Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7275
Peer-review started: May 21, 2021
First decision: June 27, 2021
Revised: June 28, 2021
Accepted: June 3, 2022
Article in press: June 3, 2022
Published online: July 26, 2022
Processing time: 415 Days and 23.6 Hours
Transarterial chemoembolization (TACE) is a recommended treatment for patients with intermediate stage hepatocellular carcinoma (HCC) but with variable treatment outcomes.
To determine factors for predicting outcomes of TACE in patients with intermediate stage B HCC.
Patients with Barcelona Clinic Liver Cancer (BCLC) stage B HCC who underwent TACE as the primary treatment were enrolled at Taichung Veterans General Hospital from January 2005 to December 2009. Patients were assigned to either the objective responder (OR) group or the non-OR group according to mRECIST criteria. Clinical and radiological characteristics were compared between the 2 groups. The overall survival of enrolled subjects was analyzed.
In 128 enrolled patients, 66 (51.6%) were in the OR group and 62 (48.4%) in the non-OR group. Compared with the non-OR group, the OR group had a significantly smaller HCC size (6.55 cm vs 9.50 cm, P = 0.001) and was within the up-to-7 criteria (50% vs 26.7%, P = 0.001). After multivariable analyses, these significant associations still existed. Overall survival rate of all the subjects averaged 20.65 ± 13.26 mo. The survival rate at 1-year was 64.8%, 2-year was 46.9%, and 3-year was 31.2%. For those patients with OR to TACE, smaller tumor size and within up-to-7 criteria were associated with significantly better overall survival. Those patients with subgroup B1 had the highest OR ratio (75%) and better overall survival (26.70 ± 12.07 mo) after TACE.
BCLC stage B HCC patients with smaller tumor size or within up-to-7 criteria had better survival outcomes to TACE. BCLC stage B subgroup is useful to predict refractoriness to TACE.
Core Tip: Transarterial chemoembolization (TACE) is the recommended treatment modality for the intermediate stage or the Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma (HCC) patients. However, due to the clinical heterogeneity in this population of patients, only some have a favorable outcome after TACE. In our study, we discovered that the intermediate stage HCC patients with smaller tumor size or within the up-to-7 criteria showed better survival outcomes for TACE. BCLC stage B subgroup is useful to predict refractoriness of HCC to TACE and to establish future therapeutic strategies.
- Citation: Lee SW, Peng YC, Lien HC, Ko CW, Tung CF, Chang CS. Clinical values of Barcelona Clinic Liver Cancer subgroup and up-to-7 criteria in intermediate stage hepatocellular carcinoma with transcatheter arterial chemoembolization. World J Clin Cases 2022; 10(21): 7275-7284
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7275.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7275
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer worldwide. The widely used algorithm for the classification of HCC is the Barcelona Clinic Liver Cancer (BCLC) staging system, based on tumor characteristics, size, number, presence of macroscopic vascular invasion or extrahepatic spread as well as the hepatic function and performance status of the patient[1,2]. The intermediate stage, or BCLC stage B, is characterized by the presence of large or multifocal HCC without evidence of macroscopic vascular invasion or extrahepatic spread. Transarterial chemoembolization (TACE) is the recommended treatment modality for these stage B patients[3,4]. Previous meta-analyses revealed the overall survival benefit of TACE[5,6]. However, due to the clinical heterogeneity in this population of patients, only some have a favorable outcome after TACE.
The up-to-7 criteria, meaning the sum of the size of the largest tumor and the number of tumors within or beyond 7, first coined by Mazzaferro et al[7], is a practical standard to select those HCC patients for liver transplantation. This criteria was further proposed by Bolondiet al[8] to identify which HCC patients in BCLC stage B could benefit from TACE.
The aim of the present study was to determine useful factors for predicting response to TACE and survival after TACE in patients with intermediate stage HCC.
HCC patients, newly-diagnosed in accordance with the American Association for the Study of Liver Disease[9] guidelines, were recruited for study at Taichung Veterans General Hospital during the period from January 2005 to December 2009. The enrolled criteria included HCC diagnosed in BCLC stage B and who underwent TACE as the primary treatment. Exclusion criteria were those diagnosed with HCC BCLC stages A, C and D and who displayed a poor performance status, missing follow-up or also receiving systemic tumor medications, like oral tyrosine kinase inhibitors. The clinical parameters of all enrolled patients, including age, gender, liver function such as total bilirubin, alanine aminotransferase, alpha-fetoprotein, presence of chronic hepatitis B virus and hepatitis C virus, cirrhotic Child-Pugh stage, and the size and numbers of the tumors, were collected.
TACE was performed with the patient’s written informed consent. A French sheath of size 5 or 6 was first inserted into the common femoral artery. Digital subtraction angiography was performed on the celiac and superior mesenteric arteries to assess the portal vein patency, vascular anatomy and tumor vascularity. All angiographic images were sent to a system of picture archiving and communication. Following the initial arterial assessment, the catheter was advanced into the lobar or segmental hepatic artery that supplied the tumor. If the initial 4 Fr or 5 Fr diagnostic catheter could be advanced into the optimal position, it was used for the TACE infusion. In cases requiring more selective catheterization, a 2.9 Fr microcatheter of Progreat (Terumo, Tokyo, Japan) was used. The TACE infusion point was chosen to enable selective tumor embolization. Once the lesion and its blood supply were identified, an emulsion of 10 to 50 mg epirubicin (Pfizer, NY, United States) and 2 mL to 60 mL Lipiodol (Guerbet, Aulnay sous Bois, France) was injected into the arterial supply of the tumor to the tumor region under fluoroscopic guidance. Administration of the emulsion was followed by embolization with a slurry of Spongostan (Ethicon, NJ, United States) until reaching stasis.
Patients were assessed every 2 mo by dynamic imaging until study end points, including death, disease progression or treatment failure after TACE. The assessment of tumor response followed the mRECIST criteria[10] in four response categories: complete response, partial response, stable disease and progressive disease. Patients with complete response or partial response were classified as the objective responder group (OR), and the patients with stable disease or progressive disease were considered the non-objective responder group (non-OR). In addition, the overall survival of enrolled subjects was collected for analysis.
Data were expressed as standard deviation of mean for each of the measured parameters. The positive rates of the 2 groups were expressed as percentages of the respective patient populations. Statistical comparisons were made using Pearson’s χ2 test for effects of the positive rate of each stratified group. Independent t-test was used to analyze continuous variables. A P value below 0.05 was considered statistically significant. Survival analyses were carried out using the Kaplan-Meier method for univariate analysis, and comparisons were subsequently performed with the log-rank test. Hazard ratios (HR) with a 95% confidence interval (CI) were calculated by multivariate Cox’s regression to examine the strengths of association between clinical parameters and tumor responses following TACE.
A total of 128 patients were enrolled, and their characteristics are shown in Table 1. The median age was 70.02 years, with male predominance (76.6%). Chronic hepatitis B virus was found in 56 patients (43.8%) and hepatitis C virus infection in 41 patients (32.0%). Child-Pugh stage A was found in 93 cases (72.7%) and Child-Pugh stage B in 35 cases (27.3%). The mean tumor nodules number was 4.20, and the mean tumor diameter was 7.98 cm. A total of 45 cases (35.2%) were within the up-to-7 criteria, and 83 cases (64.8%) were beyond the criteria. Based on tumor characteristics and cirrhotic Child-Pugh scores, enrolled subjects were further classified into four subgroups: B1, B2, B3 and B4[8].Their distributions were: B1, 40 (31.3%); B2, 62 (48.4%); B3, 11 (8.7%); and B4, 15 (11.7%).
| All (n = 128) | OR (n = 66, 51.6%) | Non-OR (n = 62, 48.4%) | P value | ||||||||
| mean ± SD | n (%) | mean ± SD | n (%) | mean ± SD | n (%) | ||||||
| Age in yr | 70.02 ± 13.04 | 70.48 ± 13.86 | 69.53 ± 12.21 | 0.6811 | |||||||
| Male sex | 98 (76.6) | 49 (74.2) | 49 (79.0) | 0.5232 | |||||||
| Viral hepatitis | 0.1322 | ||||||||||
| HBV | 56 (43.8) | 26 (39.4) | 30 (48.4) | ||||||||
| HCV | 41 (32.0) | 27 (40.9) | 14 (22.6) | ||||||||
| HBV/HCV | 4 (3.1) | 1 (1.5) | 3 (4.8) | ||||||||
| Nil | 27 (21.1) | 12 (18.2) | 15 (24.2) | ||||||||
| Cirrhosis, Child-Pugh | 0.9852 | ||||||||||
| A | 93 (72.7) | 48 (72.7) | 45 (72.6) | ||||||||
| B | 35 (27.3) | 18 (27.3) | 17 (27.4) | ||||||||
| Bilirubin in mg/dL | 1.27 ± 0.99 | 1.18 ± 0.74 | 1.35 ± 1.20 | 0.3261 | |||||||
| ALT in U/L | 64.38 ± 67.61 | 57.91 ± 43.04 | 71.27 ± 86.30 | 0.2751 | |||||||
| AFP as × 103 ng/mL | 3.42 ± 8.86 | 2.52 ± 7.89 | 4.42 ± 6.84 | 0.4341 | |||||||
| HCC number | 4.20 ± 3.24 | 3.70 ± 2.84 | 4.74 ± 3.55 | 0.0701 | |||||||
| HCC size in cm | 7.98 ± 4.01 | 6.55 ± 2.98 | 9.50 ± 4.40 | 0.0011 | |||||||
| Up-to-7 criteria | 0.0012 | ||||||||||
| Within | 45 (35.2) | 33 (50.0) | 12 (26.7) | ||||||||
| Beyond | 83 (64.8) | 33 (50.0) | 50 (73.3) | ||||||||
| Subclassification | 0.0032 | ||||||||||
| B1 | 40 (31.3) | 30 (45.5) | 10 (16.1) | ||||||||
| B2 | 62 (48.4) | 23 (34.8) | 39 (62.9) | ||||||||
| B3 | 11 (8.6) | 6 (9.1) | 5 (8.1) | ||||||||
| B4 | 15 (11.7) | 7 (10.6) | 8 (12.9) | ||||||||
Therapeutic outcomes of these patients after TACE were analyzed. Their case distributions were 24 cases (18.8%) with complete response, 42 cases (32.8%) with partial response, 30 cases (23.4%) with stable disease and 32 cases (25.0%) with progressive disease. Overall, 66 patients (51.6%) belonged to OR with TACE and 62 (48.4%) patients to non-OR with TACE.
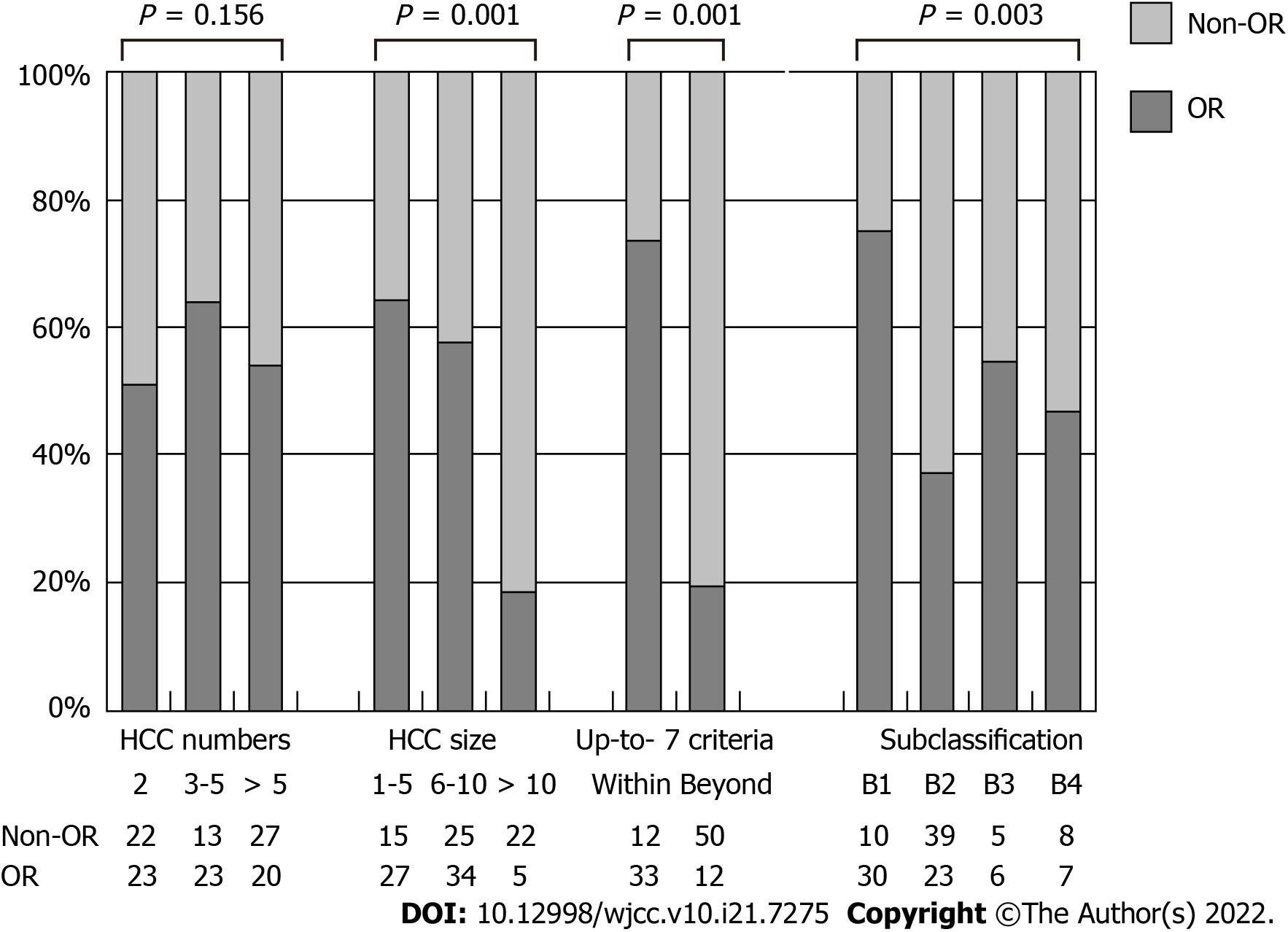
Clinical and tumor characteristics of both groups are shown in Table 1. The 2 groups shared similar factors of age, gender, ratio of chronic viral hepatitis infection and Child-Pugh stage and similar laboratory parameters like total bilirubin, alanine aminotransferase and alpha-fetoprotein. However, the OR group had a significantly smaller HCC than the non-OR group (mean tumor size, 6.55 cm vs 9.50 cm, P = 0.001). The ratio of within up-to-7 criteria was 50.0% in the OR group and 26.7% in the non-OR group. The difference was also significant (P= 0.001). On the contrary, the numbers of HCC between these 2 groups were similar (mean tumor numbers, 3.70 vs 4.74, P = 0.070). Significantly more patients with OR after TACE were in the subgroup B1 than in other subgroups (B2, B3 and B4) (45.5% vs 34.8%, 9.1% and 10.6%, respectively, P = 0.003).
Patients were further classified into subgroups according to tumor number (2, 3-5, > 5) and size (1-5 cm, 6-10 cm, > 10 cm). The subgroup percentages of OR with different HCC numbers, size or up-to-7 criteria are shown in Figure 1. Similarly, significantly more OR existed in those with smaller HCC (OR ratio, 64.2% vs 57.6% vs 18.5%, P = 0.001) and within up-to-7 criteria (73.3% vs 19.4%, P = 0.001). No association was found between OR following TACE and the HCC number (51.1% vs 63.9% vs 42.6%, P = 0.156). More OR were found with subgroup B1 compared with other subgroups (75% vs 34.8%, 9.1%, 10.6%, P = 0.003).
Associations between clinical parameters and OR following TACE based on logistic analysis are shown in Table 2. Those variables with no association were age, gender, viral hepatitis, cirrhosis Child-Pugh stage, alanine aminotransferase, alpha-fetoprotein and HCC number. On the other hand, larger HCC size (HR = 0.81, 95%CI: 0.73-0.90, P = 0.002) and the beyond up-to-7 criteria (HR = 0.24, 95%CI: 0.11-0.53, P = 0.004) had a significant negative impact on achieving OR after TACE. After multivariable analysis with adjusting other variables, these significant associations still persisted (HCC size, HR = 0.81, 95%CI: 0.72-0.91, P = 0.002; beyond up-to-7 criteria, HR = 0.25, 95%CI: 0.11-0.57, P = 0.009).
| Univariable analysis | Multivariable analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 0.99 (0.97-1.02) | 0.679 | ||
| Sex | 1.31 (0.57-2.98) | 0.523 | ||
| Viral hepatitis | 0.75 (0.48-1.17) | 0.209 | ||
| Cirrhosis, Child-Pugh stage | 0.99 (0.45-2.16) | 0.156 | ||
| ALT | 1.00 (0.99-1.01) | 0.281 | ||
| AFP | 1.00 (1.00-1.00) | 0.478 | ||
| HCC numbers | 0.90 (0.81-1.00) | 0.069 | 0.91 (0.81-1.02) | 0.086 |
| HCC size | 0.81 (0.73-0.90) | 0.002 | 0.81 (0.72-0.91) | 0.002 |
| Beyond up-to-7 criteria | 0.24 (0.11-0.53) | 0.004 | 0.25 (0.11-0.57) | 0.009 |
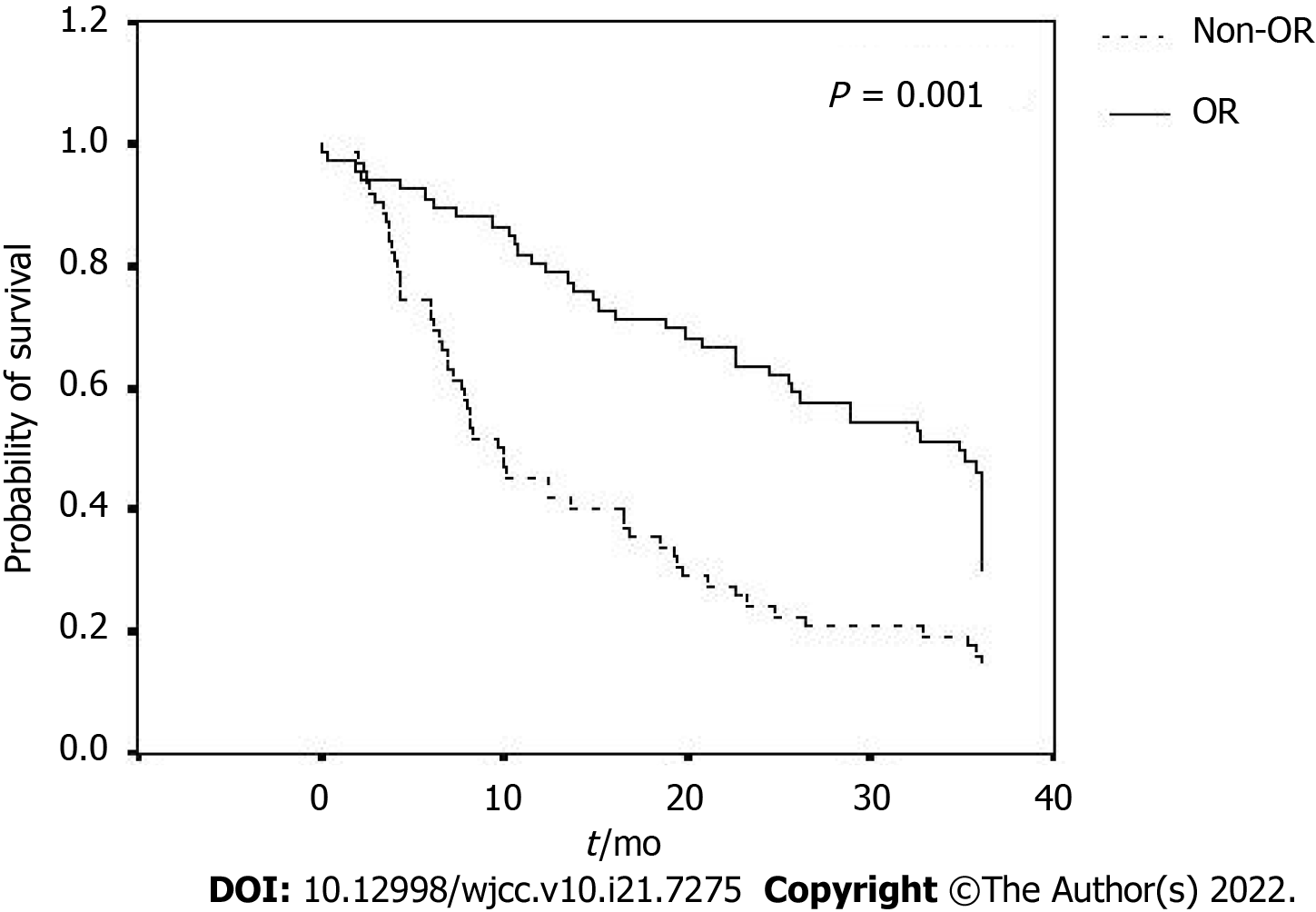
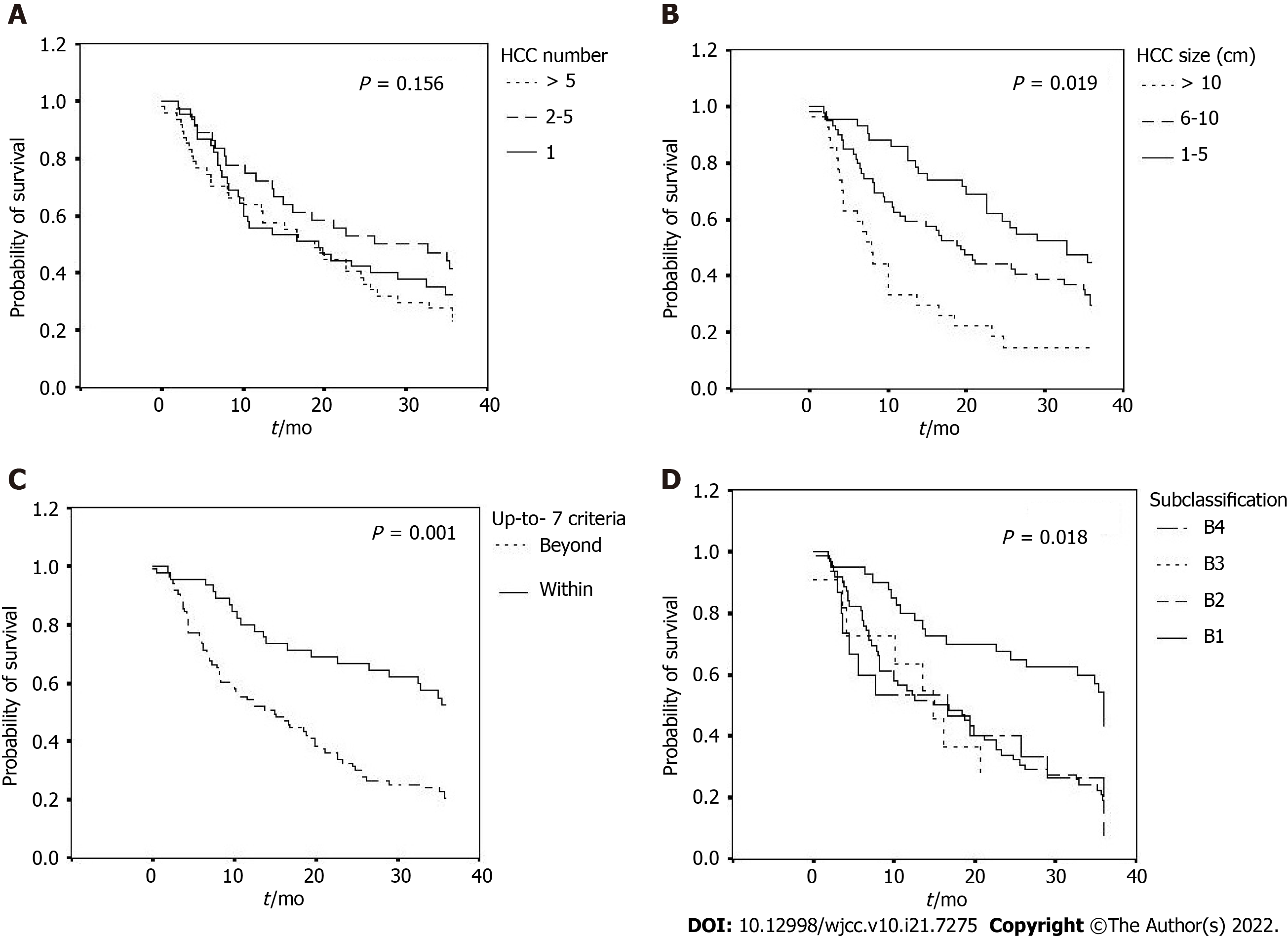
Overall survival rates of the enrolled patients are shown in Table 3, Figure 2 and Figure 3A-D. Their survival rates were 64.8% for 1-year, 46.9% for 2-year and 31.2% for 3-year, with an average overall survival period of 20.65 ± 13.26 mo. Those patients with smaller HCC, within up-to-7 criteria or the subgroup B1 had the tendency to remain alive at the 1-year, 2-year and 3-year follow-up. The OR group had a significantly longer overall survival than the non-OR group (25.80 ± 12.12 mo vs 15.18 ± 12.26 mo, P = 0.001). The longest average overall survival appeared in patients with 1 cm to 5 cm HCC followed by those with 6 cm to 10 cm and over 10 cm tumors (26.09 ± 11.48 mo vs 20.50 ± 13.35 mo vs 12.55 ± 11.71 mo, respectively, P = 0.019). Those with tumors within the up-to-7 criteria had a significantly better overall survival than those without (26.75 ± 11.97 mo vs 17.35 ± 12.80 mo, P = 0.001). However, the mean overall survival was similar among the patients with different HCC numbers (2, 3-5, > 5; 19.98 ± 13.13 mo vs 23.72 ± 13.17 mo vs 18.96 ± 13.33 mo, respectively, P = 0.156). Among the different BCLC stage B subgroups, those in subgroup B1 had a significantly better overall survival than other subgroups (B2, B3 and B4) (26.70 ± 12.07 mo vs 18.07 ± 12.73 mo, 17.38 ± 13.39 mo, 17.62 ± 14.18 mo, respectively, P = 0.018).
| All, n | ≥ 1 yr | ≥ 2 yr | ≥ 3 yr | P value | ||
| All | ||||||
| 128 | 83 (64.8) | 60 (46.9) | 40 (31.2) | |||
| HCC number | 0.629 | |||||
| 2 | 45 | 26 (57.8) | 19 (42.2) | 13 (28.9) | ||
| 3-5 | 36 | 27 (75.0) | 20 (55.6) | 15 (41.7) | ||
| > 5 | 47 | 30 (63.8) | 21 (44.7) | 12 (25.5) | ||
| HCC size in cm | 0.001 | |||||
| 1-5 | 42 | 36 (85.7) | 29 (69.0) | 17 (40.5) | ||
| 6-10 | 59 | 38 (64.4) | 26 (44.1) | 19 (32.2) | ||
| > 10 | 27 | 9 (33.4) | 5 (18.5) | 4 (14.8) | ||
| Up-to-7 criteria | 0.003 | |||||
| Within | 45 | 36 (80.0) | 31 (68.9) | 22 (48.9) | ||
| Beyond | 83 | 47 (56.6) | 29 (34.9) | 18 (21.9) | ||
| Subclassification | 0.049 | |||||
| B1 | 40 | 12 (30.0) | 8 (20.0) | 20 (50.0) | ||
| B2 | 62 | 39 (62.9) | 10 (16.1) | 13 (21.0) | ||
| B3 | 11 | 8 (72.7) | 0 | 3 (27.3) | ||
| B4 | 15 | 9 (60.0) | 2 (13.3) | 4 (26.7) | ||
HCC is the most common type of primary liver cancer worldwide, with a steady rise in incidence. Merely 30% to 40% of patients are diagnosed at an early stage and that means most patients do not benefit from curative therapies[2]. Treatment recommendations for unresectable HCC include locoregional therapy, like TACE, and systemic therapy, like sorafenib. According to the BCLC staging system, TACE is the standard care method for BCLC stage B HCC[3].
One meta-analysis reported 2-year survival benefits of TACE on HCC in 545 patients: 41% (range 19% to 63%) in the treatment group and 27% (range 11% to 50%) in the control group. The ratio of treatment-induced OR is 35% (range 16% to 61%)[5]. Another systematic review with data from more than 10000 patients with HCC undergoing TACE found the ratio of OR was 52.5%, with overall survival at 70.3% for 1-year, 51.8% for 2-year, 40.4% for 3-year and 32.4% for 5-year[6]. In our study, 66 of 128 cases achieved OR with TACE, with the OR ratio at 51.6%. The 1-year, 2-year and 3-year survival rates were 64.8%, 46.9% and 31.2%, respectively. Our results were comparable with previous studies.
TACE is performed through the injection of chemotherapy mixed with Lipiodol (ethiodized oil), followed by the obstruction of a selected hepatic artery branch that feeds HCC, resulting in ischemic necrosis of the tumor and slows tumor progression[11]. However, TACE often results in incomplete tumor necrosis. Tumor recurrence after TACE is therefore common. Additionally, repeating TACE could damage liver function resulting in worse survival. Predicting factors with no satisfactory response to TACE should be determined so that other therapeutic strategies can be applied early to these patients.
The up-to-7 criteria is used widely for the subclassification of patients who would benefit from TACE[8]. A past study suggested that first time TACE is more effective for HCC patients with nodules < 5 cm, whereas those with nodules > 5 cm have poorer response and poorer outcomes[12]. A validated prognostic scoring system, hepatoma arterial-embolization prognostic score, based on four factors including albumin, bilirubin, α-fetoprotein and tumor size, was found to be a significant predictor of the overall survival[13].
For our patients, only HCC size and the up-to-7 criteria, but not HCC number, had a significant role in determining the therapeutic outcomes of patients treated with TACE, both in tumor responses and overall survival. The ratios of OR after TACE among patients with HCC size 1-5 cm, 6-10 cm and > 10 cm were 64.2%, 57.6% and 18.5%, respectively (P = 0.001). The mean overall survival of these subgroups was 26.09 mo, 20.50 mo and 12.55 mo, respectively (P = 0.019). The ratios of OR with TACE for subjects within and beyond the up-to-7 criteria were 73.3% and 19.4% (P = 0.001), respectively, and the corresponding mean overall survival was 26.75 mo and 17.35 mo (P = 0.001).
One retrospective study in Japan enrolling 50 patients with BCLC stage B HCC who underwent TACE reported that tumor size (HR 0.997, P = 0.811) was not a meaningful factor determining tumor response[14]. The difference in results between their study and our study could be related to differences in HCC size (their mean tumor size, 3.1 cm compared with 7.98 cm in our study).
The heterogeneity of BCLC stage B HCC has led to the division of BCLC stage B into 4 subgroups (B1, B2, B3 and B4), based on tumor burden and liver function, and that corresponded to a rising severity of the disease. In this scenario, TACE is proposed as the first-line therapy for patients in subgroups B1 and B2 and as a potential option in subgroup B3 but not for subgroup B4[8]. Our results supported the best outcomes for TACE in subgroup B1 (OR ratio 75%, mean overall survival 26.7 mo), followed by B2 (OR ratio 34.8%, mean overall survival 18.07 mo), B3 (OR ratio 9.1%, mean overall survival 17.38 mo) and B4 (OR ratio 10.6%, mean overall survival 17.62 mo). For subgroups B3 and B4, other treatment options to be considered include transarterial radioembolization and oral tyrosine kinase inhibitors.
Our study has several limitations. First, this is a retrospective study, with the potential bias in patient selection or reporting. Second, our sample size was relatively small. Third, all our patients received conventional TACE. Those with TACE using drug-eluting bead therapies were not included, despite past meta-analyses reporting no difference in outcomes between the two TACE methods[15,16]. At last, the sessions of repeat TACE or adverse effects of TACE of our cases were not recorded. Further research with a larger number of patients including more variables is desirable.
In conclusion, the intermediate stage HCC patients with smaller tumor size or within the up-to-7 criteria showed better survival outcomes for TACE. The BCLC stage B subgroup is useful to predict refractoriness of HCC to TACE and to establish future therapeutic strategies.
The widely used algorithm for the classification of hepatocellular carcinoma (HCC) is the Barcelona Clinic Liver Cancer (BCLC) staging system, based on tumor characteristics, like size, number, presence of macroscopic vascular invasion or extrahepatic spread as well as the hepatic function and performance status of the patient. The intermediate stage, or BCLC stage B, is characterized by the presence of large or multifocal HCC without evidence of macroscopic vascular invasion or extrahepatic spread. Transarterial chemoembolization (TACE) is the recommended treatment modality for these stage B patients. However, due to the clinical heterogeneity in this population of patients, only some have a favorable outcome after TACE.
The up-to-7 criteria, meaning the sum of the size of the largest tumor and the number of tumors within or beyond 7, is a practical standard to select those HCC patients for liver transplantation. This criteria was further proposed to identify which HCC patients in BCLC stage B could benefit from TACE.
The aim of the present study was to determine useful factors for predicting response to TACE and survival after TACE in patients with intermediate stage HCC.
Patients with BCLC stage B HCC who underwent TACE as the primary treatment were enrolled at Taichung Veterans General Hospital from January 2005 to December 2009. Patients were assigned to either the objective responder (OR) group or the non-OR group according to mRECIST criteria. Clinical and radiological characteristics were compared between the 2 groups. The overall survival of enrolled subjects was analyzed.
In 128 enrolled patients, 66 (51.6%) were in the OR group and 62 (48.4%) in the non-OR group. Compared with the non-OR group, the OR group had a significantly smaller HCC size (6.55 cm vs 9.50 cm, P = 0.001) and was within the up-to-7 criteria (50% vs 26.7%, P = 0.001). After multivariable analyses, these significant associations still existed. Overall average survival rate of all the subjects was 20.65 ± 13.26 mo. Survival rates at 1-year were 64.8%, at 2-year were 46.9% and at 3-year were 31.2%. For those patients with OR to TACE, smaller tumor size and within up-to-7 criteria were associated with significantly better overall survival. Those patients with subgroup B1 had the highest OR ratio (75%) and better overall survival (26.70 ± 12.07 mo) after TACE.
BCLC stage B HCC patients with smaller tumor size or within up-to-7 criteria had better survival outcomes to TACE. BCLC stage B subgroup is useful to predict refractoriness to TACE.
In the future, the BCLC stage subgroup and up-to-7 criteria should have predictive value for patients with intermediate stage HCC receiving TACE.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: de la Pinta C, Spain; Zeng YY, China A-Editor: Liu X, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2873] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3595] [Article Influence: 276.5] [Reference Citation Analysis (4)] |
| 3. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 4. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 5. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2270] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 6. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodoltransarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 519] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 7. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1572] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 8. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 9. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3023] [Article Influence: 431.9] [Reference Citation Analysis (3)] |
| 10. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3301] [Article Influence: 220.1] [Reference Citation Analysis (36)] |
| 11. | Au JS, Frenette CT. Management of Hepatocellular Carcinoma: Current Status and Future Directions. Gut Liver. 2015;9:437-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Golfieri R, Renzulli M, Mosconi C, Forlani L, Giampalma E, Piscaglia F, Trevisani F, Bolondi L; Bologna Liver Oncology Group (BLOG). Hepatocellular carcinoma responding to superselectivetransarterial chemoembolization: an issue of nodule dimension? J VascIntervRadiol. 2013;24:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Kadalayil L, Benini R, Pallan L, O'Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, Palmer DH, Meyer T. A simple prognostic scoring system for patients receiving transarterialembolisation for hepatocellular cancer. Ann Oncol. 2013;24:2565-2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 14. | Katayama K, Imai T, Abe Y, Nawa T, Maeda N, Nakanishi K, Wada H, Fukui K, Ito Y, Yokota I, Ohkawa K. Number of Nodules but not Size of Hepatocellular Carcinoma Can Predict Refractoriness to Transarterial Chemoembolization and Poor Prognosis. J Clin Med Res. 2018;10:765-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, Bruix J. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 719] [Article Influence: 39.9] [Reference Citation Analysis (1)] |
| 16. | Gao S, Yang Z, Zheng Z, Yao J, Deng M, Xie H, Zheng S, Zhou L. Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |