Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.7153
Peer-review started: February 17, 2022
First decision: March 24, 2022
Revised: April 4, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: July 16, 2022
Processing time: 137 Days and 12.7 Hours
It is rare for urothelial and renal cell carcinomas to coexist in the same patient, and even rarer for them to be detected simultaneously. Because of this rarity, a standard treatment has not been established and studies about overall survival are scarce. Therefore, physicians must modify treatments according to the individual’s situation and the stage of each disease. In recent years, with advances in the instruments and techniques, minimal invasive robotic surgeries have become available for advanced-stage or high-risk patients.
An 85-year-old woman with a medical history of hypertension and hyperlipidemia visited our institution. She had visited her local hospital complaining of intermittent, painless, gross hematuria that had started 3 mo earlier. On computed tomography, a right renal mass and left proximal ureteral mass with hydro
This case report assessed the feasibility of simultaneous minimal invasive robotic surgery as an alternative to conventional open or laparoscopic surgery.
Core Tip: Upper tract urothelial carcinoma with synchronous contralateral renal cell carcinoma is extremely rare and seldom reported. There have only been a few reported cases or a small series. Therefore, no effective standard treatment has been established. In high-risk patients with multiple comorbidities or the super elderly, a conventional open or laparoscopic approach to bilateral tumor lesions can be burdensome to both patient and surgeon, considering perioperative or postoperative complications. This case report might present an attractive treatment option using a minimal invasive robot-assisted approach done simultaneously at once, and suggests that these methods can be used more widely.
- Citation: Yun JK, Kim SH, Kim WB, Kim HK, Lee SW. Simultaneous robot-assisted approach in a super-elderly patient with urothelial carcinoma and synchronous contralateral renal cell carcinoma: A case report. World J Clin Cases 2022; 10(20): 7153-7162
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/7153.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.7153
Renal cell carcinoma (RCC) and urothelial carcinoma (UC) are types of genitourinary malignancy. Upper tract UC (UTUC) has a relatively low prevalence, accounting for approximately 5% of urothelial cancers and less than 10% of renal tumors[1]. Although RCC and UTUC share common risk factors such as smoking, UTUC and synchronous contralateral RCC are very rare. As a result, the few studies of synchronous UC and RCC have been reports of individual cases or small series. A few decades ago, the term synchronous primary urological cancer (SPUC) was introduced as advanced diagnostic and surgical treatment techniques developed[2]. The overall prognosis of patients with SPUC is less favorable than that of patients with a single urological malignancy, due to the higher grade and stage of tumors at presentation[3]. Furthermore, it is important to preserve adequate renal function, especially in advanced-stage or high-risk patients with SPUC. For a patient diagnosed with bilateral urological cancer, the surgical procedures are performed simultaneously or as staged procedures according to the patient’s characteristics and comorbidities. Operating on both lesions simultaneously has the benefit of eliminating morbidity and the need for a second operation. However, the risk of perioperative surgical complications and a significant decline in renal function increases. With advances in the instruments and techniques of robot-assisted urological surgery, surgeries are available for significantly higher-risk patients, i.e. those who have multiple comorbidities or are super elderly.
An 85-year-old female visited her local hospital complaining of intermittent, painless, gross hematuria that had started 3 mo earlier.
The patient presented with the symptom of an intermittent painless, gross hematuria. The patient did not complain about any other symptoms.
The patient had a medical history of hypertension and hyperlipidemia.
The patient’s personal and family history was unremarkable.
The patient’s vital signs were stable. Clinical physical examination revealed no abnormalities.
There were no unusual findings of routine blood and biochemical examinations, except hematuria detected in a urinalysis. The baseline levels of serum creatinine level were 0.8 mg/dL.
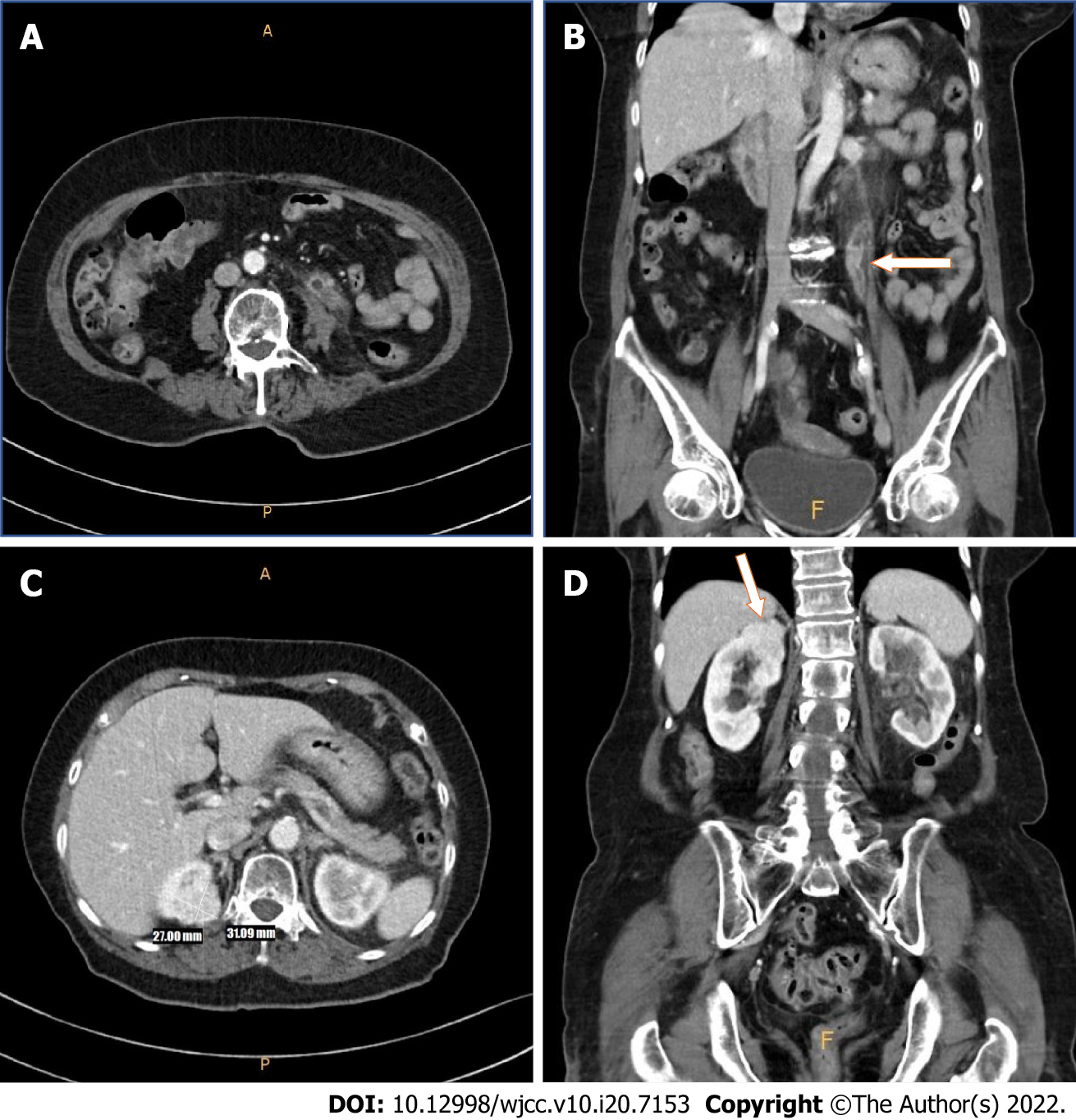
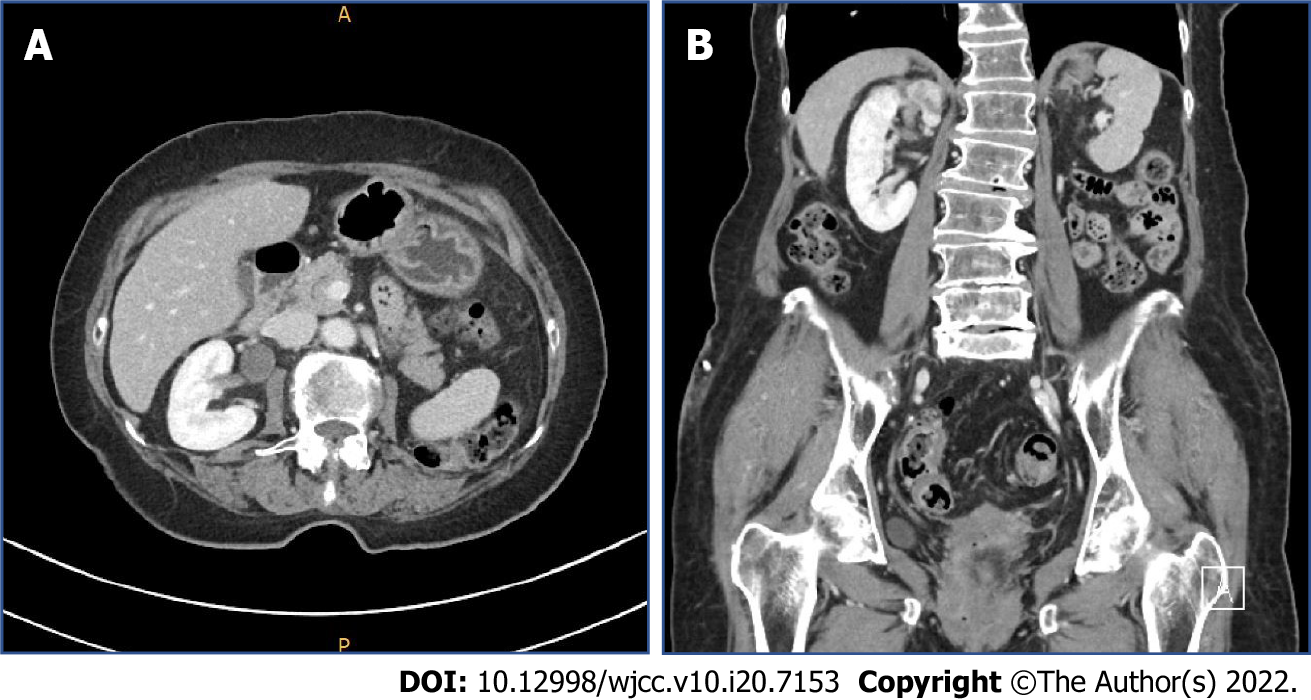
Upon initial presentation, the patient underwent an abdominal computed tomography (CT) scan, which revealed a right renal mass and segmental left proximal ureteral mass with hydronephrosis (Figure 1). The patient was referred to our institution for further urologic evaluation and management. After admission, CT urography was performed to more accurately determine the extent and size of the masses, and the presence or absence of metastases. In the right kidney, an approximately 3.1 cm-sized homogeneous enhancing mass was located in the upper pole. In addition, a 1.6- m-sized mass was observed in the left distal portion of proximal ureter, with suspicious periureteral soft tissue invasion. Perilesional fluid collection and eccentric ureteral wall thickening and fat infiltration were discovered in the proximal portion of the ureter (Figure 2).
Preoperative diagnosis was a left proximal ureter mass and right renal mass. The patient underwent simultaneous robot-assisted left nephroureterectomy right partial nephrectomy.
The surgeon was experienced in robot-assisted laparoscopic radical nephroureterectomy and partial nephrectomy. The da Vinci Xi system (Intuitive Surgical, Inc., Sunnyvale, CA, United States) was used for the operation, which was performed under general anesthesia. A transurethral catheter and nasogastric tube were placed before positioning the patient. In consideration of the patient’s renal function during surgery, a partial nephrectomy was planned to be performed first. We placed the patient in the modified lateral decubitus position at 45° (15° Trendelenburg position) the operating table was flexed appropriately. The abdomen and pelvic area were prepped and draped in a conventional sterile manner. A small incision in the periumbilical region was made for a 12 mm camera port. Using a Veress needle, pneumoperitoneum was achieved with 15 mmHg of carbon dioxide. The laparoscope was inserted into the peritoneal cavity to inspect internal injuries and adhesions. The robotic trocar and assistant port were arranged under direct vision at the lateral border of the rectus muscle, at 8-cm intervals.
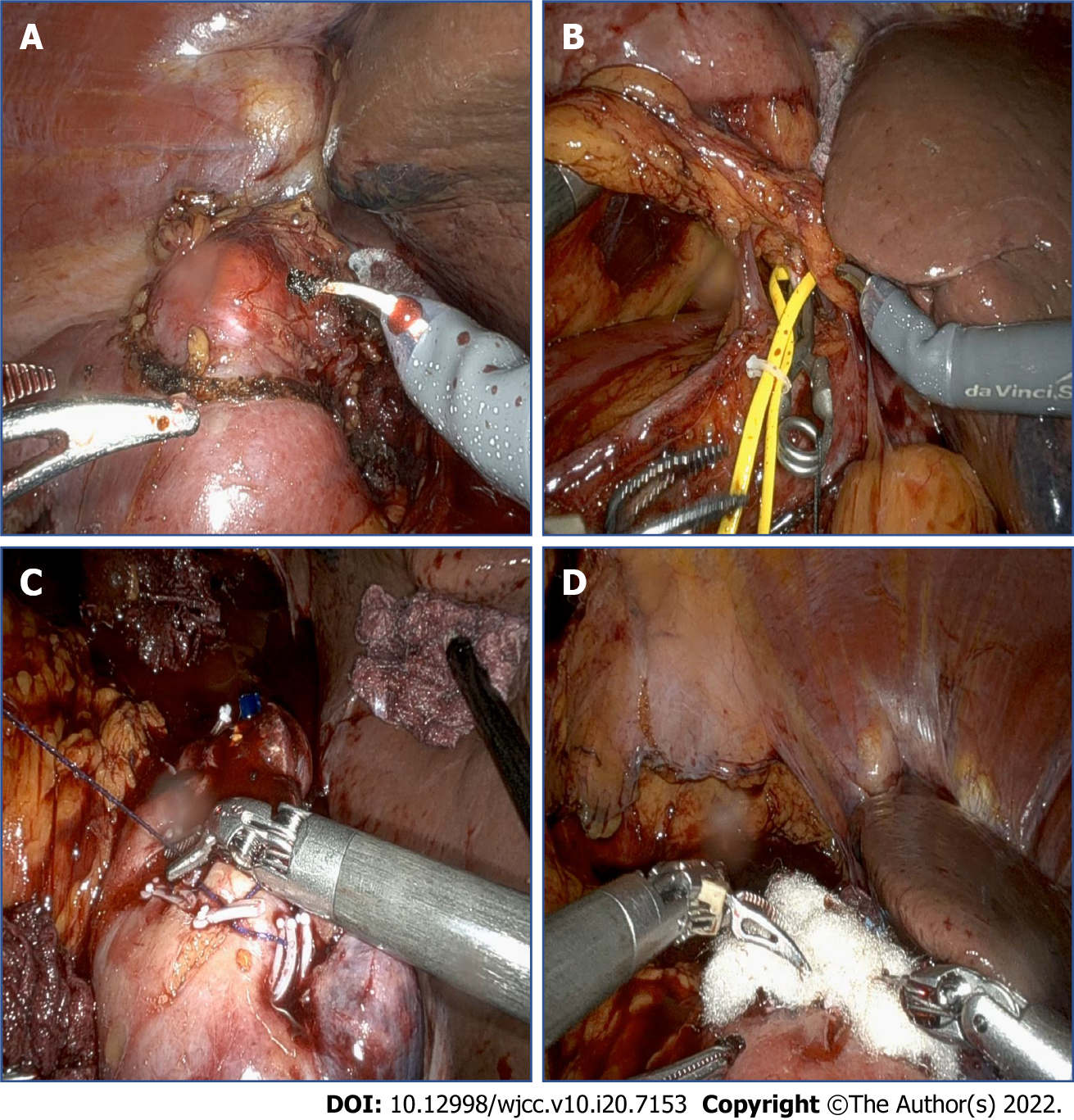
After robot docking, the line of Toldt was incised and dissected to mobilize the right colon. After opening Gerota’s fascia and performing perirenal fat dissection at the upper pole lesion, the mass was marked by cauterization with a 2-cm positive margin. Intraoperative ultrasonography was used to confirm the margins of the lesion. Selective mass hilar clamping was applied with bulldog clamps. Mass excision was completed in a row, and the inner defect and renal calyx were closed with a 40 absorbable polyglactin 910 (Vicryl; Ethicon Inc., Raritan, NJ, United States) suture and 30 absorbable Vloc (Covidien Inc., Dublin, Ireland) suture with a Hem-o-lok clip (Figure 3). A Jackson–Pratt drain (Cardinal Health, Waukegan, IL, United States) was positioned and anchored, and the skin wound was closed layer by layer.
The patient was repositioned in the contralateral decubitus position without interrupting the anesthesia. Using both the previous periumbilical and midline ports for the camera and 12 mm assistant trocar, respectively, additional 8 mm robotic trocars were placed. The robot was redocked and the line of Toldt was dissected. As previously performed on the right side, the ureter was located and dissected proximally to expose the kidney, and then cranially to the renal hilum. A Hemolok clip was placed on the ureter to prevent any spillage of malignant cells. The renal pedicle was identified, and the renal artery and vein, and gonadal vein, were dissected and ligated using another Hemolok clip. The kidney was dissected completely, and the ureter was dissected to the level of the iliac vessels.
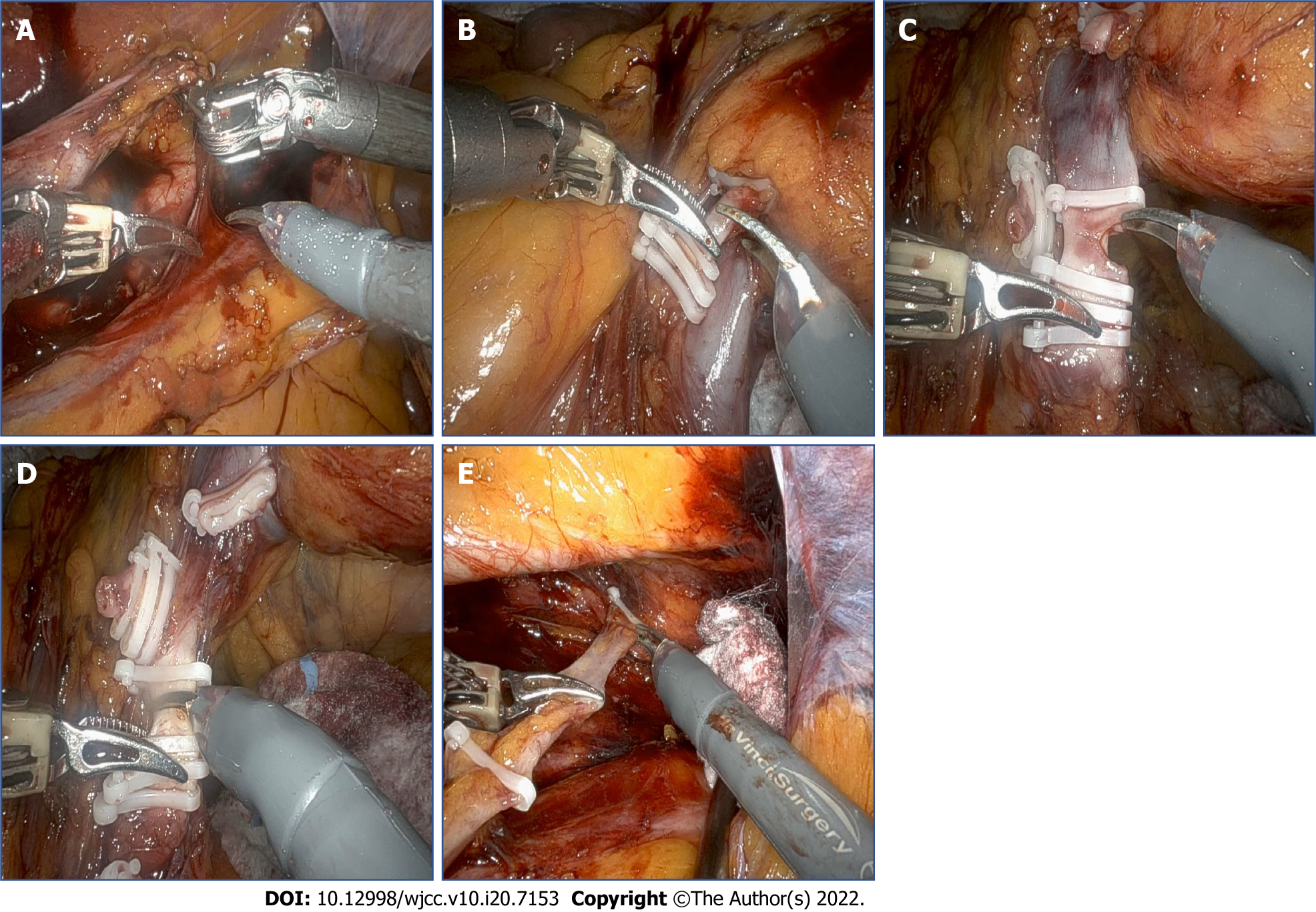
After completing the nephrectomy, the robotic visual field was moved to the lower part of the ureter. Dissection around the lower ureter and lateral urinary bladder wall was performed with traction of the ligated ureter by the robotic arm (Figure 4). Using the urethral catheter, the bladder was filled with 200 mL of saline to facilitate extravesical bladder cuff excision. After completing the excision, the bladder defect was repaired in two layers using 30 absorbable polyglactin 910 (Vicryl; Ethicon Inc.) sutures. The bladder was refilled with 200 mL of saline to confirm that there was no leakage. A Jackson–Pratt drain was positioned in the dependent portion and anchored. All of the trocars were then removed, and the robot was undocked. The specimens, including both the kidney and ureter, were removed from the extended umbilical wound by endobags. From the start of the operation until the patient regained consciousness in the recovery room, the patient’s condition had remained stable; She was transferred to the general ward.
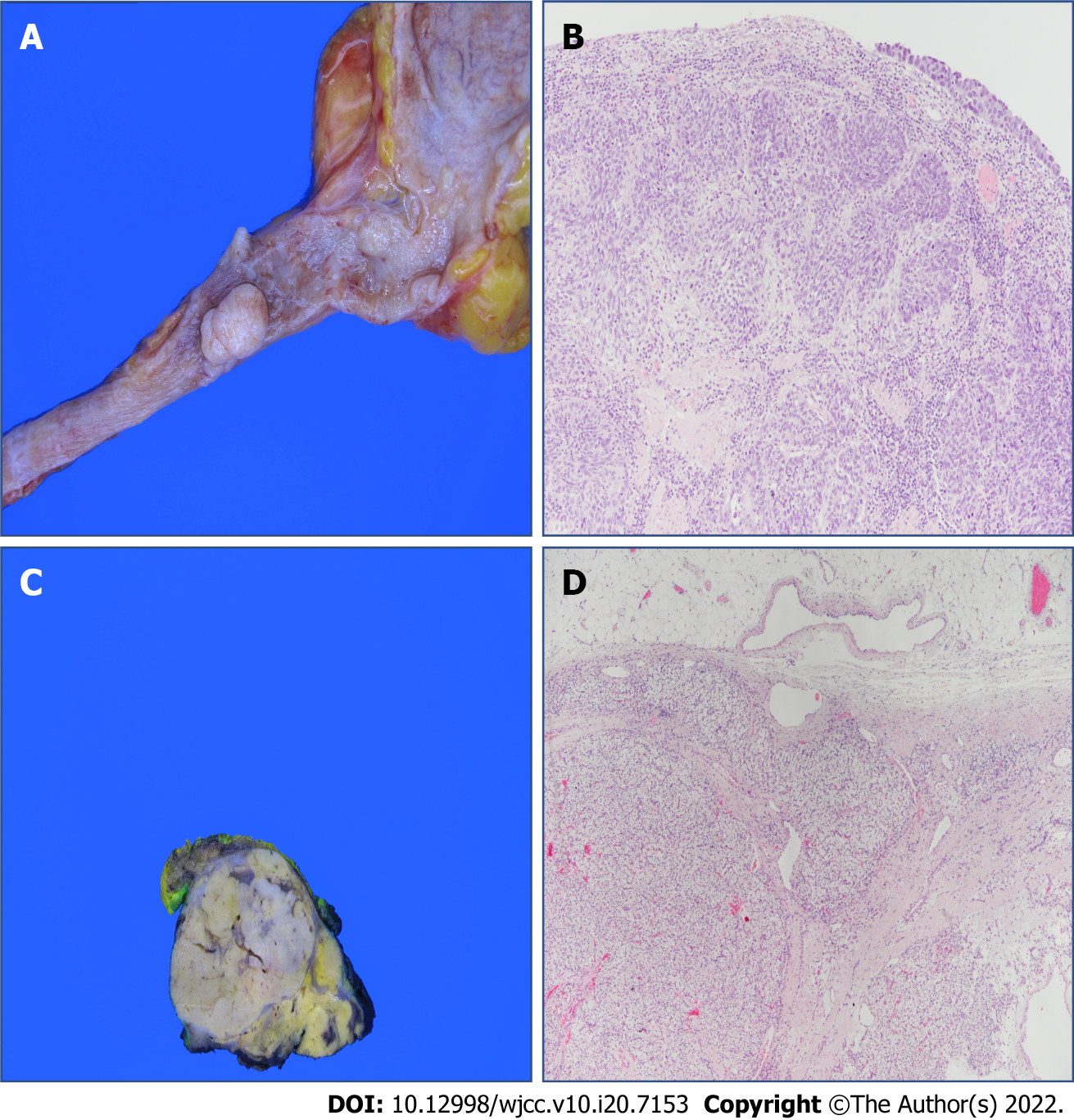
The specimen from the left proximal ureter measured 1.7 cm × 1.0 cm × 1.0 cm and was multifocal (Figure 5A). The tumor extended to the muscularis, with a negative resection margin. The final histopathological diagnosis was of high-grade papillary UC with pathological stage pT2, and invasion into the muscular layer. Specimens were stained with hematoxylin and eosin and viewed at 100 × magnification (Figure 5B). The right renal specimen measured 3.6 cm × 3.2 cm × 2.3 cm (Figure 5C). The pathological examination revealed clear cell carcinoma with Fuhrman Grade 3 and pathological stage pT3a. The tumor extended into the perinephric tissue with positive resection margins. The specimens were stained with hematoxylin and eosin and viewed at 40 × magnification (Figure 5D).
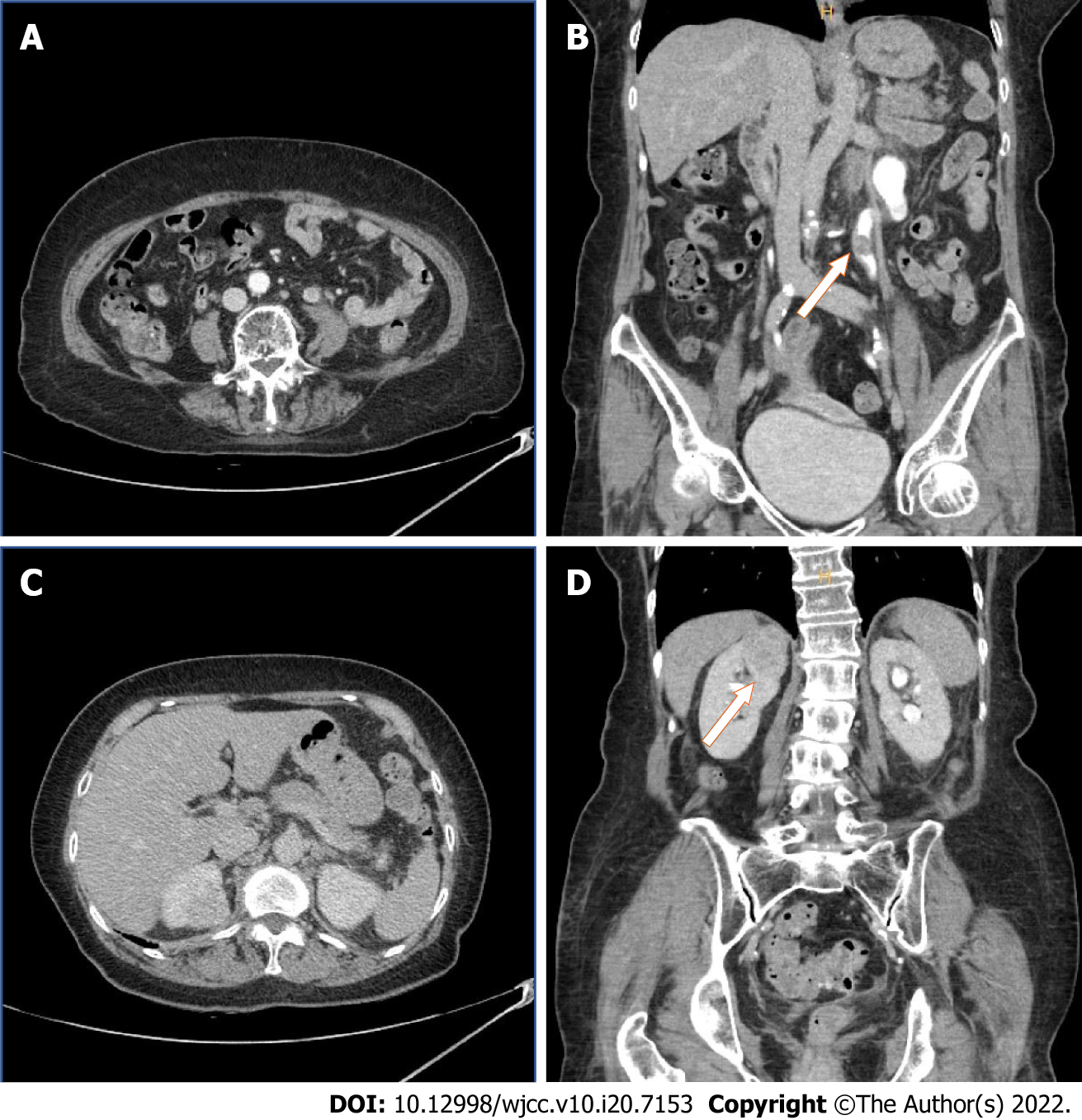
The total operation time was 268 min, with an estimated blood loss of 200 mL. The preoperative initial hemoglobin level was 10.6 g/dL and that at the 1d follow-up was 10.1 g/dL. Postoperative transfusion was unnecessary. The initial preoperative serum creatinine level was 0.8 mg/dL and that at 1 d postoperatively was 1.5 mg/dL. The patient was mobilized on day 2. The right and left Jackson–Pratt drains were removed on postoperative days 4 and 5, respectively. All stiches were removed on postoperative day 13. The urethral catheter was removed on day 19 and the patient was discharged on day 20, which was approximately 10 d later than the other patients who had undergone the same kidney surgery. During this long period of hospitalization, the serum creatinine level increased to 4.0 g/dL, and there was a complaint of edema in both lower extremities. Therefore, intensive urine output checks, fluid management, and diuretics usage were performed postoperatively. The day before discharge, the serum creatinine level had decreased to 1.5 mg/dL and the lower limb edema had improved. There were no additional complications or symptoms, which is notable given the patient’s age. At 6 mo after the operation, follow-up CT showed no specific findings (Figure 6). A further follow-up CT was planned, 6 mo after the first one.
Globally, RCC accounts for approximately 2% of cancer diagnoses and deaths, while UC of the renal pelvis or ureter accounts for 5%-7% of all genitourinary tumors. UTUCs are uncommon, accounting for only 5%-10% of all UC[1,4]. The clinical presentations of RCC and UTUC are variable, and typically nonspecific and unremarkable until the disease is advanced. Patient are often asymptomatic or have mild symptoms, including visible or invisible hematuria and flank pain[5]. To date, only about 50 cases of synchronous renal tumors have been reported in the literature and these mostly occurred ipsilaterally[6-9]. Diagnosis of UTUC with synchronous contralateral RCC, as in the present case, is extraordinarily rare; We found only 11 cases during our literature review.
UTUC accompanied by RCC is a multiple primary malignant neoplasm. This phenomenon was first described by Billroth et al[10], after which Warren et al[11] reviewed 1259 patients and set the standard for diagnosing them: malignancy of any tumors should be confirmed through histopathology. The minimum distance between two tumors should be 2 cm, and if both are located on the same site, the minimum interval should be 5 years and the possibility of metastasis should be ruled out. UTUC and RCC in the present case were discovered synchronously. Thus, we classify our case as synchronous primary multiple neoplasm, or more precisely as SPUC[2,12].
No effective standard treatment has yet been established because of the scarcity of cases. Hong et al[13] searched the literature and reported that seven cases of synchronous renal pelvis tumor and contralateral RCC had undergone renal parenchymal-sparing surgery. According to the 2020 European Association of Urology guidelines, segmental ureteral resection with wide margins or endoscopic ablation are sufficient to manage disease progression in patients with low-risk UTUC[14]. However, for patients with high-risk UTUC, radical nephroureterectomy is appropriate.
Despite undergoing a curative right partial nephrectomy, a positive surgical margin was detected in our case. However, such a margin does not necessarily predict recurrence or indicate residual disease[15-17]. Yossepowitch et al[18] studied 1344 patients who underwent nephron-sparing surgery; Surgical margins were positive in 77 of these patients (5.5%), and they had equivalent rates of local recurrence-free survival (97% vs 98%) and metastasis-free survival (95% in both groups) at 5 years to patients with negative margins. Another multi-institutional review reported conflicting data. Among 1240 patients who underwent partial nephrectomy for localized RCC, a positive surgical margin was associated with an increased risk of recurrence in those classified as high-risk (pathologic T2-T3a or Fuhrman grade III-IV disease) compared to those with negative margins (hazard ratio, 7.4, 95% confidence interval, 2.75-20.34). In patients with low-risk disease (pathologic T1 and Fuhrman grade I-II), no increased risk of recurrence was detected[19]. Although the pathological stage of our patient was pT3a and achieving a negative surgical margin is important, not all cases with positive surgical margins experience local tumor recurrence or metastatic disease progression if complete resection of the gross tumor is accomplished. A postoperative, residual, microscopic focus of RCC might survive for a long time. Renal ischemia induced by clamping of the renal artery, and adequate electrocauterization of the cortical rim of the renal parenchyma after excising the mass, might eradicate any residual tumor[18]. Lastly, considering that false-positives might arise during specimen processing, and that negative margins might only appear to be so because of sampling error, it is expected that no additional treatment other than vigilant monitoring will be required.
There is no consensus on simultaneous or staged procedures, or on which kidney should be treated first in bilateral synchronous urological cancer. Some clinical centers routinely use the staged approach for the following reasons. First, in a staged operation, the contralateral kidney can function as a backup during surgery, thus minimizing the risk of acute renal insufficiency, which would require temporary hemodialysis[20,21]. Second, based on the pathological findings and outcomes of the first surgery, the treatment strategy can be modified more easily. The Memorial Sloan Kettering Cancer Center prefers staged partial nephrectomy of the more-involved kidney first[22,23]. By contrast, the Mayo Clinic prefers a simultaneous procedure on the kidney with the more complex tumors first[24,25]. Considering the age of the patient and risk of anesthesia in our case, we opted for a simultaneous, one-stage surgical procedure. A multidisciplinary assessment indicated that two periods of general anesthesia and lengthy hospitalization would adversely affect the patient’s health. Therefore, a simultaneous robot-assisted laparoscopic nephroureterectomy with contralateral partial nephrectomy was performed.
This patient underwent robot-assisted surgery. About robotic surgery vs conventional open and laparoscopic surgery, many researches and systemic reviews were already carried out by lots of urologist. Rates of perioperative complications and postoperative efficacy were compared. Peyronnet et al[26] noted that despite its high-cost, robot-assisted partial nephrectomy has clear benefits in terms of preoperative morbidity than open approach. Larcher et al[27] demonstrated that the use of a robotic approach, compared to conventional open and laparoscopic approaches, is associated with lower rates for overall (21% vs 36%; P < 0.0001) and major (3% vs 9%; P = 0.03) complications. Shen et al[28] reported that at the period of follow up after surgery, the rates of tumor recurrence metastasis or death were lower in patients who had undergone robotic partial nephrectomy than that of open partial nephrectomy group. Robotic surgery can minimize damage to peripheral organs, blood vessels and nerves because it has achieved a sufficient field of surgical vision[29]. Furthermore, it has the advantages of minimizing the operative incision and bleeding, precise incisions and a rapid return to normal daily life for the patient. In our case, despite her old age and additional operative time due to the bilateral occurrence of tumors, no postoperative transfusion or hemodialysis was required. Considering the rarity of this case, we expect that this case report would be meaningful example for physicians considering an aggressive treatment option for super-elderly patient. Limitations of this case report included its short follow-up period and the positive surgical margin. Also, no studies on the overall survival rate following active or conservative treatment in super-elderly patients have been published. Studies with longer follow-up are needed to overcome these limitations.
Synchronous UTUC and RCC of the contralateral kidney is an extremely rare condition, and there is no standard treatment. When a surgical approach is required for both sides simultaneously, individualized nephron-sparing surgery is recommended, where possible, to preserve renal function. Our case confirms that robotic surgery can replace conventional open and laparoscopic surgery, given its favorable postoperative outcomes, complication rate and hospitalization period. In the present case, the procedure was performed accurately and safely, despite the patient being classified as super-old and having comorbidities.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao L, China; Le PH, Taiwan; lin Q, China A-Editor: Liu X, China S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. 2011;107:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | Qin J, Fan B, Chen X, Li X, Wang X, Song X. Clinicopathologic insight of synchronous primary urologic cancers. Int J Clin Exp Med. 2016;9:11458-11466. |
| 3. | Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin N Am. 2003;30:843-852. [RCA] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 567] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 4. | Padala SA, Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A, Rawla P. Epidemiology of Renal Cell Carcinoma. World J Oncol. 2020;11:79-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 624] [Article Influence: 124.8] [Reference Citation Analysis (0)] |
| 5. | Andersen JR, Kristensen JK. Ureteroscopic management of transitional cell tumors. Scand J Urol Nephrol. 1994;28:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Leveridge M, Isotalo PA, Boag AH, Kawakami J. Synchronous ipsilateral renal cell carcinoma and urothelial carcinoma of the renal pelvis. Can Urol Assoc J. 2009;3:64-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Atilgan D, Uluocak N, Parlaktas BS. Renal cell carcinoma of the kidney with synchronous ipsilateral transitional cell carcinoma of the renal pelvis. Case Rep Urol. 2013;2013:194127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Mucciardi G, Galì A, D'Amico C, Muscarà G, Barresi V, Magno C. Transitional Cell Carcinoma of the Renal Pelvis With Synchronous Ipsilateral Papillary Renal Cell Carcinoma: Case Report and Review. Urol Case Rep. 2015;3:93-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Dutta G, Silver D, Oliff A, Harrison A. Synchronous renal malignancy presenting as recurrent urinary tract infections. Case Rep Urol. 2011;2011:832673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Billroth T, Winiwarter A. Die allgemeine chirurgische Pathologie und Therapie in 51 Vorlesungen, Berlin: De Gruyter, 1906. [DOI] [Full Text] |
| 11. | Warren S. Multiple primary malignant tumors. A survey of the literature and a statistical study. Am J Cancer. 1932;16:1358-1414. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Moertel CG, DOCKERTY MB, BAGGENSTOSS AH. Multiple primary malignant neoplasms. I. Introduction and presentation of data. Cancer. 1961;14:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Hong SK, Jeong SJ, Lee SE. A case of renal transitional cell carcinoma associated with synchronous contralateral renal cell carcinoma. J Korean Med Sci. 2001;16:108-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Cowan NC, Dominguez-Escrig JL, Gontero P, Hugh Mostafid A, Palou J, Peyronnet B, Seisen T, Soukup V, Sylvester RJ, Rhijn BWGV, Zigeuner R, Shariat SF. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol. 2021;79:62-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 529] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 15. | Gill IS, Matin SF, Desai MM, Kaouk JH, Steinberg A, Mascha ED, Thornton J, Sherief MH, Strzempkowski B, Novick AC. Comparative Analysis of Laparoscopic Versus Open Partial Nephrectomy for Renal Tumors in 200 Patients. J Urology. 2003;170:64-68. [RCA] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 422] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 16. | Permpongkosol S, Colombo JR Jr, Gill IS, Kavoussi LR. Positive surgical parenchymal margin after laparoscopic partial nephrectomy for renal cell carcinoma: oncological outcomes. J Urol. 2006;176:2401-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Breda A, Stepanian SV, Liao J, Lam JS, Guazzoni G, Stifelman M, Perry K, Celia A, Breda G, Fornara P, Jackman S, Rosales A, Palou J, Grasso M, Pansadoro V, Disanto V, Porpiglia F, Milani C, Abbou C, Gaston R, Janetschek G, Soomro NA, de la Rosette J, Laguna MP, Schulam PG. Positive margins in laparoscopic partial nephrectomy in 855 cases: a multi-institutional survey from the United States and Europe. J Urol. 2007;178:47-50; discussion 50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Yossepowitch O, Thompson RH, Leibovich BC, Eggener SE, Pettus JA, Kwon ED, Herr HW, Blute ML, Russo P. Positive surgical margins at partial nephrectomy: predictors and oncological outcomes. J Urol. 2008;179:2158-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | Shah PH, Moreira DM, Okhunov Z, Patel VR, Chopra S, Razmaria AA, Alom M, George AK, Yaskiv O, Schwartz MJ, Desai M, Vira MA, Richstone L, Landman J, Shalhav AL, Gill I, Kavoussi LR. Positive Surgical Margins Increase Risk of Recurrence after Partial Nephrectomy for High Risk Renal Tumors. J Urol. 2016;196:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Funahashi Y, Hattori R, Yamamoto T, Kamihira O, Sassa N, Gotoh M. Relationship between renal parenchymal volume and single kidney glomerular filtration rate before and after unilateral nephrectomy. Urology. 2011;77:1404-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Phelan MW. Small renal mass with contralateral large renal mass: remove large renal mass first in staged fashion. Pro. J Urol. 2012;188:18-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Lowrance WT, Yee DS, Maschino AC, Cronin AM, Bernstein M, Thompson RH, Russo P. Developments in the surgical management of sporadic synchronous bilateral renal tumours. BJU Int. 2010;105:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Patel MI, Simmons R, Kattan MW, Motzer RJ, Reuter VE, Russo P. Long-term follow-up of bilateral sporadic renal tumors. Urology. 2003;61:921-925. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Blute ML, Itano NB, Cheville JC, Weaver AL, Lohse CM, Zincke H. The Effect of Bilaterality, Pathological Features And Surgical Outcome in Nonhereditary Renal Cell Carcinoma. J Urology. 2003;169: 1276-1281. [RCA] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Boorjian SA, Crispen PL, Lohse CM, Leibovich BC, Blute ML. The impact of temporal presentation on clinical and pathological outcomes for patients with sporadic bilateral renal masses. Eur Urol. 2008;54:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Peyronnet B, Khene ZE, Mathieu R, Bensalah K. Robot-assisted Versus Open Partial Nephrectomy: Do We Really Need More Evidence To End the Debate? Eur Urol Oncol. 2018;1:69-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Larcher A, Capitanio U, De Naeyer G, Fossati N, D'Hondt F, Muttin F, De Groote R, Guazzoni G, Salonia A, Briganti A, Montorsi F, Mottrie A. Is Robot-assisted Surgery Contraindicated in the Case of Partial Nephrectomy for Complex Tumours or Relevant Comorbidities? Eur Urol Oncol. 2018;1:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Shen Z, Xie L, Xie W, Hu H, Chen T, Xing C, Liu X, Xu H, Zhang Y, Wu Z, Tian D, Wu C. The comparison of perioperative outcomes of robot-assisted and open partial nephrectomy: a systematic review and meta-analysis. World J Surg Oncol. 2016;14:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | de Vermandois JAR, Cochetti G, Zingaro MD, Santoro A, Panciarola M, Boni A, Marsico M, Gaudio G, Paladini A, Guiggi P, Cirocchi R, Mearini E. Evaluation of Surgical Site Infection in Mini-invasive Urological Surgery. Open Med (Wars). 2019;14:711-718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |