INTRODUCTION
Glucocorticoids (GCs) are hormones produced by the adrenal cortex and can also be synthesized chemically. Exogenous hormones, including dexamethasone, prednisone, and methylprednisolone, are mainly used in diseases such as various allergic diseases and rheumatic immune diseases. Some GCs are derived from the adrenal cortex, such as hydrocortisone, which is mainly used for endocrine replacement therapy or as a treatment in various forms of congenital adrenal hyperplasia. GCs, which participate in many important physiological processes (such as metabolism, inflammation, immunity, and stress), are clinically used in the treatment of various types of acute and chronic inflammation, autoimmune diseases, organ transplantation, and tumors. GCs have many effects on immune regulation, and an important consideration is the influence on white blood cells, including the influence on their count and function. GCs act on nearly every cell type of the immune system, but the functional aspects of GCs differ by cell type. The mechanism of GC action on leukocytes is very complex, and studies have suggested that GCs exert anti-inflammatory and immunomodulatory effects through genomic and non-genomic mechanisms. Finally, 44 articles were selected for analysis and summary. This article summarizes the relevant research on the effects of GCs on leukocytes in recent decades.
EFFECTS OF GCs ON LEUKOCYTES: GENOMIC AND NON-GENOMIC MECHANISMS
Effects of GCs on neutrophils
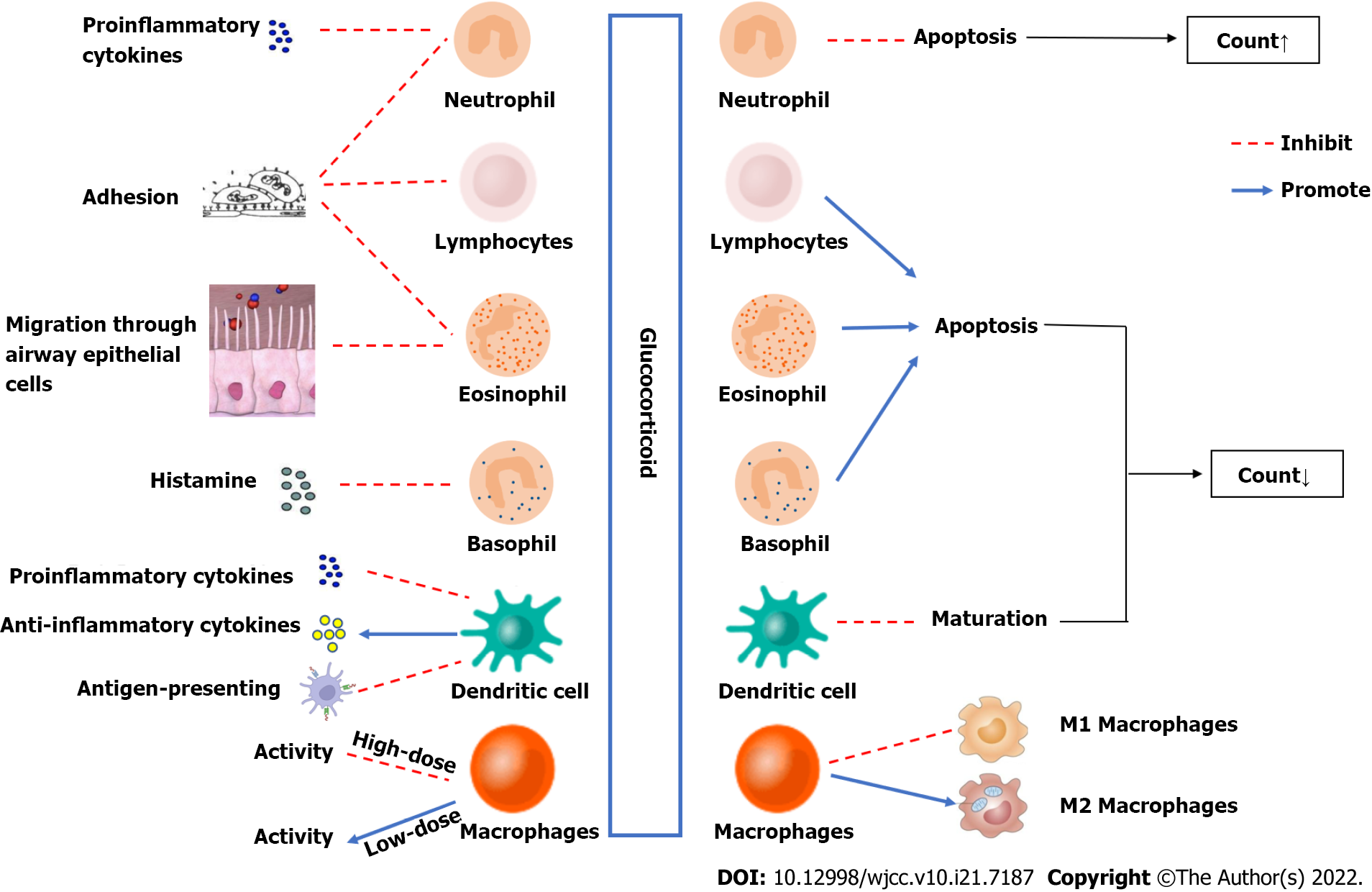
Effects of GCs on neutrophil count: Barden et al[1] found that after 24 h of dexamethasone use in healthy volunteers, the neutrophil count increased in a dose-dependent manner[1]. GCs increase the neutrophil count in peripheral blood through a variety of mechanisms of action: (1) They promote neutrophil attachment to the blood vessel walls to enter the blood circulation (the marginal pool enters the circulating pool); (2) They reduce neutrophil outflow from the circulating pool; (3) They inhibit the apoptosis of neutrophils and delay their clearance in peripheral blood; and (4) They stimulate hematopoiesis in the bone marrow and increase the production of neutrophils in the peripheral blood[2,3].
Cavalcanti et al[4] confirmed in rat experiments that endogenous GCs can promote the maturation of bone marrow neutrophils and the mobilization of neutrophils from the bone marrow into the circulation[4]. A few studies have confirmed that GCs can inhibit the apoptosis of neutrophils and delay their clearance both in vivo and in vitro[3,5]. However, the mechanism by which GCs regulate neutrophil apoptosis is still unclear. It has been suggested that GC-mediated apoptosis inhibition mechanisms may include upregulation of antiapoptotic B-cell lymphoma-2 (Bcl-2) and members of the inhibitor of apoptosis family. For example, dexamethasone has been shown to induce survival and enhance McL-1, a proapoptotic member of the Bcl-2 family, through phosphatidylinositol 3-kinase (PI3K) and P38 mitogen-activated protein kinase (P38 MAPK) in human neutrophils[6]. Research by Chapman et al[7] confirmed that GCs can promote macrophages to phagocytose apoptotic leukocytes and cause them to rapidly degrade without causing proinflammatory secretory reactions. This effect can be strengthened by 11β-hydroxysteroid dehydrogenase[7]. Additionally, GCs can stimulate bone marrow hematopoiesis and increase bone marrow neutrophil production. Endogenous GCs are one of the factors stimulating the maturation of bone marrow neutrophils and promoting the mobilization of neutrophils from the bone marrow into the circulation[3]. Human and mouse neutrophil migration depends on the induction of interleukin (IL)-8 expression, and this induction is inhibited by GCs[8]. For the migration of neutrophils, GCs inhibit this migration by attenuating the expression of CXC receptor 2 agonists such as IL-8 and CXCL18b[9]. Ricci et al[10] found in mouse experiments that GC-induced leucine zipper (GILZ) inhibited the migration of neutrophils by controlling the expression of annexin A1[10].
Effects of GCs on the intercellular adhesion of neutrophils: GCs can inhibit neutrophil-endothelial cell adhesion, and their molecular mechanism may include downregulation of GCs on cell surface adhesion factors, such as intercellular adhesion molecule-1 (ICAM-1), endothelial cell adhesion molecule-1, E-selectin, P-selectin and L-selectin, thereby affecting the leukocyte-endothelial cell interaction[4]. The stagnation of leukocytes on the endothelial surface is largely mediated by leukocyte integrins, especially β1 (late antigen-4) and β2 (lymphatic function-related antigen-1 and macrophage antigen-1), and their respective endothelial counterparts are cell adhesion molecule-1 (CAM-1), ICAM-1 and ICAM-2. In the process of inflammation, CAM, ICAM-1, and E-selectin, among other adhesion molecules, are significantly upregulated to promote the adhesion, aggregation and activation of leukocytes. Cell experiments have confirmed that GCs can inhibit the upregulation of these factors, thereby inhibiting cell adhesion. Therefore, GCs may also prohibit the expression of adhesion molecules by inhibiting the synthesis of cytokines. Most of the effects of GCs are caused by genomic mechanisms; that is, they affect cell transcription and protein expression, such as inhibiting the activation of the nuclear factor-κB (NF-κB) pathway and inducing MAPK phosphatase-1 to inhibit MAPK activation, thereby reducing the expression of cytokines, chemokines and adhesion molecules[11-13], including CD44 and integrin lymphocyte function-associated antigen 1 (LFA-1) and very late antigen 4, to inhibit neutrophil adhesion[14].
Effects of GCs on neutrophil function: Neutrophils are an important line of defense in the human body against foreign pathogens. Neutrophils are rapidly activated after encountering foreign antigens (such as viruses or bacteria), and their activation is followed by phagocytosis and degranulation. Then, enzymes in the particles enter the phagolysosome or cytoplasm. These enzymes are excreted outside of the cell and exert functions such as sterilization, lysis, and digestion of foreign bodies. In vitro studies have found that when human neutrophils are acutely exposed to methylprednisolone or hydrocortisone, their N-formyl-methionyl-leucyl-phenylalanine-induced neutrophil degranulation is obviously suppressed, and this effect is not influenced by RU486 or cycloheximide[15]. Research by Ricci et al[16] confirmed that GILZ inhibits the activation and migration of neutrophils to inflammation sites by inhibiting the MAPK pathway and the secretion of proinflammatory cytokines by neutrophils[16].
Effects of GCs on lymphocytes
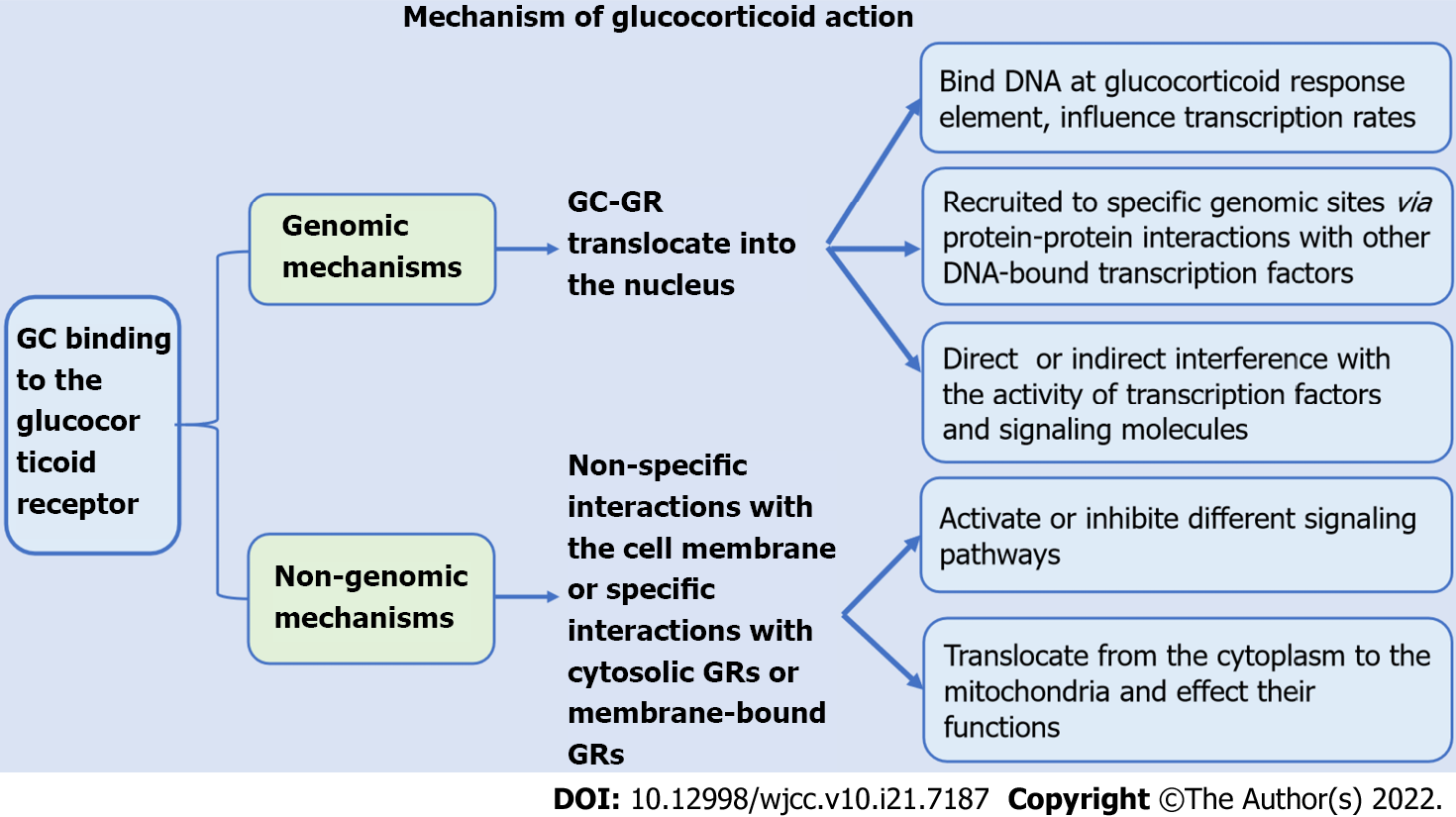
Effects of GCs on lymphocyte count: GCs can reduce peripheral blood lymphocyte counts. The peripheral blood lymphocyte count decreased significantly after short- or long-term application of GCs in both animal and clinical experiments[17]. GCs promote the apoptosis of lymphocytes and significantly decrease the lymphocyte count in peripheral blood. In vitro cell culture of mouse spleen cells and bone marrow lymphocytes revealed that dexamethasone stimulated the apoptosis of all B cell developmental subgroups, while in vivo experiments showed that immature B cells promoted GCs. Multiple injections of dexamethasone regulated the number of B cells in the bone marrow but did not affect the number of mature B cells in the body[18]. Costa et al[19] also confirmed that hydrocortisone could regulate the production of B lymphocytes[19]. Several experiments have shown that GCs are involved in regulating T cell apoptosis, and the mechanisms include genomic and nongenomic mechanisms. GCs exert their effects predominantly through the GC glucocorticoid receptor (GR). The genomic function of GCs is mainly to bind to specific GRs in the cytoplasm to form complexes and transfer to the nucleus, thus regulating the transcriptional activity of GC response genes. The GR can dimerize and directly bind DNA at GC response elements, affecting transcription rates. In addition, ligand-bound GR can be recruited to specific genomic sites via protein-protein interactions with other DNA-bound transcription factors. GCs also exert genomic effects by interfering with the activity of transcription factors and signaling molecules[20]. Genes with up- or down-regulated expression in GC-induced apoptosis include c-myc, tdag8, dig2, Bim, and PUMA[21,22]. Non-genomic effects include the physicochemical interactions of GCs with biological membranes, the effects mediated by the GC-GR complex and the GC-induced mitochondrial apoptotic pathway. These mechanisms have not been fully elucidated[21]. Multiple mouse experiments have demonstrated that pro- and antiapoptotic members of the Bcl-2 family are involved in GC-induced apoptosis in lymphocytes. Caspase-3 and caspase-8 are thought to mediate GC-induced apoptosis[21-24]. The events involved in GC-induced apoptosis include the production of ceramide, changes in intracellular sodium and potassium levels, the activation of PI3K and inositol triphosphate receptors, and the interaction of GR and other signaling proteins, such as protein kinase C and Raf[22].
Effects of GCs on lymphocyte activity: GCs can inhibit lymphocyte proliferation and reduce lymphocyte activity, thereby inhibiting cellular and humoral immunity. GCs affect the activity of transcription factors downstream of T cell receptor (TCR) activation, including NF-κB, activator protein 1 (AP-1) and nuclear factor of activated T cells[14,22]. GCs can also regulate T cell activation by regulating the functions of DCs, macrophages, and mast cells[25]. Studies have suggested that the effect of GCs on T cells is partly mediated by GILZ. GILZ regulates cell apoptosis, proliferation and differentiation by regulating transcription factors and signaling pathways related to host immunity and inflammation. For example, GILZ associates with NF-κB and inhibits NF-κB- and AP-1-dependent transcription. GILZ also binds Raf and Ras and inhibits the activation of Ras/Raf downstream targets, including MAPK1. GILZ also promotes the activity of regulatory T cells (Tregs) by activating transforming growth factor-β signaling. Ultimately, these effects inhibit T cell activation, regulate T helper Th-1, Th-2, and Th-17 cell differentiation, and reduce interferon-γ (IFN-γ) production by Th1, CD8 T, and NK cells, leading to the inhibition of cytotoxic responses[25,26]. A large number of studies have shown that GCs preferentially inhibit the responses of Th-1 cells and Th-17 cells while retaining or even promoting the functions of Th-2 cells and regulatory T cells[14]. GCs have the potential to promote Th2 cytokine production. CD4 T cells pretreated with dexamethasone produce higher levels of IL-4, IL-10 and IL-13[26]. GCs can inhibit the adhesion of lymphocytes to endothelial cell lines and inhibit the intercellular aggregation of activated lymphocytes[11]. Xing et al[27] proposed that GCs induce programmed cell death 1 (PD-1) expression in activated T cells and inhibit TCR-mediated T cell proliferation and cytokine production, including IL-2, IFN-γ and tumour necrosis factor-α (TNF-α)[27]. Studies by Okoye et al[28] confirmed that dexamethasone can affect the activity of T cells by promoting the expression of PD-1 and CTLA-4 through activated T cells, inhibiting the secretion of cytokines and inducing their apoptosis[28]. GCs can regulate the maturation and differentiation of regulatory T cell subsets. For patients with autoimmune diseases, allergies or autoinflammatory diseases, GC therapy can lead to the expansion of Treg cells[29]. Cain et al[30] found through experiments in mice that GCs regulate the expression of CXCR4 in B cells, thereby promoting their migration to the bone marrow[30].
Effects of GCs on macrophages
Effects of GCs on macrophage count: GCs have a concentration-dependent dual effect on macrophages. Low concentrations have immunostimulatory effects on macrophage functions such as adhesion, transformation, phagocytosis and cytokine production, while high concentrations exert immunosuppressive effects[31,32]. GCs can also directly induce specific changes in cell survival, proliferation and phagocytosis, thereby inhibiting cell proliferation. Ai et al[5] found that dexamethasone induced GR recruitment to the transcription factor Krüppel-like factor 9 promoter and increased mitochondrial ROS production, leading to mitochondrial-dependent apoptosis of macrophages[5].
Effects of GCs on macrophage differentiation: GCs affect the typing and differentiation of macrophages. Heideveld et al[33] confirmed that GC receptor activation differentiates monocytes into anti-inflammatory tissue macrophages with an M2 phenotype[33]. Experiments have confirmed that GCs can stimulate human and mouse macrophages to phagocytose apoptotic substances, and monocytes can change their intracellular composition under the induction of GCs, regulate cell skeletal reorganization and adhesion, and thus transform into a highly phagocytic monocyte-derived macrophage (MDMφ) phenotype[31]. In animal models of arthritis and acute lung injury, GCs have been shown to inhibit the differentiation of macrophages to the M1 phenotype[9].
Effects of GCs on macrophage function: GCs can stimulate the ability of macrophages to swallow apoptotic substances. Exposure of mouse and human macrophages to GCs for 24 h leads to increased uptake of apoptotic bodies[34]. GCs initiate gene programs in monocytes and macrophages to promote the phagocytosis of apoptotic cells and debridement cells[14]. GCs have been shown to inhibit the production of several proinflammatory cytokines in human monocytes and macrophages, including IL-1β, IL-6, IL-12, TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF)[35].
Effects of GCs on eosinophils
Effects of GCs on eosinophil count: GC application can reduce the number of peripheral blood eosinophils. The application of inhaled corticosteroids can reduce the number of eosinophils in the peripheral blood circulation and tracheal mucosa of asthma patients[36]. When GCs are used in Crohn's disease, chronic obstructive pulmonary disease, eosinophilic bronchitis, eosinophilic gastroenteritis, nephrotic syndrome and other diseases, a decrease in the patient's eosinophil count can be observed[37-39]. GCs can promote the apoptosis of eosinophils[9,31]. Cell experiments indicate that without the involvement of cytokines, GCs can accelerate the apoptosis of eosinophils, while GCs can reverse the cell survival induced by TNF-α and antagonize low-dose GM-CSF and IL-5 inhibition of apoptosis on eosinophils but cannot reverse the effects induced by IL-3, IL-5, and GM-CSF at the concentration that produces the maximum anti-apoptotic effect[40]. The findings of Hong et al[41] showed that GC-induced eosinophilia is caused by CXCR4-dependent migration of eosinophils to the bone marrow[41].
Effects of GCs on eosinophil function: In mouse eosinophils, dexamethasone and budesonide reduce the expression of basal CD11b in a concentration-dependent manner, thereby affecting the adhesion of eosinophils[42]. A study found that preincubation of cells with different concentrations of budesonide can also effectively downregulate the expression of LFA-1 and Mac-1 induced by GM-CSF on eosinophils and downregulate the migration of eosinophils through airway epithelial cells[43]. Studies have found that the level of IL-5 in sputum decreases after prednisone or prednisolone treatment, further affecting the recruitment, activation and survival of eosinophils[37].
Effects of GCs on basophils
GCs can cause a decrease in peripheral blood basophil count. Barden et al[1] found that peripheral blood basophil hormone decreased significantly after 4 h of dexamethasone use in healthy volunteers[1]. GCs can inhibit the release of histamine from basophils, increase the transcription of leukocyte protease inhibitors, and reduce the basophil count[31]. Thus, GCs can promote the apoptosis of basophils[9].
Effects of GCs on dendritic cells
Both in vivo and in vitro, GCs can inhibit dendritic cell (DC) maturation and weaken the activity of DCs[31]. Studies have shown that the application of inhaled corticosteroids can rapidly reduce the number of peripheral blood DCs in patients with allergic rhinitis, and the DC activation markers CD86 and CD80 are reduced to varying degrees, suggesting that GCs inhibit DC activation[44]. GCs inhibit DC function, reduce the expression of class II MHC and costimulatory molecules, reduce proinflammatory cytokines and increase the secretion of anti-inflammatory cytokines. GCs can also improve the ability of DCs to capture antigens but inhibit their function as antigen-presenting cells[25,45].
The role of GCs on leukocytes is very important, and a lack of GCs or excessive GCs in the body will cause abnormal states of the body. Impaired adrenocortical axis integrity in vivo can lead to immunodeficiencies. For example, patients with deficits in anterior pituitary function and variable immune deficiency presenting with adrenocorticotropin deficiency have decreased B cells, persistent hypoglobulinemia, and susceptibility to infection. The patient's symptoms improved significantly after hydrocortisone replacement therapy[46,47]. Elevated leukocytes and neutrophils and decreased lymphocytes can be observed in patients with Cushing's syndrome. During remission or hormonal control of the disease, a significant decrease in neutrophil counts and in the hemoglobin concentration together with a rise in lymphocyte numbers was observed[48].
CONCLUSION
GCs increase peripheral blood neutrophil counts through genomic and non-genomic mechanisms and inhibit cell adhesion and neutrophil activation and secretion. Moreover, GCs reduce the counts of lymphocytes, eosinophils, basophils, and mononuclear macrophages, reduce cell activity, regulate the distribution of T cell subsets, and inhibit the expression of proinflammatory factors and chemokines. GCs increase peripheral blood neutrophil counts through genomic and non-genomic effects and reduce the numbers of lymphocytes, eosinophils, basophils, and monocytes. GCs also regulate cell activity and affect cells. The processes of adhesion, activation, secretion and differentiation inhibit the expression of proinflammatory factors and chemokines (Figure 1). The mechanisms of action of GCs include effects on intracellular transcription and protein expression, effects on mitochondria, physical and chemical interactions with biological membranes, and receptor-mediated interactions with signal proteins (Figure 2). Clinically, due to the immunosuppressive effect of GCs, patients treated with GCs are prone to coinfection. For patients on GC therapy, the effects of GCs on white blood cells are similar to the effects of bacterial infections on white blood cells, which may lead to misdiagnosis of patients suffering from infection and overuse of antibiotics. Therefore, it is very important to identify whether patients who use GCs have infections. As a consequence, we suggest that clinicians should be more cautious in assessing the presence of infection in children with long-term use of GCs and avoid overuse of antibiotics in the presence of elevated leukocytes. The role of GCs is very important, and a lack of or excess of GCs in the body can cause abnormalities. In recent years, many studies have examined intracellular transcription and the cellular pathways related to the effects of GCs on white blood cells, but these processes are not yet fully clear. Further research on these mechanisms will help to develop new therapeutic strategies.
Figure 1 Mechanism of glucocorticoid action.
Figure 2 Effects of glucocorticoids on leukocytes.
GC: Glucocorticoid; GR: Glucocorticoid receptor.