Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7333
Peer-review started: April 6, 2022
First decision: May 11, 2022
Revised: May 18, 2022
Accepted: June 26, 2022
Article in press: June 26, 2022
Published online: July 26, 2022
Processing time: 95 Days and 22.6 Hours
China ranks 120th worldwide for the incidence of breast cancer and 163rd for mortality. Early screening, diagnosis, and timely determination of the optimal treatment plan can help ensure clinical efficacy and prognosis.
To investigate the relationship between quantitative magnetic resonance imaging parameters, apparent diffusion coefficient value, pathological immunohistochemical status, and patient prognosis.
A total of 108 patients with breast cancer (breast cancer group) and 110 patients with benign breast tumors (benign group) confirmed by pathological examination at our Hospital from September 2013 to August 2016 were selected. All patients had undergone preoperative magnetic resonance imaging (MRI) examinations, and the quantitative parameters of MRI and apparent diffusion coefficient (ADC) values for the two groups were compared. The MRI quantitative parameters and ADC values of patients with different estrogen receptor (ER), progesterone receptor, and human epidermal growth factor receptor-2 expression were statistically analyzed. The relationship between the quantitative parameters of MRI and ADC values and patient recurrence was analyzed using receiver operating curves.
The measured values of the quantitative parameters of MRI- Ktrans, Kep, and Ve in the breast cancer group were higher than those in the benign group; the ADC value in the breast cancer group was lower than that in the benign group, and the difference was statistically significant (P < 0.05). The Ktrans, Ve, and ADC values in patients with ER-positive breast cancer were significantly lower than those in patients with negative ER expression (P < 0.05). After 5 years of follow-up, 22 patients with breast cancer experienced postoperative recurrence. The Kep, Ve, and ADC values of the recurrence group were significantly lower than those of the non-recurrence group, and the difference was statistically significant (P < 0.05).
MRI quantitative parameters and ADC are related to the expression of breast cancer-related immunological receptor factors and have certain clinical value in assessing postoperative recurrence in patients.
Core Tip: The high sensitivity and repeatability of magnetic resonance imaging make it more widely used in disease screening and treatment. Its advantages in soft tissue resolution and radiation make it more meaningful for lesions that cannot be clarified by clinical and ultrasound techniques in breast examination. Especially, diffusion weighted imaging has more advantages in cell structure and cell membrane integrity.
- Citation: Wang Z, Ren GY, Yin Q, Wang Q. Correlation of magnetic resonance imaging quantitative parameters and apparent diffusion coefficient value with pathological breast cancer. World J Clin Cases 2022; 10(21): 7333-7340
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7333.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7333
Breast cancer is one of the most common malignant tumors in women worldwide[1], and disease staging at the time of diagnosis is crucial for the prognosis of patients[2]. Studies have also found that different prognostic factors, such as human epidermal growth factor receptor-2 (HER-2); progesterone receptor (PR); estrogen receptor (ER); and cell proliferation antigen marker (Ki-67) also affect the treatment and prognosis of breast cancer[3,4]. The advantages that magnetic resonance imaging (MRI) has over the resolution and radiation of soft tissue make it extremely useful for lesions that cannot be clarified by clinical or ultrasonic breast examination techniques. In particular, diffusion-weighted imaging (DWI) has advantages in terms of cell structure and cell membrane integrity. Various influencing factors have different effects on the diagnosis and prognosis of the disease, and various quantitative parameters have different correlations with prognostic factors and cancer classification. Therefore, this study explored the relationship between MRI quantitative parameters and apparent diffusion coefficient (ADC) values of different prognostic factors in patients with breast cancer and benign breast tumors confirmed by pathological examination to provide clinical value for evaluating postoperative recurrence.
A total of 108 patients with breast cancer (breast cancer group) and 110 patients with benign breast tumors (benign group) confirmed by pathological examination in our hospital from September 2013 to August 2016 were selected.
The inclusion criteria were set according to the diagnostic criteria of the “National Comprehensive Cancer Network Breast Cancer Clinical Practice Guidelines 2010, First Edition”. Patients aged 19–65 years, diagnosed with breast cancer and benign breast tumors, underwent surgical treatment in our hospital. The tumors were confirmed by postoperative pathological examination. Patients also underwent X-ray mammography, MRI examination, ultrasound examination, and complete examination data before surgery. All patients were followed up for > 5 years. Exclusion criteria included: (1) Patients with recurrent breast cancer after surgery; (2) Patients with malignant tumors in other parts of the body, which had metastasized to the breast; (3) Patients who received preoperative chemoradiotherapy; (4) Patients who failed to receive postoperative follow-up; and (5) Patients with a lack of imaging data. This study was approved by the Medical Ethics Committee.
The American Society for Clinical Oncology[5] has defined the invasive positive tumor nucleus < 1% as PR and ER negative, and vice versa. HER-2 expression scores of 0 and + were negative, 3+ was positive, and 2+ were further analyzed by Fluorescent in situ Hybridization (FISH). If FISH was positive, gene amplification was performed; otherwise, the results were negative.
The patients were asked to take the prone position, put their arms on the side of their heads, and the positioning line was aligned with the patient's nipple. The conventional examination was performed using 3.0T MRI (Siemens Skyra, Germany), followed by DWI scanning. The transverse T2-weighted image parameters were: TR/TE, 4270/66 ms, layer thickness, 4.0 mm; layer spacing, 4.8 mm; fOV, 34 cm × 34 cm; and number of incentives, 2. The T1-weighted image parameters were: TR/TE, 6.02/2.43 ms; layer thickness, 1.2 mm; fOV, 34 cm × 34 cm; and number of incentives, 2. The DWI parameters were: TR/TE, 8070/82 ms; layer spacing, 6 mm; layer thickness, 4.0 mm; fOV, 34 cm × 34 cm; and number of incentives, 2; matrix, 10.2 cm × 19.2 cm. The two b values were: 0 s/mm2 and 800 s/mm2. The 4D tissue processing software was used to correct the artifacts first and then input the T1 map image data to fit the image to obtain the quantitative T1 value. Transfusions such as the internal thoracic artery, obtain function, select ROI (obvious lesion area), and avoid necrotic area were combined with T1 value, using Tolfs model to measure Ve, Ktrans, Kep, select ROI, and to calculate the average.
In this study, the measurement indexes such as Ktrans, Kep, Ve, and ADC values were assessed whether they were normally distributed, and they were all in line with an approximately normal or normal distribution, which was represented by mean ± SD. A t-test was used to compare the two groups of data. The discrete data were expressed as percentages, and the comparison was performed using the χ2 test. The receiver operating characteristic (ROC curve) was drawn to analyze the value of each index in predicting postoperative recurrence. The professional SPSS 21.0 software (IBM Corp., Armonk, NY, USA) was used for data processing, with a test level α = 0.05.
The age, body mass index, distribution of affected side, comorbidities, and menopausal status were compared between the breast cancer and benign tumor groups, and the difference was not statistically significant (P > 0.05, Table 1).
| Factor | Breast cancer group (n = 108) | Benign group (n = 110) | t/χ2 | P value |
| Age (mean ± SD, yr) | 46.1 ± 7.0 | 47.5 ± 8.4 | -1.336 | 0.183 |
| Body mass index (mean ± SD, kg/m2) | 24.6 ± 2.5 | 24.3 ± 2.6 | 0.868 | 0.386 |
| Affected side distribution | 0.902 | 0.342 | ||
| Left side | 58 (53.7) | 52 (48.15) | ||
| Right | 50 (46.3) | 58 (53.7) | ||
| Hypertension | 0.618 | 0.432 | ||
| Yes | 11 (10.19) | 15 (13.89) | ||
| No | 97 (89.81) | 95 (87.96) | ||
| Diabetes | 2.062 | 0.151 | ||
| Yes | 6 (5.56) | 12 (11.11) | ||
| No | 102 (94.44) | 98 (90.74) | ||
| Hyperlipidemia | 2.221 | 0.136 | ||
| Yes | 17 (15.74) | 10 (9.26) | ||
| No | 91 (84.26) | 100 (92.59) | ||
| Menopause | 0.930 | 0.335 | ||
| Yes | 18 (16.67) | 24 (22.22) | ||
| No | 90 (83.33) | 86 (79.63) |
The Ktrans, Kep, and Ve values of the breast cancer group were higher than those of the benign group. The values of Ktrans, Kep and Ve in the breast cancer group were higher than those in the benign group, and the ADC value in the breast cancer group was lower than that in the benign group; the difference was statistically significant (P < 0.05) (Table 2).
| Groups | Ktrans (min-1) | Kep (min-1) | Ve | ADC (× 10-3 mm2/s) |
| Breast cancer group (n = 108) | 0.481 ± 0.113 | 0.577 ± 0.120 | 0.764 ± 0.170 | 0.741 ± 0.184 |
| Benign group (n = 110) | 0.264 ± 0.096 | 0.408 ± 0.113 | 0.528 ± 0.150 | 1.109 ± 0.241 |
| t value | 15.290 | 10.707 | 10.874 | -12.655 |
| P value | 0.000 | 0.000 | 0.000 | 0.000 |
The values of Ktrans, Ve, and ADC in patients with ER positive breast cancer were significantly lower than those in patients with negative expression, and the difference was statistically significant (P < 0.05). The Kep value of patients with breast cancer with PR-positive expression was significantly lower than that of patients with negative expression, and the difference was statistically significant (P < 0.05). The Kep value of HER-2 positive breast cancer patients was higher than that of negative breast cancer patients, and the difference was statistically significant (P < 0.05). The Ve value of HER-2 positive breast cancer patients was significantly lower than that of HER-2 negative breast cancer patients, and the Kep value was significantly higher than that of HER-2 negative breast cancer patients; the difference was statistically significant (P < 0.05, Table 3).
| Express the situation | n | Ktrans (min-1) | Kep (min-1) | Ve | ADC (× 10-3 mm2/s) |
| ER | |||||
| Positive | 74 | 0.462 ± 0.105 | 0.563 ± 0.112 | 0.740 ± 0.166 | 0.713 ± 0.170 |
| Feminine | 34 | 0.522 ± 0.100 | 0.607 ± 0.109 | 0.816 ± 0.154 | 0.802 ± 0.168 |
| t value | -2.698 | -1.912 | -2.259 | -2.536 | |
| P value | 0.008 | 0.059 | 0.026 | 0.013 | |
| PR | |||||
| Positive | 62 | 0.468 ± 0.108 | 0.542 ± 0.115 | 0.751 ± 0.165 | 0.721 ± 0.180 |
| Feminine | 46 | 0.499 ± 0.111 | 0.624 ± 0.116 | 0.782 ± 0.158 | 0.768 ± 0.178 |
| t value | -1.458 | -3.651 | -0.983 | -1.348 | |
| P value | 0.148 | 0.000 | 0.328 | 0.180 | |
| HER-2 | |||||
| Positive | 34 | 0.461 ± 0.107 | 0.621 ± 0.116 | 0.713 ± 0.166 | 0.719 ± 0.181 |
| Feminine | 74 | 0.490 ± 0.110 | 0.557 ± 0.109 | 0.787 ± 0.162 | 0.751 ± 0.178 |
| t value | -1.283 | 2.777 | -2.188 | -0.863 | |
| P value | 0.202 | 0.006 | 0.031 | 0.390 | |
After 5 years of follow-up, a total of 22 patients with breast cancer had postoperative recurrence. The measured values of Kep, Ve and ADC in the recurrence group were significantly lower than those in the non-recurrence group. The difference between the two groups was statistically significant (P < 0.05, Table 4).
| Recurrence | n | Ktrans (min-1) | Kep (min-1) | Ve | ADC (× 10-3 mm2/s) |
| Relapse | 22 | 0.457 ± 0.111 | 0.528 ± 0.109 | 0.698 ± 0.155 | 0.663 ± 0.181 |
| No recurrence | 86 | 0.487 ± 0.107 | 0.590 ± 0.112 | 0.781 ± 0.165 | 0.761 ± 0.176 |
| t value | -1.165 | -2.329 | -2.130 | -2.317 | |
| P value | 0.247 | 0.022 | 0.035 | 0.022 |
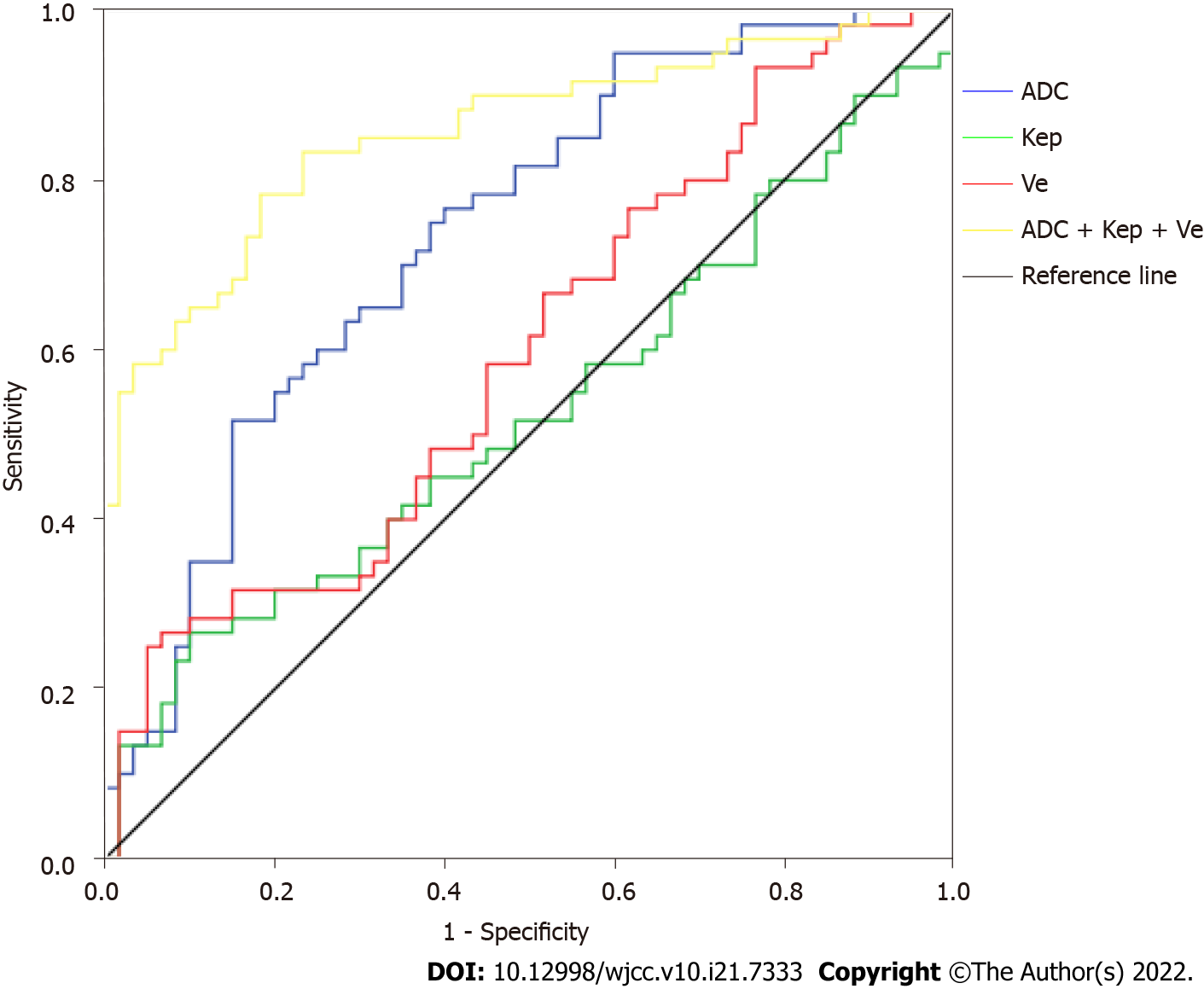
The ROC curve was drawn using the Kep, Ve, and ADC values. The results showed that the area under the curve (AUC) value of the Kep, Ve, and ADC values for predicting postoperative recurrence were 0.599, 0.572, and 0.739, respectively. The AUC value of Kep + Ve + ADC value for predicting postoperative recurrence was 0.858 (Table 5, Figure 1).
| Index | Critical value | AUC | Sensitivity (%) | Specificity (%) | Missed diagnosis rate (%) | Misdiagnosis rate (%) |
| Kep (min-1) | 0.561 | 0.599 | 65.19 | 55.40 | 34.81 | 44.60 |
| Ve | 0.725 | 0.527 | 58.04 | 51.06 | 41.96 | 48.94 |
| ADC (×10-3 mm2/s) | 0.718 | 0.739 | 71.57 | 61.09 | 28.43 | 38.91 |
| ADC + Kep + Ve | - | 0.858 | 83.67 | 77.18 | 16.33 | 22.82 |
Angiogenesis, or the formation of new blood vessels, is essential for the occurrence and development of breast cancer. Vascular endothelial growth factor, which encourages angiogenesis, is released during hypoxic stress, resulting in increased capillary osmotic pressure. This allows small molecules to quickly pass through the vascular wall (along with any contrast agent) into the tissue gap, resulting in a shortened T1 value. At this time, early lesions can be significantly enhanced[6,7].
The high sensitivity and repeatability of MRI make it more widely used in disease screening and treatment determination[8]. There are two methods for the quantitative and semi-quantitative analysis of breast diseases. In particular, quantitative analysis has important value for identifying benign and malignant tumors, prognosis, and evaluation of chemotherapy effects. However, due to research limitations, the current MRI experiments mainly focus on semi-quantitative parameters[9,10]. The research on quantitative parameters is relatively small, and the research results of various studies are different[10]. In this study, quantitative MRI parameters and ADC values of different prognostic factors in patients with breast cancer or benign breast tumors confirmed by pathological examination were compared, and the MRI quantitative parameters and ADC values of patients with different expressions of ER, PR, and HER-2 were statistically analyzed. The relationship between the quantitative parameters of MRI and ADC values and the recurrence of patients was analyzed using the ROC curve, with the aim of providing a reference for clinical diagnosis and treatment.
The results showed that the measured values of Ktrans, Kep, and Ve of the breast cancer group were higher than those of the benign group, while the ADC value was lower than that of the benign group, which was consistent with the results of Martincich et al[11]. In this study, the Ktrans, Ve, and ADC values of patients with ER-positive breast cancer were significantly lower than those of patients with ER-negative breast cancer. The Kep values of patients with HER-2 positive breast cancer were significantly higher than that of patients with HER-2 negative breast cancer[12]. In contrast, the Ve values of patients with HER-2 positive breast cancer were significantly higher than that of patients with HER-2 negative breast cancer. The Kep value of patients with PR-positive breast cancer was significantly lower than that of patients with negative expression, consistent with Lee et al[13]. In contrast to previous experiments, we found that after 5 years of follow-up, the Kep, Ve, and ADC values of patients in the recurrence group were significantly lower than those of patients without recurrence. The ROC curve results showed that the AUC values of Kep, Ve, and ADC values for predicting postoperative recurrence were 0.599, 0.572, and 0.739, respectively. The AUC value of the Kep + Ve + ADC value for predicting postoperative recurrence was 0.858. The reason for this may be that the sample size was not large enough, resulting in a decrease in the estimation accuracy of the data. In addition, the influence of blood flow factors, such as hypertension, was not considered.
The sensitivity of patients with breast cancer with PR and ER-positive expression to endocrine therapy was significantly higher than that of patients with negative expression, and their survival rate was also higher, therefore the prognosis was relatively better[14-16]. HER-2, as a growth factor receptor with tyrosine kinase activity, is less expressed in normal tissues, and its expression can be used as an important indicator of breast cancer and prognosis in the clinical setting[17,18]. Ki-67, a nuclear antigen, can effectively mark cell proliferation. In this study, when the expression of Ki-67 was higher, the possibility of tumor recurrence was greater. Previous studies have found that Ki-67 is inversely proportional to the ADC value, and AUC can more intuitively compare the predictive value of other parameters[19,20]; in other words, when the expression of Ki-67 is higher, the ADC value is lower, which also has a certain effect on our prediction of recurrence.
In summary, this study shows a correlation between MRI quantitative parameters, ADC, and the expression of immune receptor factors related to breast cancer. Therefore, the prognosis of patients can be evaluated by detecting Kep, Ve, and ADC values, which have clinical significance.
It has been reported that the 5-year survival rate of patients with breast cancer in stage I is 100% but is only 20% in stage IV; with the evolution of the disease, the total survival period gradually decreases.
This study analyzed the magnetic resonance imaging (MRI) quantitative parameters and apparent diffusion coefficient (ADC) values of patients with different estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) expression.
This study aimed to investigate the relationship between quantitative MRI parameters.
A total of 108 patients with breast cancer and 110 patients with benign breast tumors confirmed by pathological examination were selected. All patients had undergone preoperative MRI examinations, and the MRI quantitative parameters and ADC values of patients with different ER, PR, and HER-2 expression were statistically analyzed.
The measured values of the quantitative parameters of MRI- Ktrans, Kep, and Ve in the breast cancer group were higher than those in the benign group.
MRI quantitative parameters and ADC are related to the expression of breast.
This study explored the relationship between MRI quantitative parameters and ADC values of different prognostic factors in patients with breast cancer and benign breast tumors confirmed by pathological examination to provide clinical value for evaluating postoperative recurrence.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anemona L, Italy; Campos-da-Paz M, Brazil S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomarkers Prev. 2017;26:444-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 804] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 2. | Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group. Breast-cancer screening--viewpoint of the IARC Working Group. N Engl J Med. 2015;372:2353-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 559] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 3. | Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ; Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206-2223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2486] [Cited by in RCA: 2678] [Article Influence: 223.2] [Reference Citation Analysis (0)] |
| 4. | Deniz F, Dilek K, Hande M, Umit UM, Handan K. Ki-67 and caspase expression in breast carcinoma: does variance in locational sampling exist? Int J Clin Exp Pathol. 2015;8:11305-11313. [PubMed] |
| 5. | Partovi S, Sin D, Lu Z, Sieck L, Marshall H, Pham R, Plecha D. Fast MRI breast cancer screening - Ready for prime time. Clin Imaging. 2020;60:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Negrão EMS, Souza JA, Marques EF, Bitencourt AGV. Breast cancer phenotype influences MRI response evaluation after neoadjuvant chemotherapy. Eur J Radiol. 2019;120:108701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Leithner D, Moy L, Morris EA, Marino MA, Helbich TH, Pinker K. Abbreviated MRI of the Breast: Does It Provide Value? J Magn Reson Imaging. 2019;49:e85-e100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Hu X, Huang W, Fan M. Emerging therapies for breast cancer. J Hematol Oncol. 2017;10:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Li SP, Padhani AR, Taylor NJ, Beresford MJ, Ah-See ML, Stirling JJ, d'Arcy JA, Collins DJ, Makris A. Vascular characterisation of triple negative breast carcinomas using dynamic MRI. Eur Radiol. 2011;21:1364-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Koo HR, Cho N, Song IC, Kim H, Chang JM, Yi A, Yun BL, Moon WK. Correlation of perfusion parameters on dynamic contrast-enhanced MRI with prognostic factors and subtypes of breast cancers. J Magn Reson Imaging. 2012;36:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Martincich L, Deantoni V, Bertotto I, Redana S, Kubatzki F, Sarotto I, Rossi V, Liotti M, Ponzone R, Aglietta M, Regge D, Montemurro F. Correlations between diffusion-weighted imaging and breast cancer biomarkers. Eur Radiol. 2012;22:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Makkat S, Luypaert R, Stadnik T, Bourgain C, Sourbron S, Dujardin M, De Greve J, De Mey J. Deconvolution-based dynamic contrast-enhanced MR imaging of breast tumors: correlation of tumor blood flow with human epidermal growth factor receptor 2 status and clinicopathologic findings--preliminary results. Radiology. 2008;249:471-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Lee HS, Kim SH, Kang BJ, Baek JE, Song BJ. Perfusion Parameters in Dynamic Contrast-enhanced MRI and Apparent Diffusion Coefficient Value in Diffusion-weighted MRI:: Association with Prognostic Factors in Breast Cancer. Acad Radiol. 2016;23:446-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Ali SH, O'Donnell AL, Balu D, Pohl MB, Seyler MJ, Mohamed S, Mousa S, Dandona P. Estrogen receptor-alpha in the inhibition of cancer growth and angiogenesis. Cancer Res. 2000;60:7094-7098. [PubMed] |
| 15. | Suo S, Zhang D, Cheng F, Cao M, Hua J, Lu J, Xu J. Added value of mean and entropy of apparent diffusion coefficient values for evaluating histologic phenotypes of invasive ductal breast cancer with MR imaging. Eur Radiol. 2019;29:1425-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Kim JY, Kim SH, Kim YJ, Kang BJ, An YY, Lee AW, Song BJ, Park YS, Lee HB. Enhancement parameters on dynamic contrast enhanced breast MRI: do they correlate with prognostic factors and subtypes of breast cancers? Magn Reson Imaging. 2015;33:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Sun J, Wang Q, Wang L, Gui L, Li Q, Luo Y, Zhang S, Zhang P. [A prospective study of bone loss in early stage postmenopausal breast cancer treated with aromatase inhibitors]. Zhonghua Zhong Liu Za Zhi. 2020;42:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1138] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 19. | Park EK, Cho KR, Seo BK, Woo OH, Cho SB, Bae JW. Additional Value of Diffusion-Weighted Imaging to Evaluate Prognostic Factors of Breast Cancer: Correlation with the Apparent Diffusion Coefficient. Iran J Radiol. 2016;13:e33133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Thompson JL, Wright GP. The role of breast MRI in newly diagnosed breast cancer: An evidence-based review. Am J Surg. 2021;221:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |