Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.7138
Peer-review started: January 20, 2022
First decision: March 16, 2022
Revised: April 8, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: July 16, 2022
Processing time: 165 Days and 19.1 Hours
Aneurysm compression, diabetes, and traumatic brain injury are well-known causative factors of oculomotor nerve palsy (ONP), while cases of ONP induced by neurovascular conflicts have rarely been reported in the medical community. Here, we report a typical case of ONP caused by right posterior cerebral artery (PCA) compression to increase neurosurgeons’ awareness of the disease and reduce misdiagnosis and recurrence.
A 54-year-old man without a known medical history presented with right ONP for the past 5 years. The patient presented to the hospital with right ptosis, diplopia, anisocoria (rt 5 mm, lt 2.5 mm), loss of duction in all directions, abduction, and light impaired pupillary reflexes. Magnetic resonance angio
Vascular compression of the ON is a rare pathogeny of ONP that may be refractory to drug therapy and ophthalmic strabismus surgery. MVD is an effective treatment for ONP induced by neurovascular compression.
Core Tip: We report a rare case of typical oculomotor nerve palsy caused by neurovascular conflicts. Magnetic resonance imaging of the cranial nerve showed that the right posterior cerebral artery loop was in direct contact with the cisternal segment of the oculomotor nerve. We performed microvascular decompression to decompress the neurovascular compression excluding myasthenia gravis, aneurysm, and other eye diseases. Furthermore, the ocular symptoms were significantly relieved 3 mo after surgery. Additionally, we conducted a systematic review of the studies on oculomotor palsy and put forward our own interpretations of related results.
- Citation: Zhang J, Wei ZJ, Wang H, Yu YB, Sun HT. Microvascular decompression for a patient with oculomotor palsy caused by posterior cerebral artery compression: A case report and literature review . World J Clin Cases 2022; 10(20): 7138-7146
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/7138.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.7138
Oculomotor nerve palsy (ONP) can be caused by an aneurysm, intracranial space-occupying lesion, or diabetes mellitus. Cases of ONP induced by neurovascular conflicts were not studied in depth by pioneering scholars, so studies on these particular cases are limited. In 1988, Kojo et al[1] described the first case of ONP due to vascular compression of the ON, which was successfully relieved by microvascular decompression (MVD). Subsequently, only anecdotal cases have been documented in related studies. Since the development and extensive application of cranial nerve magnetic resonance imaging (MRI), the diagnosis of such atypical neurovascular compression was simplified, which contributed to the classification of nervous system diseases[2,3].
Here, we report a case of ONP caused by neurovascular conflict and present a systematic review of the literature on ONP caused by neurovascular compression.
A 54-year-old man presented with worsening symptoms of right ptosis, diplopia, and ophthalmoplegia for 5 years.
The patient’s symptoms started 5 years ago with progressive ptosis, diplopia, and ophthalmoplegia of the right eye. The patient had once undergone treatment with neostigmine intravenous injection and ophthalmic strabismus surgery in another hospital.
The patient was diagnosed with hyperlipidemia 3 years ago and received no treatment.
The patient had a clear personal and family history.
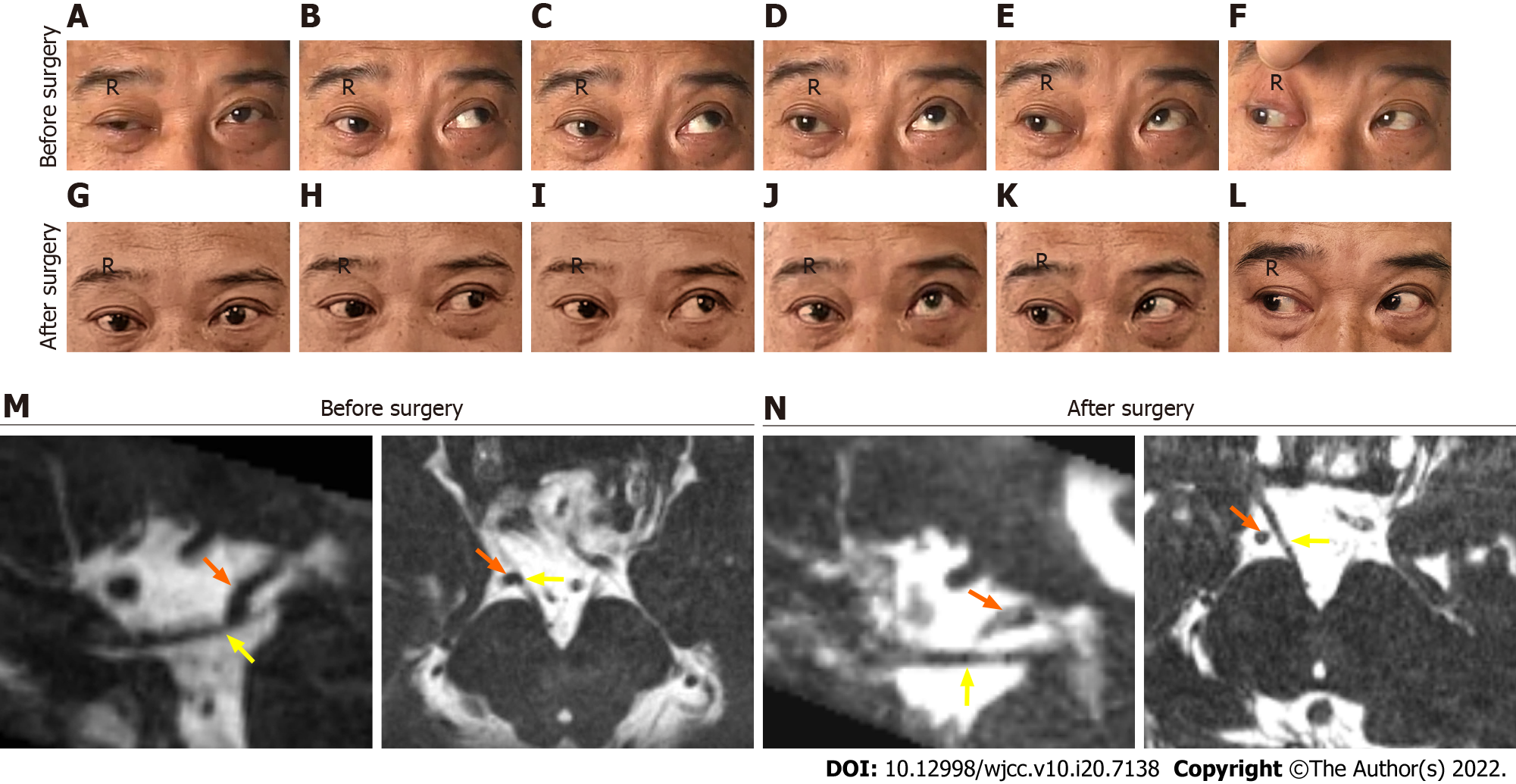
The patient’s width of the right palpebral fissure was significantly larger than that of the contralateral eye, and his right ocular motility weakened in primary position, adduction, medial-to-upper left, elevation, and medial-to-upper right (Figure 1A-E) except abduction (Figure 1F). Additionally, the right pupil was 5 mm and showed a sluggish response to direct and consensual pupillary light reflexes, while the left pupil was 2.5 mm and reacted sensitively.
The neuro-ophthalmologic evaluation, fundoscopy, and blood glucose level were negative.
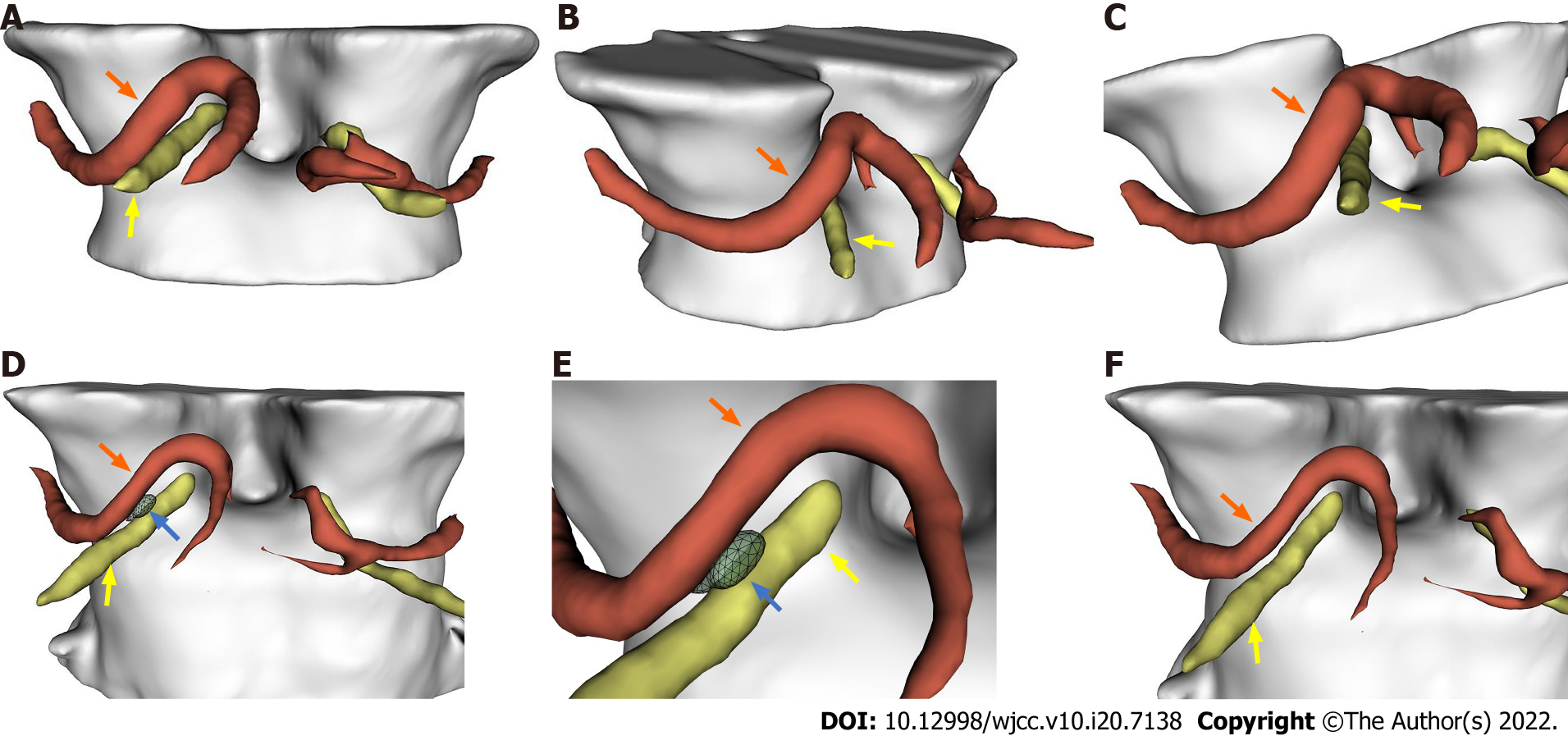
No aneurysm or phlebangioma was detected through magnetic resonance angiography (MRA) and computed tomography venography (CTV), but cranial nerve MRI (Figure 1M) and a three-dimensional (3D)-model of the cisternal segment (Figure 2A-C) revealed that the right posterior cerebral artery (PCA) loop was in direct contact with the cisternal segment of the right ON.
The diagnosis of ONP caused by neurovascular conflict was based on laboratory and typical imaging findings.
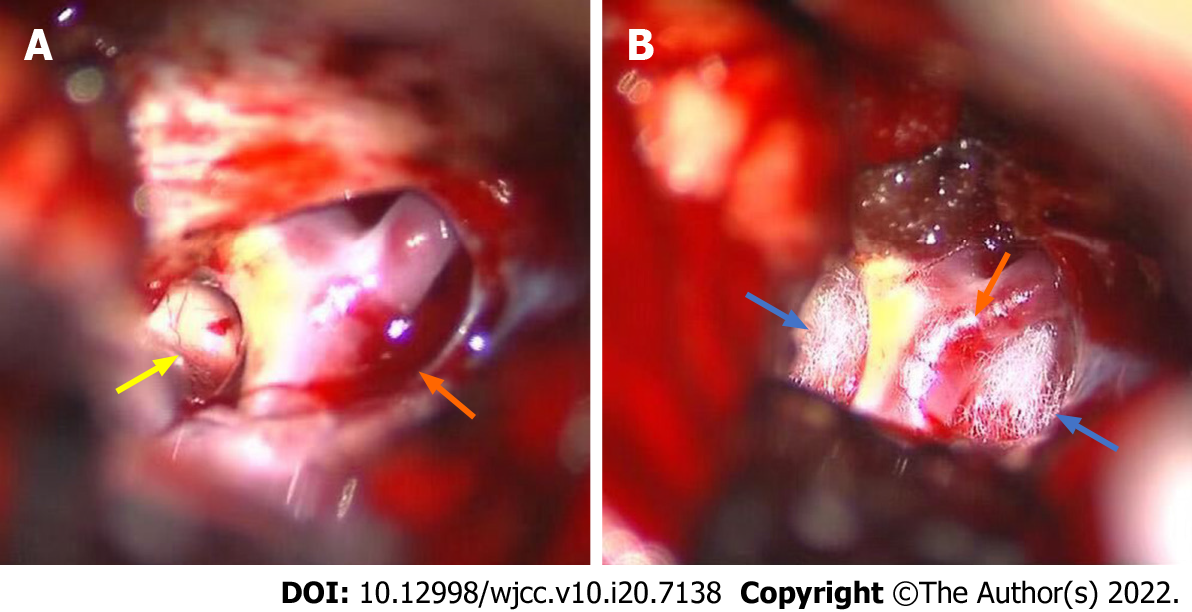
Surgical MVD was performed to decompress the neurovascular compression. The incision mark was labeled from 1 cm below the anterior ear zygomatic arch to the forepart of the parietal tuber, and then we performed a right-sided subtemporal craniotomy to approach the cisterna ambiens. Regarding the tentorial notch, the interpeduncular cistern and ventrolateral brainstem were identified, and then the overlaying arachnoid was sharply opened (Figure 3A). We also observed that the right PCA compressed the ON with a distinct indentation (Figure 3B), indicating that the right PCA and its bifurcation were the culprit vessels. The results of intraoperative exploration were consistent with that of the preoperative cranial nerve MRI.
We found that the patient’s symptoms were not altered immediately after MVD. However, the right eyestrain and diplopia were relieved when the patient was discharged. At the 1-year neuro-ophthalmologic follow-up, the patient’s ocular symptoms were significantly relieved at 3 mo after surgery, and the results of the postoperative cranial nerve MRI (Figure 2N) and 3D model imaging showed a small cerebrospinal fluid space between the right ON and the ipsilateral PCA (Figure 2D-F). Furthermore, the right pupil diameter and pupillary light reflexes returned to normal again. The patient’s right ocular motility to different directions and the symmetry of palpebral fissures in ONP were also fully recovered (Figure 1G-I and K), but we noticed mild deficits in elevation (Figure 1J).
We described the successful treatment by vascular decompression of the ON to improve the ocular movements of a 54-year-old male patient. The patient has a protracted course of the disease and was recalcitrant to conservative treatment and ophthalmic strabismus surgery. After analyzing his conditions, it was decided that MVD would results in an overall satisfactory outcome.
The definitive pathophysiology of ONP caused by vascular compression has not been clearly investigated by scholars in the medical field. However, some studies suggest that mechanical compression caused by abnormal structures, such as aneurysms and intracranial lesions, can result in axonal membrane instability and nerve ischemia, which would further induce segmental demyelination and ephaptic neural transmission hyperexcitability[4]. Additionally, an acute reaction of pulsations on the ON may clarify the acute presentation of ONP[5], whereas the ischemic effect of pulsations and the atherosclerotic deformation associated with aging may explain the chronic and persistent forms of this condition[6,7].
Reviewing the related literature, we identified that ONP caused by non-aneurysmal vascular compression has for long been defined as persistent palsy or as oculomotor neuromyotonia (ONM) with an intermittent presentation[7]. We identified 43 cases (Supplementary Table 1 and Table 1) of oculomotor involvement attribute to isolated neurovascular conflict, whereas only 12 cases, including our current case, were treated with MVD (Table 1). Among these 12 cases, only 5 patients[8-11], including our current case, involved compressive effects by the simple neurovascular conflict without vascular variation or atherosclerotic changes. The responsible vessels of the other 7 cases were confirmed to have atherosclerotic changes or accompanied by vascular tortuosity or dilation (Table 1). According to our statistical analyses, the culprit vessels were PCA in 17 cases (17/43, 39.53%). In 11 patients (11/43, 25.58%) they were posterior communicating artery (PcomA), in 6 patients (6/43, 13.95%) they were basilar artery (BA), in 2 they were (2/43, 4.65%) superior cerebellar artery (SCA), and only in 1 case (1/43, 2.33%) a trigeminal artery was identified. These statistical results are consistent with the anatomy of the ON demonstrated in previous studies. Related studies have shown that neurovascular conflict occurs most often in the cisternal segment of the ON, and especially the branches arising from the P1 and P2 segments of the PCA have a close intimate relationship with the dorsal surface of the nerve[12,13]. Interestingly, Liang et al[3] found that SCA is most likely to be in direct contact with the ON, followed by PCA, accounting for 58.9% and 55.1% of diagnosed cases respectively. However, only 7 of 392 nerves were compressed by PcomA. These findings considerably differ from our statistical data that shows PCA and PcomA are the most common culprit vessels to induce ONP, and there are many possibilities that can be related to these differences. Firstly, the PCA (7/216, 3.24%) might more easily form a “curve” induced by neurovascular conflict than that of SCA (5/231, 2.16%)[3]. A study conducted by Krayenbuhl et al[14] suggested that in about 55% of elderly patients, the PCA was a “curve” that generally develop to tortuosity and dilation, which easily leads to neurovascular conflict. Secondly, the ON originates from the midbrain and traverses between the PCA and the SCA, then runs parallelly to the PcomA[15]. So, the ON often contacts the medial trunk of the PCA when it travels to the interpeduncular fossa, while the SCA spreads out beneath the oculomotor nerve and has little contact with the nerve[16]. Additionally, the ON, PcomA, and anterior choroidal artery are firmly surrounded by the arachnoid membrane at the interpeduncular cistern segment[17], which makes it easier for the PcomA to generate a pulsation to the nerve. Hence these anatomical features may be able to increase the incidence of vascular compression by the PcomA and PCA, especially when atherosclerotic changes or vascular variations occur. Furthermore, the incidence of PcomA concomitant with infundibular dilatation is as high as 24.6% (64/260)[17,18]. A study conducted by Epstein et al[19] suggests that the infundibular dilatation of the PcomA should be regarded as a normal anatomical condition but not an aneurysm because it is generally considered to be a congenital or acquired anatomical variation. We have followed this principle in the process of counting the number of blood vessels, which might the differences in the number of PcomA between our study and Liang et al's[3]. Other reasons may be related to the various conditions of the selected cases. Further clinical research with more cases are required to illuminate these problems.
| Ref. | Case number | Age/sex | Culprit vessel | Vascular anomaly | Clinical Presentation | Past history | Treatment/outcome |
| Kojo et al[1], 1988 | 1 | 47/F | Left PcomA | Tortuous and dilated | 1 d, left blepharoptosis and diplopia | Migrainous headache and cerebral aneurysm | MVD, improved at 28 d after surgery |
| Nakagawa et al[12], 1991 | 1 | 59/M | Bilateral PCA and postoperativearachnoidaladhesions | Atherosclerosis | Left-sided, 1 mo, ophthalmoplegia | None | MVD, the left-sided ocular symptoms were improved at 1 mopostoperatively (mild residual diplopia) |
| Right-sided, 2 d, ophthalmoplegia | MVD, the right-sided ocular movement and ptosis were improved at 1.5 mo postoperatively (mild residual ptosis) | ||||||
| Mulderink et al[25], 2001 | 1 | 69/M | Left PcomA | Atherosclerosis anddilatation | Left ophthalmoplegia | Chronic headaches | MVD, improved at 3 mo after surgery |
| Babbitz et al[26], 2005 | 1 | 36/M | Left PcomA | Atherosclerosis anddilatation | 2 mo, left retro-orbital headache, left ptosis, external ophthalmoplegia, and diplopia | Headache, left ptosis, external ophthalmoplegia and diplopia | Primary treatment: steroids (ineffective); Follow-up treatment: MVD and recovered completely at 8 d after surgery |
| Suzuki et al[24], 2008 | 1 | 78/M | Left PCA and SCA | Atherosclerosis | Left ptosis and eye movement with papillary dilatation | ICPC aneurysm | MVD, improved at 1 mo after surgery |
| Inoue et al[8], 2012 | 1 | 62/F | Left PCA and SCA | None | 4 yr, vertical diplopia and partial ophthalmoparesis | None | MVD, diplopia and anisocoria disappeared within 1 wk postoperatively, the ocular movement was improved by the time the patient discharged |
| Kheshaifati et al[9], 2016 | 1 | 16/M | Right PCA | None | 1 yr, right ptosis, mydriasis and ophthalmoplegia | None | MVD, improved at 3 mo after surgery |
| Fukami et al[33], 2018 | 1 | 70/F | Left PcomA | Infundibular dilatation | Headache, left ptosis and mild anisocoria | None | MVD, left ptosis disappeared 3 mo postoperatively |
| Onuma et al[11], 2020 | 1 | 70/F | Left PcomA | None | 14 d, severe diplopia | None | MVD, resolved within 1 mo of surgery |
| Pomeraniec et al[10], 2020 | 1 | 71/F | Left PCA | None | 1 yr, left eye diplopia | Cushing’s disease | MVD, remained unchanged at 1 yr follow-up |
| Haider et al[15], 2019 | 1 | 76/F | Left PcomA | tortuous | 3 wk, left-sided incomplete ptosis | Hypertension | MVD, completely resolved |
Our literature review revealed that the diagnosis of ONP caused by neurovascular conflict should be performed with neuro-imaging examinations, including cranial nerve MRI, MRA, angiography, and CTV. Surgical treatment and conservative medication are reported as options of administration, but there is not an expert consensus or official guidelines for the treatment of such cases. Furthermore, how to correctly make the indications and find opportunities suitable for surgical approaches are still controversial issues in the community. However, opinions on the therapy method are relatively uniform and are relatively aligned among scholars that conducted research on these clinical conditions. For intermittent presentation, such as ONM, Stockman et al[20] demonstrated that an attempt of conservative medication with carbamazepine is suitable to be performed and can reach a response rate of more than 87.8%. For ONP patients with acute onset, Shimizu et al[21] reported that the administration of steroids could relieve the inflammatory state associated with vascular compression to assist the patient's recovery. MVD is only considered applicable for some patients with ONP who did not respond well to conservative treatment[22]. Additionally, MVD is also available for ONM, but it should be avoided when risks exceed benefits[7]. Until now, just anecdotal evidence recommended that MVD is an effective operative method (Table 1), and there are still some controversial opinions regarding the use of surgical approaches. Other similar studies pointed out that MVD probably should be considered for older patients once the neurovascular conflict is identified in MRI[9,12,23,24]. Although the specific reasons are not mentioned in their studies, we speculate that it is related to cerebral arteriosclerosis changes of culprit vessels in elderly patients diagnosed with ONP. It is well known that cerebral arteriosclerosis is a predisposing factor that can lead to ONP. The medical community also agrees that the severity of arteriosclerosis increases with age, which is an irreversible condition[25]. Therefore, surgical intervention is ultimately needed to solve this issue. Interestingly, other studies reported the case of 4 ONP patients with culprit vascular atherosclerosis who successfully achieved clinical recovery after MVD, indicating that the vascular arteriosclerotic changes contribute to ON dysfunction and proving the efficacy of MVD as an effective therapy approach[12,24-26].
Although the arteriosclerotic changes are not observed in our case, the patient has a history of hyperlipidemia, which is a risk factor for this disease. This information sheds light on the need to amplify the application of MVD surgical interventions to treat ONP. A study conducted by Pomeraniec et al[10] observed that longer intervals between diagnosis and surgical intervention were associated with a lower possibility of neurofunctional rehabilitation. Moreover, a review of 319 cases with isolated ONP induced by aneurysms and administered with surgical decompression revealed that 64% of patients got their neurological functions completely restored when surgical intervention was carried out within 2 wk of symptom onset, 30% within 2 - 4 wk and 14% after 1 mo[27]. These studies convey a meaningful message to us that earlier detection and surgical intervention can be effective in treating patients with ONP.
Regarding the use of surgical approaches for patients with ONP, both sub temporal craniotomy and orbitozygomatic craniotomy have been documented in previous studies[8,10,12,24]. Compared with orbitozygomatic craniotomy, we believe that subtemporal craniotomy provides wide surgical access to arrive at the base of the middle cranial fossa, superior petroclival region, and their adjacent structures, which is more suitable for detecting complex vascular malformations in interpeduncular cisterns[8,12,24]. The patients with ONP treated by MVD do not report immediate relief of ocular symptoms, and improvements in quality of life have been reported usually after 3 mo of recovery (Table 1). However, these post-treatment conditions are different from those suffering from trigeminal neuralgia and hemifacial spasm that come with symptoms that can be alleviated immediately after MVD. Similar results were achieved in our case, and the success of our methodology may be attributed to different reasons. We referred to the causes of delayed healing of hemifacial spasms and the anatomical features of the ON[16,28-30] and concluded that culprit vessels usually give off arterioles to innervate the nerve. These arterioles are easily stimulated and constricted by intraoperative traction or subarachnoid blood oozing, which may result in transient nerve ischemia. The process of ischemia recovery may take some time and go through several periods of changing gradually. Secondly, the neurovascular conflict contributes to local inflammation and demyelination. However, these symptoms are likely to be alleviated after MVD, although it might take some time to regain neuro-potentials from the hyperexcitability state[31,32]. Furthermore, in some previously reported cases, a visible indentation that indicates severe neuropathy could be observed on the surface of the ON. This indentation was induced by long-term vascular compression in some cases, including in ours[9,33], so we infer that the delay restoration is related to the time it completely disappeared.
ONP caused by neurovascular conflict is rare in clinical practices. A further understanding of ONP’s etiology can provide a reliable basis for subsequent precise treatments. We recommend that any patient with a pupil-involving palsy undergo high-quality cranial nerve MRI and angiographic analysis to detect the microanatomical relationship between vessels and nerves. Furthermore, MVD should be considered if the neurovascular conflict is identified in MRI, but the correlation between surgical timing and clinical prognosis still requires further investigation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohey NM, Egypt; Osawa I, Japan A-Editor: Zhu JQ, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
| 1. | Kojo N, Lee S, Otsuru K, Takagi S, Shigemori M, Watanabe M. [A case of ophthalmoplegic migraine with cerebral aneurysm]. No Shinkei Geka. 1988;16:503-507. [PubMed] |
| 2. | Sun X, Liang C, Liu C, Liu S, Deng K, He J. Oculomotor paralysis: 3D-CISS MR imaging with MPR in the evaluation of neuralgic manifestation and the adjacent structures. Eur J Radiol. 2010;73:221-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Liang C, Du Y, Lin X, Wu L, Wu D, Wang X. Anatomical features of the cisternal segment of the oculomotor nerve: neurovascular relationships and abnormal compression on magnetic resonance imaging. J Neurosurg. 2009;111:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Bizilis JC, Simonin A, Lind CR. Delayed oculomotor nerve palsy associated with a ruptured anterior communicating aneurysm: Case report. J Clin Neurosci. 2021;90:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Joshi S, Tee WWH, Franconi C, Prentice D. Transient oculomotor nerve palsy due to non-aneurysmal neurovascular compression. J Clin Neurosci. 2017;45:136-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Jo YS, Kim SK, Kim DH, Kim JH, Na SJ. Complete Oculomotor Nerve Palsy Caused by Direct Compression of the Posterior Cerebral Artery. J Stroke Cerebrovasc Dis. 2015;24:e189-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Belotti F, Zanin L, Fontanella MM, Panciani PP. The oculomotor neurovascular conflict: Literature review and proposal of management. Clin Neurol Neurosurg. 2020;195:105920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Inoue T, Hirai H, Shimizu T, Tsuji M, Shima A, Suzuki F, Matsuda M. Ocular neuromyotonia treated by microvascular decompression: usefulness of preoperative 3D imaging: case report. J Neurosurg. 2012;117:1166-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Kheshaifati H, Al-Otaibi F, Alhejji M. Microvascular Decompression for Oculomotor Nerve Palsy: A Case Report and Literature Review. World Neurosurg. 2016;88:695.e1-695.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Pomeraniec IJ, Ding D, Ksendzovsky A, Liu KC. Microvascular decompression of the posterior cerebral artery for treatment of oculomotor nerve palsy. J Cerebrovasc Endovasc Neurosurg. 2020;22:85-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Onuma K, Yanaka K, Akimoto Y, Yamano A, Nakamura K, Ishikawa E. Microvascular Decompression for Oculomotor Nerve Palsy due to Nonaneurysmal Vascular Compression. World Neurosurg. 2021;145:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Nakagawa H, Nakajima S, Nakajima Y, Furuta Y, Nishi O, Nishi K. Bilateral oculomotor nerve palsies due to posterior cerebral arterial compression relieved by microvascular decompression--case report. Neurol Med Chir (Tokyo). 1991;31:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Uz A, Tekdemir I. Relationship between the posterior cerebral artery and the cisternal segment of the oculomotor nerve. J Clin Neurosci. 2006;13:1019-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Krayenbuhl HAY. MG: Cerebral Angiography. London, Butterworth & Co Ltd 1968; 34. |
| 15. | Haider AS, Gottlich C, Sumdani H, Layton KF, Doughty K. Acute Oculomotor Nerve Palsy Caused by Compression from an Aberrant Posterior Communicating Artery. Cureus. 2019;11:e3920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Margolin E, Freund P. Third Nerve Palsies: Review. Int Ophthalmol Clin. 2019;59:99-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Dimopoulos VG, Fountas KN, Feltes CH, Robinson JS, Grigorian AA. Literature review regarding the methodology of assessing third nerve paresis associated with non-ruptured posterior communicating artery aneurysms. Neurosurg Rev. 2005;28:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Ebina K, Ohkuma H, Iwabuchi T. An angiographic study of incidence and morphology of infundibular dilation of the posterior communicating artery. Neuroradiology. 1986;28:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Epstein F, Ransohoff J, Budzilovich GN. The clinical significance of junctional dilatation of the posterior communicating artery. J Neurosurg. 1970;33:529-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Stockman AC, Dieltiëns M, Janssens H, Van Lammeren M, Beelen L, Van Bellinghen V, Cassiman C. Ocular Neuromyotonia: Case Reports and Literature Review. Strabismus. 2018;26:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Shimizu M, Tozaka N, Ishii A, Mamada N, Terada M, Takuma H, Tamaoka A. Third nerve palsy due to local inflammation associated with vascular compression: A case series. J Neurol Sci. 2016;367:365-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Wang J, Gong XY, Sun Y, Hu XY. Prevalence of nerve-vessel contact at cisternal segments of the oculomotor nerve in asymptomatic patients evaluated with magnetic resonance images. Chin Med J (Engl). 2010;123:989-992. [PubMed] |
| 23. | Miller MJ, Mark LP, Ho KC, Haughton VM. Anatomic relationship of the oculomotor nuclear complex and medial longitudinal fasciculus in the midbrain. AJNR Am J Neuroradiol. 1997;18:111-113. [PubMed] |
| 24. | Suzuki K, Muroi A, Kujiraoka Y, Takano S, Matsumura A. Oculomotor palsy treated by microvascular decompression. Surg Neurol. 2008;70:210-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Mulderink TA, Bendok BR, Yapor WY, Batjer HH. Third nerve paresis caused by vascular compression by the posterior communicating artery. J Stroke Cerebrovasc Dis. 2001;10:139-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Babbitz JD, Harsh GR 4th. Concomitant ectatic posterior communicating artery and tentorial meningioma as a source of oculomotor palsy: case report. Neurosurgery. 2005;57:E1316; discussion E1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Leivo S, Hernesniemi J, Luukkonen M, Vapalahti M. Early surgery improves the cure of aneurysm-induced oculomotor palsy. Surg Neurol. 1996;45:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Saito S, Møller AR, Jannetta PJ, Jho HD. Abnormal response from the sternocleidomastoid muscle in patients with spasmodic torticollis: observations during microvascular decompression operations. Acta Neurochir (Wien). 1993;124:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Sanders DB. Ephaptic transmission in hemifacial spasm: a single-fiber EMG study. Muscle Nerve. 1989;12:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Nanda A, Javalkar V, Zhang S, Ahmed O. Long term efficacy and patient satisfaction of microvascular decompression and gamma knife radiosurgery for trigeminal neuralgia. J Clin Neurosci. 2015;22:818-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Xia L, Zhong J, Zhu J, Dou NN, Liu MX, Li ST. Delayed relief of hemifacial spasm after microvascular decompression. J Craniofac Surg. 2015;26:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Deng Z, Liu R, Liu Y, Wang Z, Yu Y, Zhang L. Factors That May Affect Delayed Relief Of Trigeminal Neuralgia After Microneurosurgery And The Long-Term Outcomes Associated With Delayed Relief. J Pain Res. 2019;12:2817-2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Fukami S, Akimoto J, Fukuhara H, Kohno M. Microvascular Decompression for Oculomotor Nerve Palsy Due to Compression by Infundibular Dilatation of Posterior Communicating Artery. World Neurosurg. 2018;119:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |