythemia]. Bull Acad Natl Med. 2007;191:519-30; discussion 530. [PubMed] |
| 4. | Mora B, Passamonti F. Developments in diagnosis and treatment of essential thrombocythemia. Expert Rev Hematol. 2019;12:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2722] [Cited by in RCA: 4498] [Article Influence: 899.6] [Reference Citation Analysis (0)] |
| 6. | Barbui T, Thiele J, Gisslinger H, Finazzi G, Vannucchi AM, Tefferi A. The 2016 revision of WHO classification of myeloproliferative neoplasms: Clinical and molecular advances. Blood Rev. 2016;30:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Accurso V, Santoro M, Mancuso S, Napolitano M, Siragusa S. The Essential Thrombocythemia in 2020: What We Know and Where We Still Have to Dig Deep. 2020. |
| 8. | Tefferi A, Vannucchi AM, Barbui T. Essential thrombocythemia treatment algorithm 2018. Blood Cancer J. 2018;8:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, Rodeghiero F, Randi ML, Bertozzi I, Vannucchi AM, Antonioli E, Gisslinger H, Buxhofer-Ausch V, Finazzi G, Gangat N, Tefferi A, Barbui T. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117:5857-5859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 10. | Gül C, Kürüm T, Demir M, Ozbay G, Vural O, Iqbal O, Fareed J. Acute myocardial infarction in a patient with essential thrombocythemia treated with glycoprotein IIb/IIIa inhibitor. Clin Appl Thromb Hemost. 2004;10:77-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Alioglu E, Tuzun N, Sahin F, Kosova B, Saygi S, Tengiz I, Turk U, Ozsan N, Ercan E. Non ST-segment elevation myocardial infarction in patient with essential thrombocythemia. Thromb J. 2009;7:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Bildirici U, Celikyurt U, Ural E. Essential thrombocythemia: a case of acute ST-segment elevation myocardial infarction in a young female. Clin Cardiol. 2009;32:104-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Zhang Z, Wan X, Liu Y, Lin X, Ni Z, Yang X, Zhang L. Non-ST-segment elevation myocardial infarction in a patient with essential thrombocythemia treated with glycoprotein IIb/IIIa inhibitor: a case report. Clin Appl Thromb Hemost. 2011;17:532-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J. 2015;36:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 15. | Găman MA, Cozma MA, Dobrică EC, Crețoiu SM, Găman AM, Diaconu CC. Liquid Biopsy and Potential Liquid Biopsy-Based Biomarkers in Philadelphia-Negative Classical Myeloproliferative Neoplasms: A Systematic Review. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |