Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7365
Peer-review started: May 10, 2022
First decision: May 30, 2022
Revised: June 5, 2022
Accepted: June 14, 2022
Article in press: June 14, 2022
Published online: July 26, 2022
Processing time: 60 Days and 4.3 Hours
It is estimated that about 30% of esophageal cancer (EC) patients are over 70 years old. Therefore, there is less evidence on the diagnosis and management of elderly EC patients. It is important to explore how elderly EC patients benefit from radical radiochemotherapy regimens, including the target area of radiotherapy (RT), radiation dose and fraction, and choice of chemotherapy drugs.
To compare the efficacy of involved-field intensity-modulated RT (IF-IMRT) combined with S-1 vs RT alone in the treatment of elderly EC patients in terms of safety, short-term response, and survival.
Thirty-four EC patients aged > 70 years were prospectively enrolled between December 2017 and December 2019. Based on the random number table, they were divided into an IF-IMRT + S-1 group and an IF-IMRT alone group, with 17 patients in each group. All patients were treated with IF-IMRT at a dose of 50.4-56 Gy in 28-30 fractions (1.8-2 Gy/fraction, 5 fractions/wk). Oral S-1 was admini
As of April 2022, these 34 patients had been followed up for 15.2-32.5 mo, with a median follow-up period of 24.5 mo. Complete efficacy indicators were obtained from all the patients. The objective response rate was 88.2% vs 76.5%, respectively, in the IF-IMRT + S-1 group and the RT alone group, where as the disease control rate was 100% vs 82.4%, respectively. The incidence of adverse events including grade 1-2 fatigue, granulocytopenia, thrombocytopenia, anemia, radiation esophagitis, radiation-induced skin injury, and radiation-induced lung injury was not significantly different between these two groups, so was the incidence of the grade 3 radiation esophagitis (0% vs 5.7%). The rate of progressive disease (PD) was 52.9% (n = 9) in the IF-IMRT + S-1 group and 64.7% (n = 11) in the RT alone group. The median progression-free survival (PFS) was 23.4 mo vs 16.3 mo, and the 2-year PFS rate was 42% vs 41.2%. The median overall survival (OS) was 27.0 mo vs 23.0 mo, and the 2-year OS rate was 58.8% vs 47.1%. Multivariate analysis showed that age was a significant prognostic factor (P = 0.0019); patients aged < 75 years had a significant survival advantage over patients aged ≥ 75 years. The locations of EC also affected the prognosis. In the IF-IMRT + S-1 group, the number of chemotherapy cycles was a significant prognostic factor (P = 0.0125), and the risk of PD was significantly lower in EC patients who had received 6 cycles of chemotherapy than those who had received 2-5 cycles of chemotherapy.
Compared with IF-IMRT alone, IF-IMRT + S-1 shows the benefits of preventing PD and prolonging survival without increasing adverse reactions. Therefore, this concurrent radiochemotherapy deserves clinical application.
Core Tip: Esophageal cancer (EC) represents the second most common gastrointestinal cancer in China, there is less evidence on the diagnosis and management of elderly EC patients. It is important to explore how elderly EC patients benefit from radical radiochemotherapy regimens, including the target area of radiotherapy (RT), radiation dose and fraction, and choice of chemotherapy drugs. Compared with involved-field intensity-modulated RT (IF-IMRT) alone, IF-IMRT + S-1 shows the benefits of preventing progressive disease and prolonging survival without increasing adverse reactions. Therefore, this concurrent radiochemotherapy deserves clinical application.
- Citation: Liu LH, Yan MH, Di YP, Fu ZG, Zhang XD, Li HQ. Comparison of involved-field intensity-modulated radiotherapy combined with S-1 vs radiotherapy alone for elderly patients with esophageal cancer. World J Clin Cases 2022; 10(21): 7365-7375
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7365.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7365
Esophageal cancer (EC) represents the second most common gastrointestinal cancer in China, with 320000 newly-diagnosed cases and 300000 new cancer deaths in 2020[1]. It is estimated that about 30% of EC patients are over 70 years old[2-4]. Radical surgery remains the primary treatment for early EC, and neoadjuvant chemoradiotherapy followed by surgery and radical chemoradiotherapy are the standard treatments for locally advanced EC. However, many elderly EC patients are unwilling or intolerant to undergo surgery due to organ dysfunction and/or underlying diseases (e.g., cardiopulmonary diseases). In addition, EC patients aged ≥ 70 years had been ruled out by most large-scale randomized clinical trials. Therefore, there is less evidence on the diagnosis and management of elderly EC patients. A pooled analysis of the treatment options and outcomes in elderly EC patients in a recent systematic review showed that, compared with palliative treatment or no treatment, radical chemoradiotherapy or neoadjuvant treatment combined surgery had significantly better survival benefits in elderly EC patients[5]. Zhao et al[6] compared the outcomes of elderly EC patients who received radical radiotherapy (RT) combined with intravenous chemotherapy vs RT alone, and found that radical radiochemotherapy was superior in clinical complete response (CR) rate (34.6% vs 18.6%, P = 0.044), median overall survival (OS) (24.6 mo vs 19.4 mo, P = 0.018), and progression-free survival (PFS) (15.3 mo vs 10.6 mo, P = 0.008) over RT alone; however, the former had significantly higher incidence of grade 3 esophagitis (5.8% vs 1.4 %) and hematological toxicities (9.8% vs 0%, P < 0.05).
Therefore, it is important to explore how elderly EC patients benefit from radical radiochemotherapy regimens, including the target area of RT, radiation dose and fraction, and choice of chemotherapy drugs. In this prospective, randomized, controlled trial, we attempted to compare the safety and efficacy of involved-field intensity-modulated RT (IF-IMRT) combined with tegafur-gimeracil-oteracil potassium capsules (S-1) vs RT alone in the treatment of elderly esophageal squamous cell carcinoma.
Patients with pathologically confirmed locally advanced EC who were treated in our hospital from December 2017 to December 2019 were prospectively enrolled. The inclusion criteria were: (1) Aged ≥ 70 years; (2) With pathologically confirmed esophageal squamous cell carcinoma; (3) Treatment-naive; (4) With a clinical stage of cT2-3N0-2M0; (5) Unable or refusing to undergo surgical resection; (6) With contraindications to radiochemotherapy; (7) Able to understand the content of the informed consent forms for RT and chemotherapy; (8) Without any medical history of other malignant tumors; and (9) With complete medical records. The exclusion criteria were: (1) Younger than 70 years; (2) With pathologically confirmed esophageal non-squamous cell carcinoma; (3) With the risk of esophageal perforation and/or gastrointestinal bleeding, as suggested by examinations and tests; (4) With early (stage I) EC; (5) With distant metastasis; (6) With a history of prior surgery, RT, and/or chemotherapy, or with a history of other malignant tumors; (7) With contraindications for radiochemotherapy; (8) Undergoing conventional/three-dimensional conformal RT and/or receiving chemotherapy for no more than 4 cycles; and (9) With incomplete medical data. Based on the random number table, they were divided into an IF-IMRT + S-1 group and an IF-IMRT alone group, with 17 cases in each group. The median age was 76 years (range 72-80 years). Eighteen patients (52.9%) were older than 75 years, and 29 (85.3%) were males. The general data of the patients are shown in Table 1.
| Variables | Total (n = 34) | CRT (n = 17) | Radiation (n = 17) | P value |
| Age (yr) | 0.275 | |||
| Median (range) | 76.0 (72.0-80.0) | 77.0 (75.0-80.0) | 75.0 (72.0-80.0) | |
| Age | 0.731 | |||
| ≤ 75 yr | 16 (47.1) | 7 (41.2) | 9 (52.9) | |
| > 75 yr | 18 (52.9) | 10 (58.8) | 8 (47.1) | |
| Gende | 1.000 | |||
| Female | 5 (14.7) | 3 (17.6) | 2 (11.8) | |
| Male | 29 (85.3) | 14 (82.4) | 15 (88.2) | |
| Primary tumor location | 1.000 | |||
| Cervical | 3 (8.8) | 1 (5.9) | 2 (11.8) | |
| Distal third | 14 (41.2) | 7 (41.2) | 7 (41.2) | |
| Middle third | 11 (32.4) | 6 (35.3) | 5 (29.4) | |
| Proximal third | 6 (17.6) | 3 (17.6) | 3 (17.6) | |
| cTNM stage | 0.282 | |||
| II | 12 (35.3) | 4 (23.5) | 8 (47.1) | |
| III | 22 (64.7) | 13 (76.5) | 9 (52.9) | |
| Gastric tube insertion | 1.000 | |||
| No | 23 (67.6) | 11 (64.7) | 12 (70.6) | |
| Yes | 11 (32.4) | 6 (35.3) | 5 (29.4) | |
| Follow-up (mo) | 0.293 | |||
| Median (IQR) | 24.5 (15.2-32.5) | 26.0 (19.0-36.0) |
RT: IF-IMRT (56-60 Gy/28-30 fractions; 50.4-2.0 Gy/28-30 fractions, 5 fractions/wk) was administered in both groups. Prophylactic irradiation was not performed on the lymphatic drainage region.
Oral chemotherapy: Oral chemotherapy regimen in the IF-IMRT + S-1 group was as follows: S-1 40-60 mg bid was administered for 14 consecutive days and then withdrawn for 7 d, during which RT was concurrently applied in week days from Monday to Friday. After RT, S-1 was maintained for 4 cycles (21 d made up a cycle) as the consolidation chemotherapy. No chemotherapy was given in the IF-IMRT alone group.
The treatment efficacy was evaluated using the benchmarks of the Response Evaluation Criteria in Solid Tumors version 1.1, which included CR, partial response (PR), stable disease (SD), and progression disease (PD); the objective remission rate (ORR) was calculated using the following formula: ORR = (CR + PR)/total cases × 100%. Chest computed tomography (CT), magnetic resonance imaging, and positron emission tomography-CT (if necessary) were performed after induction chemotherapy and 1 mo after concurrent chemoradiotherapy. Adverse reactions were graded according to the United States National Cancer Institute Common Toxicity for Adverse Events version 3.0.
The primary outcome measures included PFS, OS, ORR after induction chemotherapy, and ORR after concurrent radiochemotherapy. The secondary outcome measure was the adverse reactions after radiochemotherapy. All patients were followed up by telephone or outpatient visits.
Descriptive statistics was performed for all subjects. Categorical variables are presented as n (%) and continuous variables are reported as median and interquartile range. The variables were compared using the χ2 test (for categorical variables), one-way analysis of variance (for normally distributed data), and Kruskal-Wallis test (if a skewed distribution was met). All analyses were performed using the R statistical software (v4.4.1; R Core Team) and Free Statistics software (v1.4). A two-tailed test was used and P < 0.05 was considered statistically significant.
A total of 34 patients with locally advanced esophageal squamous cell carcinoma were included in this study, and efficacy indicators were obtained in all patients. In the IF-IMRT + S-1 group, 10 patients (58.8%) completed 6 cycles of chemotherapy and 7 (41.2%) received 2-5 cycles of induction chemotherapy.
The ORR was 88.2% vs 76.5%, respectively, in the IF-IMRT + S-1 group and the IF-IMRT alone group. The disease control rate (DCR) was 100% (PR 82.4%; and SD 11.8%) in the IF-IMRT + S-1 group and 82.4% (CR 5.9%; PR 70.6%; SD 5.9%; and PD 17.6%, all 3 cases had mediastinal lymph node progression) in IF-IMRT alone group (Table 2).
| Total (n = 34) | CRT (n = 17) | Radiation (n = 17) | P value | |
| Fatigue | 0.273 | |||
| 0 | 14 (41.2) | 8 (47.1) | 6 (35.3) | |
| Grade 1 | 18 (52.9) | 7 (41.2) | 11 (64.7) | |
| Grade 2 | 2 (5.9) | 2 (11.8) | 0 (0) | |
| Granulocytopenia | 0.450 | |||
| 0 | 15 (44.1) | 6 (35.3) | 9 (52.9) | |
| Grade 1 | 16 (47.1) | 10 (58.8) | 6 (35.3) | |
| Grade 2 | 3 (8.8) | 1 (5.9) | 2 (11.8) | |
| Thrombocytopenia | 1.000 | |||
| 0 | 29 (85.3) | 14 (82.4) | 15 (88.2) | |
| Grade 1 | 5 (14.7) | 3 (17.6) | 2 (11.8) | |
| Anemia | 0.342 | |||
| 0 | 10 (29.4) | 3 (17.6) | 7 (41.2) | |
| Grade 1 | 17 (50.0) | 10 (58.8) | 7 (41.2) | |
| Grade 2 | 7 (20.6) | 4 (23.5) | 3 (17.6) | |
| Esophagitis | 0.865 | |||
| 0 | 16 (47.1) | 9 (52.9) | 7 (41.2) | |
| Grade 1 | 15 (44.1) | 7 (41.2) | 8 (47.1) | |
| Grade 2 | 2 (5.9) | 1 (5.9) | 1 (5.9) | |
| Grade 3 | 1 (2.9) | 0 (0) | 1 (5.9) | |
| Gastrointestinal reactions | 0.012 | |||
| 0 | 22 (64.7) | 7 (41.2) | 15 (88.2) | |
| Grade 1 | 12 (35.3) | 10 (58.8) | 2 (11.8) | |
| Radiation pneumonitis | 0.653 | |||
| 0 | 27 (79.4) | 13 (76.5) | 14 (82.4) | |
| Grade 1 | 6 (17.6) | 4 (23.5) | 2 (11.8) | |
| Grade 2 | 1 (2.9) | 0 (0) | 1 (5.9) | |
| Dermatitis | 0.601 | |||
| 0 | 30 (88.2) | 16 (94.1) | 14 (82.4) | |
| Grade 1 | 4 (11.8) | 1 (5.9) | 3 (17.6) | |
| Short-term efficacy | 0.409 | |||
| ORR | 28 (82.4) | 15 (88.2) | 13 (76.5) | |
| CR | 2 (5.9) | 1 (5.9) | 1 (5.9) | |
| PR | 26 (76.5) | 14 (82.4) | 12 (70.6) | |
| SD | 3 (8.8) | 2 (11.8) | 1 (5.9) | |
| PD | 3 (8.8) | 0 (0) | 3 (17.6) |
The adverse events included grade 1-2 fatigue (53% in the IF-IMRT + S-1 group vs 64.7% in the RT alone group), grade 1-2 granulocytopenia (64.7% vs 47.1%), grade 1 thrombocytopenia (17.6% vs 11.8%), grade 1-2 anemia (82.3% vs 58.8%), grade 1-2 radiation esophagitis (47.1% vs 53%), grade 3 radiation esophagitis (0% vs 5.7%), grade 1-2 radiation-induced skin injury (5.9% vs 17.6%), and grade 1-2 radiation-induced lung injury (23.5% vs 17.7%), and all of them showed no significant difference between the two groups. Nasogastric intubation was performed before RT in 10 patients (58.8%) in the IF-IMRT + S-1 group and only in 2 patients (11.8%) in the IF-IMRT alone group (Table 2).
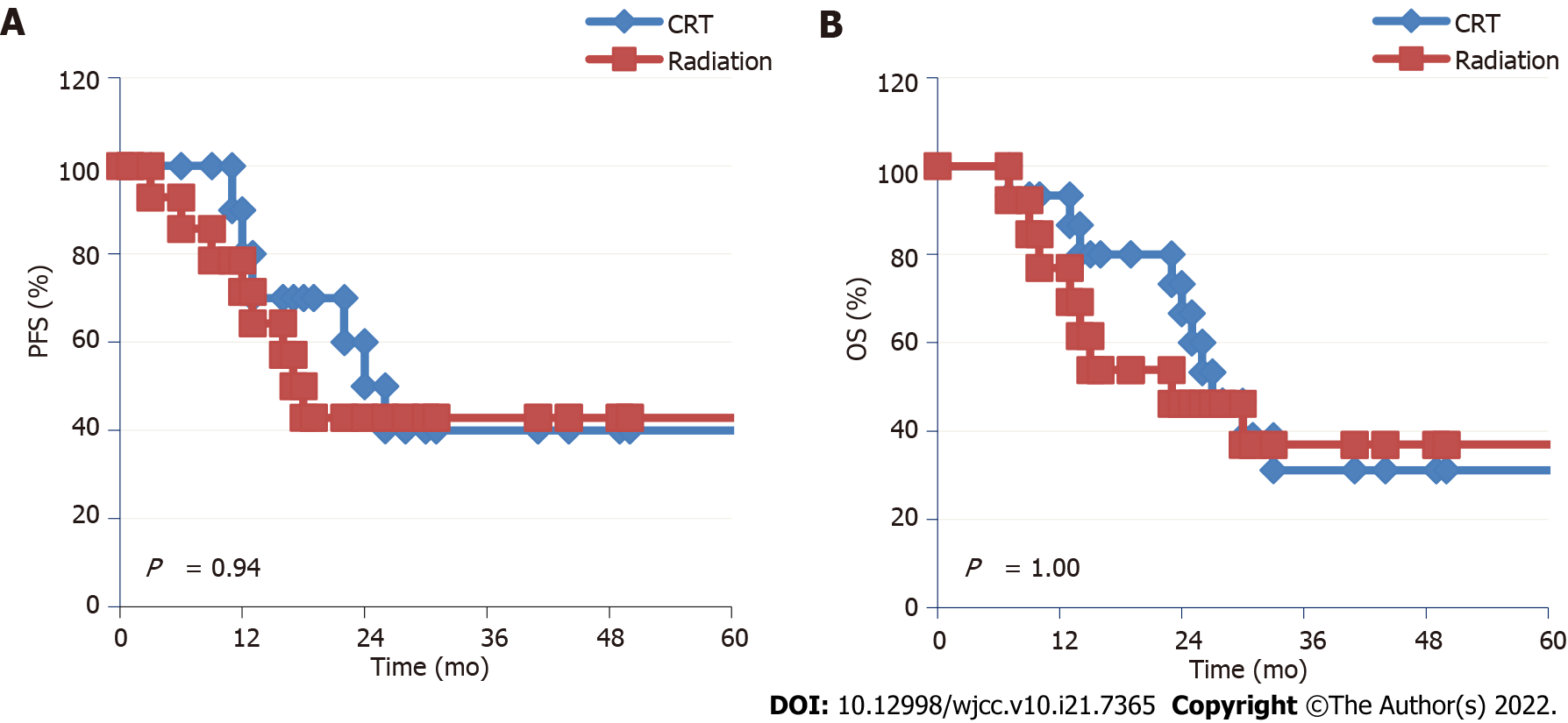
All patients were followed up as of April 2022. The 34 patients were followed up for 15.2-32.5 mo, with a median follow-up period of 24.5 mo. Nine patients (52.9%) in the IF-IMRT + S-1 group experienced PD, including EC progression in 6 cases (35.3%), EC progression + lymph node metastasis in 1 case (5.9%), distant metastasis in 2 cases (11.8%), lung metastasis in 1 case (5.9%), and brain metastasis in 1 case (5.9%). Eleven patients (64.7%) in the RT alone group experienced PD, including EC progression in 4 cases (23.5%), lymph node metastasis in 5 cases (29.4%), EC progression + lymph node metastasis in 1 case (5.9%), and distant metastasis to the liver in 1 case (5.9%). There were 12 deaths in the IF-IMRT + S-1 group, including 5 deaths (29.4%) due to tumor progression, 2 (11.8%) due to gastrointestinal bleeding, and 5 (29.4%) due to non-tumor causes such as cardiopulmonary diseases. Ten patients in the RT alone group died, including 5 deaths (29.4%) due to tumor progression, 2 (11.8%) due to gastrointestinal bleeding, 2 (11.8%) due to liver failure following liver metastases, and 1 patient (5.9%) due to esophageal fistula. The median PFS was 23.4 mo vs 16.3 mo, and the 2-year PFS rate was 47.1% vs 41.2% in the IF-IMRT + S-1 group and RT alone group. The median OS was 27.0 mo vs 23.0 mo, and the 2-year OS rate was 58.8% vs 47.1% in the two groups (Figures 1A and B).
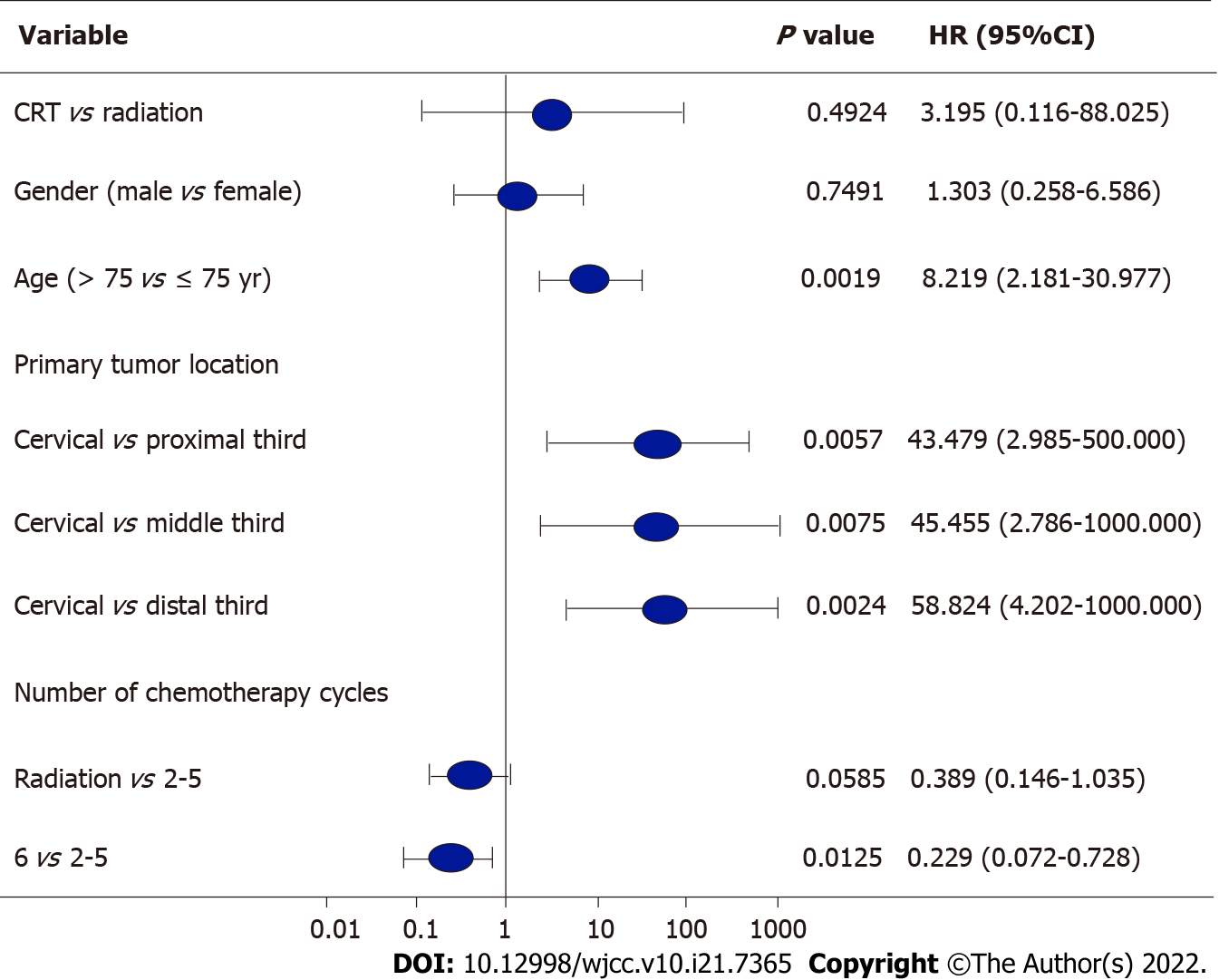
Age was a significant prognostic factor (P = 0.0019); patients aged < 75 years had a significant survival advantage over patients aged ≥ 75 years. The locations of EC also affected the prognosis. The prognosis of patients with cervical EC was worse than that of patients with tumors located in upper (P = 0.0057), middle (P = 0.0075), and lower thoracic segments (P = 0.0024). In the IF-IMRT + S-1 group, the number of chemotherapy cycles was a significant prognostic factor (P = 0.0125), and the risk of PD was significantly lower in EC patients who had received 6 cycles of chemotherapy than those who had received 2-5 cycles of chemotherapy [hazard ratio (HR) = 0.229; 95% confidence (CI): 0.072-0.728] (Figure 1 and Figure 2).
With the aging of the population, the incidence of elderly ECs been increasing rapidly. Treatments for elderly EC patients are risky as many older patients are complicated with cardiopulmonary diseases. Elderly patients were often ruled out in clinical studies, making the treatment of EC in these patients particularly challenging due to the lack of clinical evidence[7]. A propensity score-matched analysis compared the efficacy of chemoradiotherapy with RT alone for non-surgical EC patients aged ≥ 65 years and found that the 3-year OS rate was 21.8% vs 6.4% and the 5-year OS rate was 12.7% vs 3.5%, showing significant differences. In five subgroups based on the age stratification (65-69; 70-74; 75-79; 80-84; ≥ 85 years), the 3- and 5-year OS showed significant benefits in the chemoradiotherapy group compared with the RT alone group (all P < 0.05). The authors thus concluded that chemoradiotherapy could sig
The radiation dose and volume can directly affect the incidence of adverse reactions and treatment efficacy. A dose-related retrospective study on EC showed that there was no significant difference in locoregional failure (52% vs 56%) or 2-year OS rate (31% vs 40%) between the high-dose and standard-dose groups[14,15]. The ARTDECDO study randomly enrolled EC patients to receive radical concurrent chemoradiotherapy with different radiation doses. The results suggested that increased radiation dose on the primary tumor to 61.6 Gy did not significantly improve local control compared with 50.4 Gy, but increased the incidence of toxicity; in addition, there was no OS benefit. The 3-year local PFS rates of the low-dose group and high-dose group of squamous cell carcinoma patients were 75% and 79% (P > 0.05), while the incidence of the common grade 4 and 5 toxicities was 12% and 5% in the low-dose group, lower than those (14% and 10%) in the high-dose group[16]. The application of RT technology can also affect the prognosis of elderly EC patients. One study compared 3DCRT with IMRT in elderly (> 65 years) EC patients and found that the IMRT group had lower cardiac mortality and overall mortality[17]. With the wider application of IMRT, individualized precision RT for EC has improved the local control rate and survival time compared with conventional RT; however, the treatment failure is attributed to local recurrence within the irradiation field and distant metastasis[18]. Target volume delineation during the precise RT for EC includes involved-field irradiation (IFI) and elective nodal irradiation (ENI). ENI has a larger range of irradiation field, which increases the incidence of grade 3 or higher radiation esophagitis and radiation pneumonitis[19-22]. In contrast, IFI can reduce the irradiation range and dose volume; theoretically, it can reduce radiation-induced damage to the esophagus, lung, heart, and spinal cord, increase the treatment completion rate, thus making it possible for patients to tolerate systemic chemotherapy. IFI has been widely used in the delineation of target volumes for RT of lung cancer and has been found to improve the efficacy while reducing side effects[23]. When applied in the RT of locally advanced EC, IFI achieved survival benefits in terms in OS, PFS, and longitudinal critically refracted comparable to those in the RTOG study; in addition, the side effects of IFI are lower than those of ENI[24-26]. Jing et al[27] enrolled 137 elderly (> 70 years) patients: 54 patients (39.4%) received ENI and 83 (60.6%) received IFI, and found that IFI reduced RT-induced toxicities without sacrificing OS in these patients. However, for elderly patients with esophageal squamous cell carcinoma, there is currently no high-level evidence to support the efficacy and safety of IFI. With the aging of the society and the increasing demand for high quality of life, multidisciplinary management of tumor patients has been emphasized by clinicians. Individualized precision treatment may ensure the therapeutic efficacy and meanwhile minimize the toxicities in elderly EC patients, thus having become a hot research topic in recent years. In the present study, the radiation dose ranged from 50.4-56 Gy. By IMRT, the radiation target area could be more precise and conformal, during which the IFI technique is applied in the target area. In our study, no prophylactic ENI was performed on the lymphatic drainage area, which reduced the radiation range and thus minimized the RT-related toxicities. Only one patient (5.7%) in the RT alone group suffered from grade 3 radiation esophagitis, and all the other toxicities were of grade 1 or 2, suggesting that IFI is safe for elderly EC patient.
Currently, there are no guidelines for the chemotherapy regimens used in the concurrent chemoradiotherapy for elderly EC patients with underlying diseases, and the tolerability of chemotherapeutic drugs is a primary consideration in choosing a regimen. In elderly EC patients who were treated with platinum combined with 5-fluorouracil, the treatment was often interrupted due to the high incidence of grade 3 or higher myelosuppression[9-13]. Therefore, concurrent RT and intravenous dual-drug chemotherapy are highly toxic in elderly EC patients. S-1 is an oral fluoropyrimidine; when used con
Previous studies have demonstrated that age was associated with prognosis of elderly EC patients. Zhang et al[30] found that the chemoradiotherapy was significantly superior in survival benefits to the RT alone in patients younger than 72 years, but no significant differences were reported between these two treatment regimens in patients older than 72 years. Jingu et al[31] reported that in EC patients aged over 80 years, concurrent chemotherapy and RT showed no significant OS benefit compared with RT alone. A dual-arm, open-label, randomized, multicenter phase III clinical trial is currently underway for EC patients aged over 70 years to explore the best treatment options for elderly EC patients[32]. In the present study, it was found that age was a significant prognostic factor (P = 0.0019): Patients aged < 75 years had a significant survival advantage over patients aged ≥ 75 years. The locations of EC also affected the prognosis. The prognosis of patients with cervical EC was worse than those of patients with tumors located in upper (HR = 0.0057; 95%CI: 2.985-500.000; P = 0.0057), middle (HR = 0.0075; 95%CI: 2.786-1000.000; P = 0.0075), and lower thoracic segments (HR = 0.0024; 95%CI: 4.202-1000.000; P = 0.0024).
In summary, compared with IF-IMRT alone, IF-IMRT + S-1 prevents PD and increases survival benefits without increasing toxicities. Therefore, this concurrent radiochemotherapy deserves clinical application. However, this study was limited by its short follow-up period, and long-term follow-up is needed to determine the patients’ survivals and tumor recurrence/metastasis. In addition, chemotherapy regimens, the optimal dose of radical RT, and the range/fractionation of the RT for EC in elderly patients require further clinical research.
Esophageal cancer (EC) represents the second most common gastrointestinal cancer in China, with 320000 newly-diagnosed cases and 300000 new cancer deaths in 2020. It is estimated that about 30% of EC patients are over 70 years old. There is less evidence on the diagnosis and management of elderly EC patients.
It is important to explore how elderly EC patients benefit from radical radiochemotherapy regimens, including the target area of radiotherapy (RT), radiation dose and fraction, and choice of chemotherapy drugs. In this prospective, randomized, controlled trial, we attempted to compare the safety and efficacy of involved-field intensity-modulated RT (IF-IMRT) combined with tegafur-gimeracil-oteracil potassium capsules (S-1) vs RT alone in the treatment of elderly esophageal squamous cell carcinoma.
To compare the efficacy of IF-IMRT combined with S-1 vs RT alone in the treatment of elderly EC patients in terms of safety, short-term response, and survival.
Patients with pathologically confirmed locally advanced EC. Based on the random number table, they were divided into an IF-IMRT + S-1 group and an IF-IMRT alone group, with 17 cases in each group.
The objective response rate was 88.2% vs 76.5%, respectively, in the IF-IMRT + S-1 group and the RT alone group, where as the disease control rate was 100% vs 82.4%, respectively. The rate of progressive disease (PD) was 52.9% (n = 9) in the IF-IMRT + S-1 group and 64.7% (n = 11) in the RT alone group. The median progression-free survival (PFS) was 23.4 mo vs 16.3 mo, and the 2-year PFS rate was 42% vs 41.2%. The median overall survival (OS) was 27.0 mo vs 23.0 mo, and the 2-year OS rate was 58.8% vs 47.1%.
Compared with IF-IMRT alone, IF-IMRT + S-1 shows the benefits of preventing PD and prolonging survival without increasing adverse reactions. Therefore, this concurrent radiochemotherapy deserves clinical application.
In addition, chemotherapy regimens, the optimal dose of radical RT, and the range/fractionation of the RT for EC in elderly patients require further clinical research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozawa S, Japan; Saragoni L, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64628] [Article Influence: 16157.0] [Reference Citation Analysis (176)] |
| 2. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21368] [Article Influence: 2136.8] [Reference Citation Analysis (3)] |
| 3. | National Cancer Institute. Cancer Stat Facts: Esophageal Cancer. [cited 23 March 2022]. Available from: https://seer.cancer.gov/statfacts/html/esoph.html. |
| 4. | Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 998] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 5. | Mantziari S, Teixeira Farinha H, Bouygues V, Vignal JC, Deswysen Y, Demartines N, Schäfer M, Piessen G. Esophageal Cancer in Elderly Patients, Current Treatment Options and Outcomes; A Systematic Review and Pooled Analysis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Zhao Q, Hu G, Xiao W, Chen Y, Shen M, Tang Q, Ning X. Comparison of definitive chemoradiotherapy and radiotherapy alone in patients older than 75 years with locally advanced esophageal carcinoma: A retrospective cohort study. Medicine (Baltimore). 2017;96:e7920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Tapias LF, Muniappan A, Wright CD, Gaissert HA, Wain JC, Morse CR, Donahue DM, Mathisen DJ, Lanuti M. Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann Thorac Surg. 2013;95:1741-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Xia X, Gao Q, Ge X, Liu Z, Di X, Sun X, Yang Y. Chemoradiotherapy Is Superior to Radiotherapy Alone in Esophageal Cancer Patients Older Than 65 Years: A Propensity Score-Matched Analysis of the SEER Database. Front Oncol. 2021;11:736448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Chen M, Shen M, Lin Y, Liu P, Liu X, Li X, Li A, Yang R, Ni W, Zhou X, Zhang L, Xu B, Lin J, Chen J, Tian Y. Adjuvant chemotherapy does not benefit patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Radiat Oncol. 2018;13:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Li J, Gong Y, Diao P, Huang Q, Wen Y, Lin B, Cai H, Tian H, He B, Ji L, Guo P, Miao J, Du X. Comparison of the clinical efficacy between single-agent and dual-agent concurrent chemoradiotherapy in the treatment of unresectable esophageal squamous cell carcinoma: a multicenter retrospective analysis. Radiat Oncol. 2018;13:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Zhao L, Zhou Y, Pan H, Yin Y, Chai G, Mu Y, Xiao F, Lin SH, Shi M. Radiotherapy Alone or Concurrent Chemoradiation for Esophageal Squamous Cell Carcinoma in Elderly Patients. J Cancer. 2017;8:3242-3250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Guo JH, Chen MQ, Chen C, Lu HJ, Xu BH. Efficacy and toxicity of nimotuzumab combined with radiotherapy in elderly patients with esophageal squamous cell carcinoma. Mol Clin Oncol. 2015;3:1135-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Ohba A, Kato K, Ito Y, Katada C, Ishiyama H, Yamamoto S, Ura T, Kodaira T, Kudo S, Tamaki Y. Chemoradiation therapy with docetaxel in elderly patients with stage II/III esophageal cancer: A phase 2 trial. Adv Radiat Oncol. 2016;1:230-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kim HJ, Suh YG, Lee YC, Lee SK, Shin SK, Cho BC, Lee CG. Dose-Response Relationship between Radiation Dose and Loco-regional Control in Patients with Stage II-III Esophageal Cancer Treated with Definitive Chemoradiotherapy. Cancer Res Treat. 2017;49:669-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Zhang W, Luo Y, Wang X, Han G, Wang P, Yuan W, Dai SB. Dose-escalated radiotherapy improved survival for esophageal cancer patients with a clinical complete response after standard-dose radiotherapy with concurrent chemotherapy. Cancer Manag Res. 2018;10:2675-2682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Hulshof MCCM, van Laarhoven HWM; ARTDECO study group. Reply to C. Pöttgen et al and Y.-H. Lin et al. J Clin Oncol. 2021;39:3882-3883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Lin SH, Zhang N, Godby J, Wang J, Marsh GD, Liao Z, Komaki R, Ho L, Hofstetter WL, Swisher SG, Mehran RJ, Buchholz TA, Elting LS, Giordano SH. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer. 2016;122:917-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Streeter OE Jr, Martz KL, Gaspar LE, Delrowe JD, Asbell SO, Salter MM, Roach M 3rd. Does race influence survival for esophageal cancer patients treated on the radiation and chemotherapy arm of RTOG #85-01? Int J Radiat Oncol Biol Phys. 1999;44:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 876] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 20. | Wang S, Liao Z, Chen Y, Chang JY, Jeter M, Guerrero T, Ajani J, Phan A, Swisher S, Allen P, Cox JD, Komaki R. Esophageal cancer located at the neck and upper thorax treated with concurrent chemoradiation: a single-institution experience. J Thorac Oncol. 2006;1:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Yamashita H, Okuma K, Wakui R, Kobayashi-Shibata S, Ohtomo K, Nakagawa K. Details of recurrence sites after elective nodal irradiation (ENI) using 3D-conformal radiotherapy (3D-CRT) combined with chemotherapy for thoracic esophageal squamous cell carcinoma--a retrospective analysis. Radiother Oncol. 2011;98:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Kato K, Nakajima TE, Ito Y, Katada C, Ishiyama H, Tokunaga SY, Tanaka M, Hironaka S, Hashimoto T, Ura T, Kodaira T, Yoshimura K. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for Stage II-III esophageal carcinoma. Jpn J Clin Oncol. 2013;43:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Fernandes AT, Shen J, Finlay J, Mitra N, Evans T, Stevenson J, Langer C, Lin L, Hahn S, Glatstein E, Rengan R. Elective nodal irradiation (ENI) vs. involved field radiotherapy (IFRT) for locally advanced non-small cell lung cancer (NSCLC): A comparative analysis of toxicities and clinical outcomes. Radiother Oncol. 2010;95:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Zhao KL, Ma JB, Liu G, Wu KL, Shi XH, Jiang GL. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: is elective nodal irradiation necessary? Int J Radiat Oncol Biol Phys. 2010;76:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Ma JB, Song YP, Yu JM, Zhou W, Cheng EC, Zhang XQ, Kong L. Feasibility of involved-field conformal radiotherapy for cervical and upper-thoracic esophageal cancer. Onkologie. 2011;34:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Liu M, Zhao K, Chen Y, Jiang GL. Evaluation of the value of ENI in radiotherapy for cervical and upper thoracic esophageal cancer: a retrospective analysis. Radiat Oncol. 2014;9:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Jing W, Zhu H, Guo H, Zhang Y, Shi F, Han A, Li M, Kong L, Yu J. Correction: Feasibility of Elective Nodal Irradiation (ENI) and Involved Field Irradiation (IFI) in Radiotherapy for the Elderly Patients (Aged ≥ 70 Years) with Esophageal Squamous Cell Cancer: A Retrospective Analysis from a Single Institute. PLoS One. 2016;11:e0147453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Voncken FEM, van der Kaaij RT, Sikorska K, van Werkhoven E, van Dieren JM, Grootscholten C, Snaebjornsson P, van Sandick JW, Aleman BMP. Advanced Age is Not a Contraindication for Treatment With Curative Intent in Esophageal Cancer. Am J Clin Oncol. 2018;41:919-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Xu C, Xi M, Moreno A, Shiraishi Y, Hobbs BP, Huang M, Komaki R, Lin SH. Definitive Chemoradiation Therapy for Esophageal Cancer in the Elderly: Clinical Outcomes for Patients Exceeding 80 Years Old. Int J Radiat Oncol Biol Phys. 2017;98:811-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Zhang P, Xi M, Zhao L, Shen JX, Li QQ, He LR, Liu SL, Liu MZ. Is there a benefit in receiving concurrent chemoradiotherapy for elderly patients with inoperable thoracic esophageal squamous cell carcinoma? PLoS One. 2014;9:e105270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Jingu K, Takahashi N, Murakami Y, Ishikawa K, Itasaka S, Takahashi T, Isohashi F, Sakayauchi T, Ogawa K; JROSG Working Subgroup of Gastrointestinal Cancers. Is Concurrent Chemotherapy With Radiotherapy for Esophageal Cancer Beneficial in Patients Aged 80 Years or Older? Anticancer Res. 2019;39:4279-4283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Li C, Wang X, Han C, Wang P, Pang Q, Chen J, Sun X, Wang L, Zhang W, Lin Y, Ge X, Zhou Z, Ni W, Chang X, Liang J, Deng L, Wang W, Zhao Y, Xiao Z. Correction to: A multicenter phase III study comparing Simultaneous Integrated Boost (SIB) radiotherapy concurrent and consolidated with S-1 versus SIB alone in elderly patients with esophageal and esophagogastric cancer - the 3JECROG P-01 study protocol. BMC Cancer. 2019;19:492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |