Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.7082
Peer-review started: December 17, 2021
First decision: January 26, 2022
Revised: February 10, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: July 16, 2022
Processing time: 199 Days and 13.9 Hours
Pyogenic liver abscesses are insidious in the early stage. Some cases progress rapidly, and the patient’s condition can worsen and even become life-threatening if timely treatment is not provided. Surgery and prolonged antibiotic treatment are often required if the abscess is large and liquefied and becomes separated within the lumen.
We report a case of bacterial liver abscess with a poor outcome following pharmacological treatment, review the literature related to the use of platelet-rich plasma (PRP) in the treatment of hepatic impairment and partial hepatectomy in animals, and discuss the prognostic features of surgical incision and drainage combined with PRP in the treatment of bacterial liver abscesses. This is the first case describing the use of PRP in the treatment of a bacterial liver abscess in humans, providing new ideas for the treatment of this condition.
This case highlights the importance of surgical treatment for bacterial liver abscesses that are well liquefied and poorly managed medically. PRP may produce antimicrobial effects and promote the regeneration and repair of liver tissue.
Core Tip: Previously, pyogenic liver abscesses were typically treated with antibacterial drugs, percutaneous puncture and drainage, or surgical incision and drainage; treatment by surgical incision and drainage combined with platelet-rich plasma (PRP) has not been reported. By reviewing the relevant literature and combining the previously reported benefits of PRP on liver function improvement and liver tissue regeneration and repair, we treated a large abscess with complete liquefaction and a poor response to antibacterial drug treatment by surgical incision and drainage combined with PRP at our hospital. The patient was discharged after 7 d of treatment without pus cavity flushing and with no recurrence during follow-up. Therefore, we consider that PRP may have direct antimicrobial and promotional effects on liver tissue regeneration, but this needs to be confirmed in a clinical case–control study with a larger sample.
- Citation: Wang JH, Gao ZH, Qian HL, Li JS, Ji HM, Da MX. Treatment of pyogenic liver abscess by surgical incision and drainage combined with platelet-rich plasma: A case report. World J Clin Cases 2022; 10(20): 7082-7089
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/7082.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.7082
The ageing of the population and the application of broad-spectrum antibiotics and immunosuppressive drugs in recent years have led to an increase in the incidence of pyogenic liver abscesses (PLAs). Early symptoms of PLAs are often atypical; the disease is difficult to diagnose and progresses rapidly, with approximately 15% of patients developing sepsis and even life-threatening infectious shock due to a lack of timely treatment. In some cases, PLA liquefaction is followed by separation, and anti-infective drugs combined with puncture drainage are ineffective, necessitating surgical treatment. Simple surgical incision and drainage require repeated double-cannula flushing after the procedure, which is inconvenient, requires prolonged postoperative antibacterial drug use and a long hospital stay, and is associated with a slow recovery.
In this study, we report a case of PLA treated by surgical incision and drainage combined with an injection of platelet-rich plasma (PRP). After the operation, the patient did not require double-cannula flushing or prolonged antibacterial drug administration. To date, no similar cases in the domestic or foreign literature have been identified.
A 67-year-old man visited the emergency department of our hospital for “repeated fever for more than 10 d”. The patient did not present with any signs of abdominal pain or vomiting.
For more than 10 d, the patient had chills and a fever (38.3 °C) due to a cold. The patient took some medications (unspecified by the patient), and his symptoms were slightly alleviated. He experienced repeated episodes of chills and fever in the afternoons, with a maximum body temperature of 38.8 °C, along with occasional nausea and vomiting of the gastric contents. His symptoms were not alleviated after vomiting or taking oral cold and flu medications. Therefore, he came to our hospital for further diagnosis and treatment. Abdominal computed tomography (CT) performed at the outpatient clinic revealed a liver abscess. The patient was admitted to the hospital for treatment on April 15, 2020. Since the onset of the disease, the patient had a poor appetite, a normal urine output, and no significant weight loss.
The patient had a history of coronary stent implantation due to occlusion of the descending branch of the coronary artery (myocardial infarction) in 2008. After the operation, he began taking oral medications, including clopidogrel (Plavix) 75 mg qd, simvastatin 10 mg qd, and aspirin 100 mg qd. He had discontinued the aspirin on his own accord 1 mo prior to his visit. Additionally, his history included cerebral infarction, which occurred in 2019, and the muscle strength in his left arm and leg was rated grade 4 and grade 3, respectively, after admission to our hospital.
The patient had no relevant personal or family history.
The patient was conscious with stable vital signs: Body temperature, 36.4 °C; pulse rate, 83 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 130/80 mmHg. His abdomen was soft, with tenderness in the upper right quadrant. No rebound pain was noted, and muscle tone was normal. The liver was not palpable under the costal margin. Pain was induced in the liver region on percussion. No oedema was noted in either lower limb.
Blood analysis revealed the following: White blood cell count, 6.90 × 109/L; neutrophil percentage, 84.4%; haemoglobin, 122 g/L; and platelet count, 316 × 109/L. The procalcitonin was 0.61 ng/mL. Biochemical analysis revealed the following: Total protein, 70.3 g/L; albumin, 27.5 g/L; creatinine, 50.9 mol/L; urea nitrogen, 3.12 mol/L; prothrombin time, 15.6 s; prothrombin activity percentage, 57%; activated partial prothrombin time, 44.4 s; and D-dimer, 1.25 μg/mL. The findings of routine urine and stool examinations were normal, as were the findings of electrocardiography and chest X-ray examinations (Table 1).
| Admission | Postoperative day 3 | Normal range | |
| White blood cells | 6.90 × 109/L | 9.72 × 109/L | 4-10 × 109/L |
| Neutrophil percentage | 84.4% | 81.70% | 45%-77% |
| Haemoglobin | 122 g/L | 116 g/L | 120-160 g/L |
| Platelets | 316 × 109/L | 244 × 109/L | 100-3009/L |
| Procalcitonin | 0.61 ng/ml | 0.29 ng/ml | 0-0.5 ng/ml |
| Total protein | 40 g/L | 70.3 g/L | 60-80 g/L |
| Albumin | 27.5 g/L | 34.8 g/L | 34-48 g/L |
| Creatinine | 50.9 μmol/L | 61 μmol/L | 56-130 μmol/L |
| Urea nitrogen | 3.12 μmol/L | 3.35 μmol/L | 3-7 μmol/L |
| Prothrombin time | 15.6 s | 13.4 s | 13-16 s |
| Prothrombin activity percentage | 57% | 63% | 70%-100% |
| Activated partial thromboplastin time | 44.4 s | 38.4 s | 32-44 s |
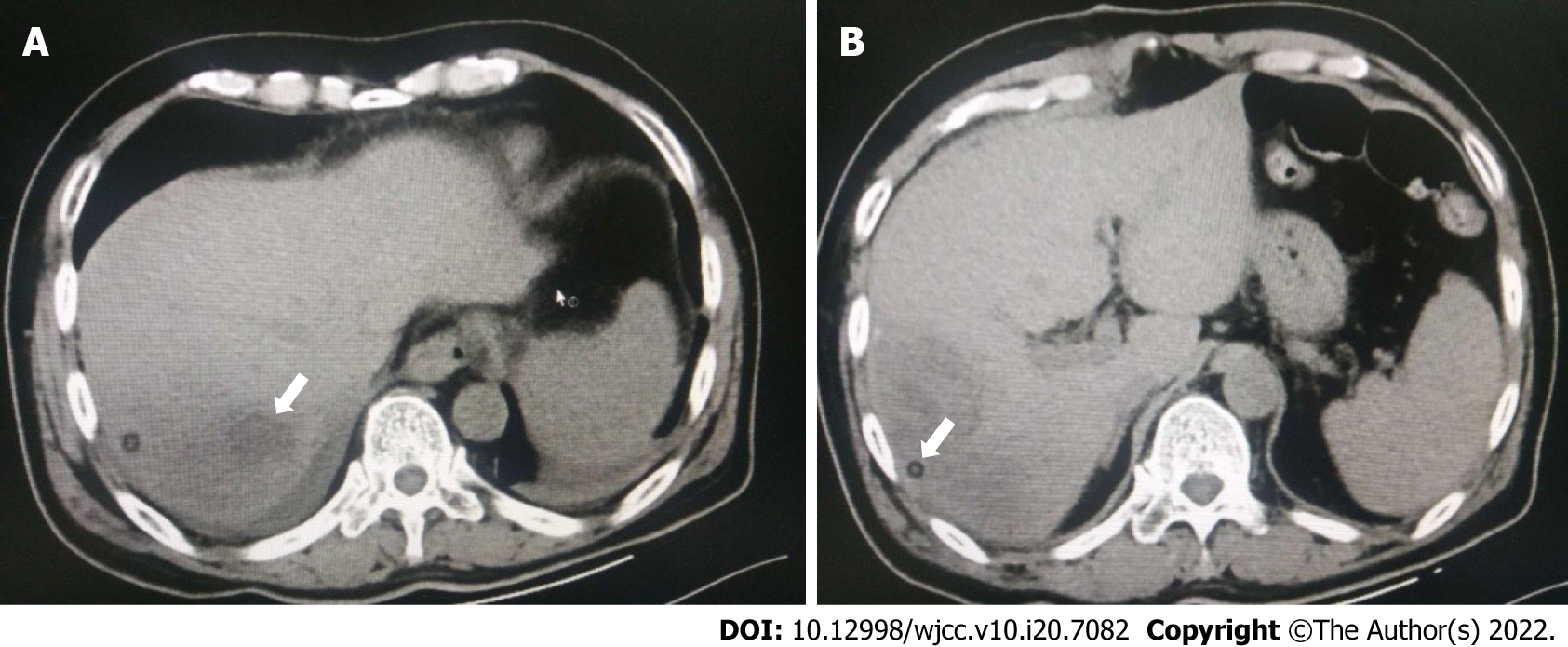
CT images showed multiple low-attenuation masses with unclear boundaries in the right lobe of the liver. A lesion measuring 9 cm × 8 cm was located in the upper posterior segment of the right lobe. A diagnosis of a liver abscess was considered (Figure 1).
A massive multilocular abscess in segments VI and VII of the right liver.
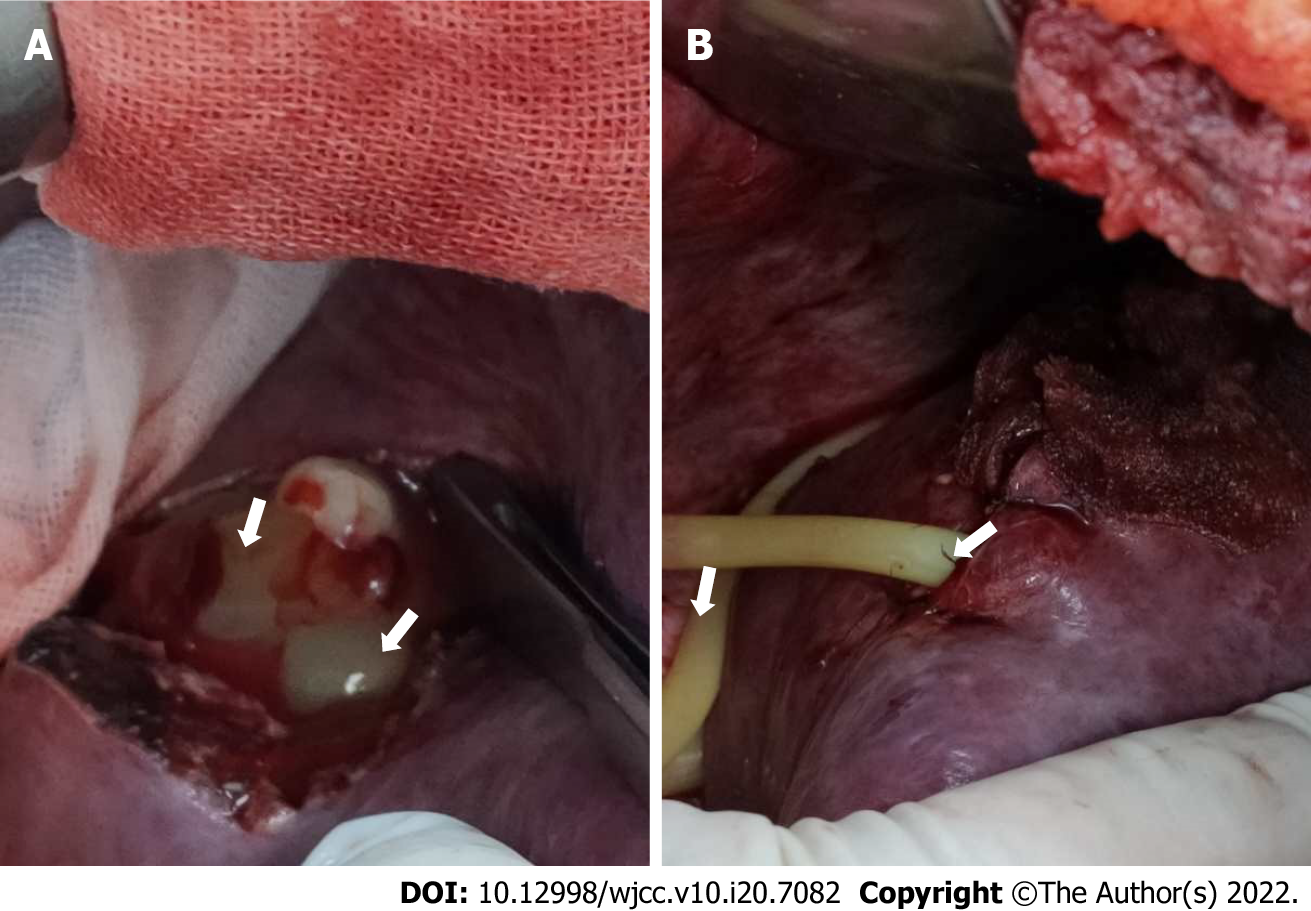
After admission, the patient was empirically given intravenous ceftazidime (2 g every 12 h) as anti-infective therapy and 30 kcal/(kg.d) of enteral and parenteral nutrition support (a full nutrition mixture). The patient’s highest body temperature on the second day after admission was 37.5 °C. He had a poor appetite. Anti-infective therapy alone was not effective. After obtaining consent from the patient’s family members, the following procedures were performed under general anaesthesia on April 21, 2020: Incision, irrigation, and drainage of the liver abscess, injection of PRP into the abscess cavity, and abdominal drainage. The intraoperative findings showed that the surface of the abscess cavity had adhered to the diaphragm and the abdominal wall. After separating the ligamentum teres hepatis, falciform ligament, and right triangular ligament, a 20-mL syringe was used to puncture the swollen hepatic surface. Whitish-grey pus was collected for bacterial culture, and an electronic scalpel was used to extend the incision and excise a portion of the tissue for pathological examination. Blunt separation was performed to separate the septa in the abscess cavity. After the pus was thoroughly drained and aspirated, the abscess cavity was rinsed with iodophor and normal saline. No bile leakage or bleeding was noted. One abdominal drainage tube was placed in the cavity to drain any newly formed pus. The abscess cavity was filled with 80 mL of prepared PRP. Haemostatic gauze was used to cover the operative field to prevent leakage of the PRP; the gauze was secured along the edges of the cavity to complete the procedure (Figure 2). The total intraoperative blood loss volume was 50 mL.
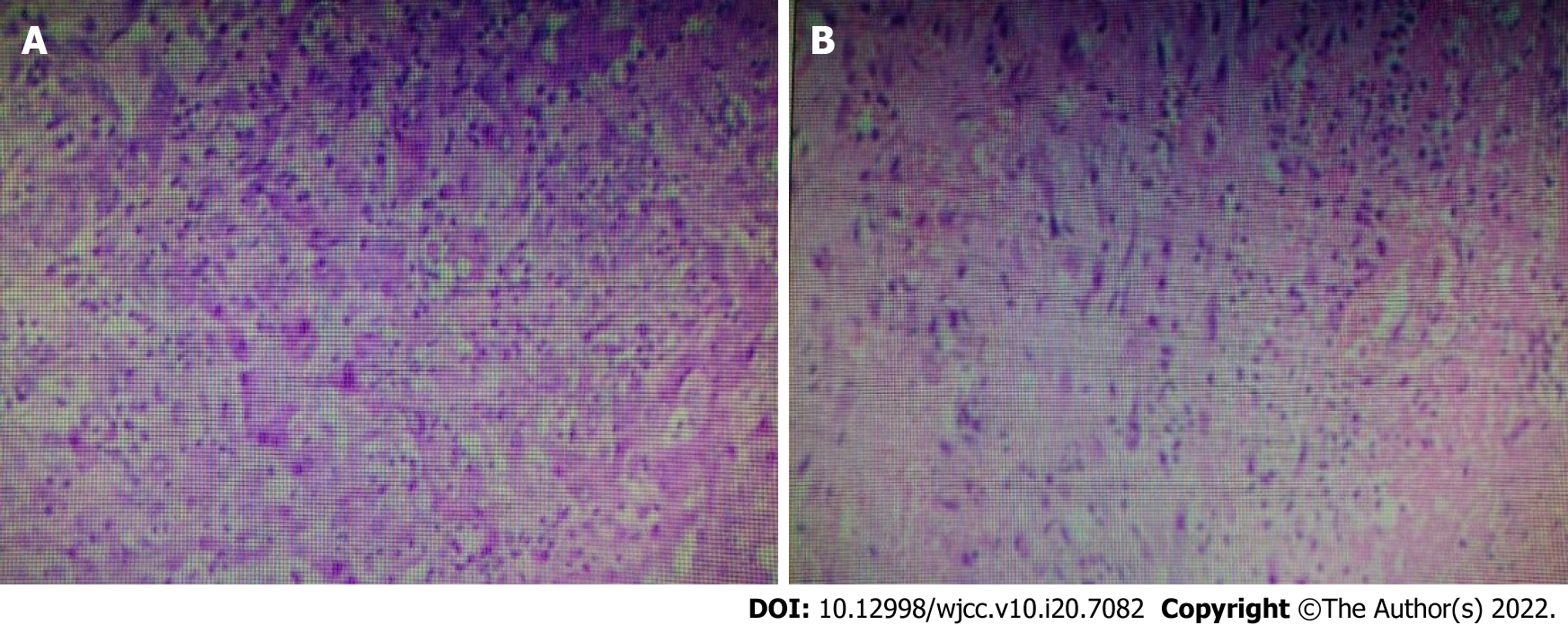
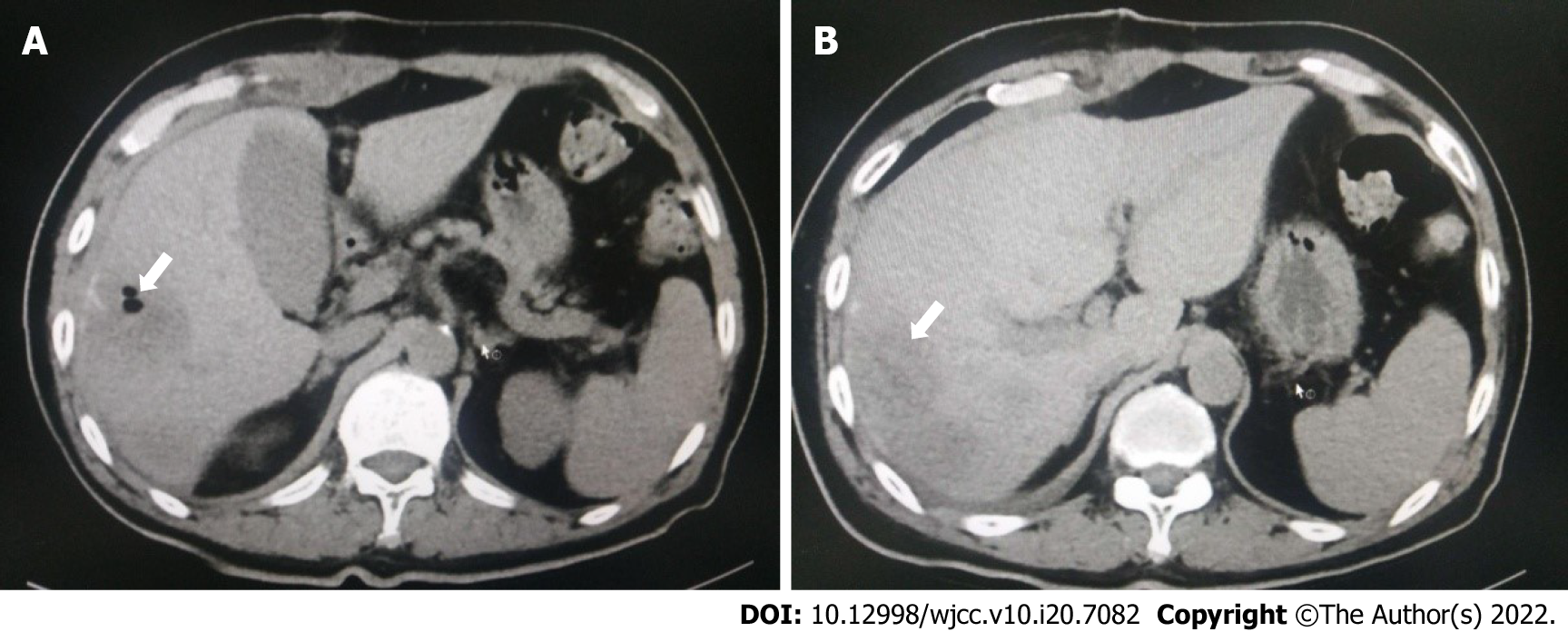
After the procedure, the patient did not have a fever. On the fourth postoperative day, Klebsiella pneumoniae (K. pneumoniae) was found in the drainage fluid on culture, which was sensitive to ceftazidime. Anti-infective therapy was administered, and supportive treatment was continued. Postoperative pathology showing a liver abscess with reactive proliferation of liver cells (Figure 3). After a follow-up CT scan (Figure 4) was performed on April 24, 2020, the drainage tube was removed. On April 28, 2020, another CT examination was performed (Figure 5). Antimicrobial treatment was discontinued, the sutures were removed, and the patient was discharged from the hospital. There was no recurrence on follow-up at 1 mo, 3 mo, 6 mo, or 1 year after surgery.
PLA is a type of purulent inflammation of the liver caused by the invasion of pyogenic bacteria and is most often caused by bloodstream and biliary tract infections. The condition has an incidence of approximately 2.3 per 100000 and is more common in men than in women[1]. In recent years, as a result of the ageing of the population and an increase in the incidence of diseases such as diabetes and malignant tumours, the incidence of PLAs has increased annually. Most cases of PLA can be cured with a combination of percutaneous liver abscess puncture or drainage and antibacterial therapy. In cases of a separated abscess due to delayed diagnosis, the combination of antibacterial drugs and puncture for treatment is often ineffective and has a poor prognosis, thus necessitating surgery[2].
Traditional surgeries for treating PLAs include surgical incision and drainage or partial hepatectomy. In this case study, the abscess was well liquefied, had a large surface area, and was separated; therefore, surgical drainage was indicated. After incising and flushing the PLA, we injected PRP to promote the growth of liver tissue in the cavity.
First developed in the 1990s, PRP is one of the most innovative autologous blood products used to promote tissue healing and regeneration. PRP is prepared by centrifuging blood drawn from one of the patient’s peripheral veins and concentrating the platelets. The long-term effects of platelet activation are manifested by the expression of > 30 growth factors[3]. The level of platelets in PRP is three to five times higher (enriched) than the basal plasma level; additionally, PRP contains elevated concentrations of autologous growth factors [e.g., vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), platelet-derived growth factor, transforming growth factor β-1, hepatocyte growth factor (HGF), and basic fibroblast growth factor], proteins and peptides (e.g., fibrinogen, fibronectin, osteonectin, osteocalcin, vitronectin, and thrombospondin), and certain chemokines and cytokines (e.g., interleukin-1 and platelet factor 4)[3]. Therefore, many PRP types have been used by clinicians for many years due to their advantageous effects on wound healing, cellular mitogenesis, osteogenesis, and angiogenesis[4]. PRP also exerts beneficial effects in terms of promoting tissue regeneration, mitigating infection due to the antibiotic-like action of the included leukocytes, and decreasing pain and blood loss[5].
PRP infusion causes notable liver regeneration in experimental animal models of liver dysfunction, as validated by a significant decrease in levels of liver enzymes (serum aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transferase, and lactate dehydrogenase). PRP provides significant protection against liver fibrosis mainly via antifibrotic, antiapoptotic, and anti-inflammatory pathways[5,6]. Immediate contact between platelets and hepatocytes can evoke the release of soluble factors from platelets, such as IGF-1 and HGF, which are considered crucial mediators of liver recovery[7]. It has also been found that exogenous platelets improve liver recovery. Furthermore, growth factors, such as IGF-1, VEGF, and HGF, contribute to hepatocyte proliferation induced by platelets[8] and promote hepatocyte mitosis, which eventually enhances liver recovery.
Oxidative stress is caused by a shift in the balance between oxidants and antioxidant cytokines and is caused by the overproduction of reactive oxygen species (ROS)[5]. A balance between ROS and the main antioxidant products (superoxide dismutase, catalase, and glutathione peroxidase) is needed to avoid damage from oxidative stress[5]. Partial hepatectomy causes liver injury and tissue loss due to an increase in ROS, which can lead to a reduction not only in cell growth but also in mitotic activity by inhibiting proteins of the cell cycle and disrupting DNA synthesis[9-11]. Histopathological analysis in rats after partial hepatectomy showed that PRP did not provide any tissue improvement in liver regeneration but significantly reduced oxidative stress-induced apoptosis[9].
Under normal physiological conditions, a variety of components in platelets act synergistically on local cells, producing a haemostatic effect; additionally, they stimulate the proliferation, differentiation, and migration of damaged tissue cells; promote collagen matrix deposition and vascular regeneration at the damaged site; shorten the bleeding, inflammation, and proliferation phases of the wound repair process; promote the proliferation and differentiation of cells in the muscular and vascular systems; accelerate epidermal growth in wounds; and promote the repair of damaged tissues and wound healing[12]. Compared with whole blood, PRP generally has 3-5 times more growth factors and differentiation factors[13]. Approximately 70% of the growth factors in PRP are activated within 10 min, and almost 100% are released within 1 h. Very few growth factors are released during the platelet survival period (8-10 d); this may be related to the rapid patient recovery and absence of fever observed after surgery.
Empirical anti-infective treatment can be provided if effective pathogens cannot be identified during the early stage of a PLA. In China, PLAs are most often caused by K. pneumoniae (accounting for 77.1% of all cases)[1]. Empirically, third-generation cephalosporins, fluoroquinolones, carbapenems, and aminoglycosides are often used in the early stage of PLAs[14]. Specific antibacterial drugs can be selected in the later stage based on drug sensitivity. In the present case, ceftazidime was used as an anti-infective treatment for 6 d before surgery. Cultures of intraoperatively collected specimens suggested the involvement of K. pneumoniae, which is sensitive to ceftazidime. Three days after surgery, all of the patient’s indicators were normal. The intravenous infusion of antibacterial drugs was continued for 7 d, and no oral antibacterial drugs were administered. CT performed 14 d after discharge showed that the patient was recovering well. Related literature indicates that antibacterial treatment for PLAs should be provided for 4-6 wk[15]. However, the duration of antibacterial treatment for the patient in this case was significantly shorter. The main considerations for treatment are the complete removal of liquefied necrotic tissue during surgery and the activation of a large number of anti-inflammatory cytokines via PRP injection. This treatment method effectively removed harmful bacteria in a timely manner[14].
To date, PRP has been utilized as a part of therapeutic and surgical approaches in numerous fields, including dentistry, orthopaedics, neurosurgery, ophthalmology, and maxillofacial and cosmetic surgery[16]. A literature review did not yield any reports on the use of PRP in abdominal surgery. Considering that the abdominal cavity is closed, it is necessary to ensure absolute sterility. We found reports on the use of PRP in the treatment of diseases related to assisted reproduction[17], which provides reliable evidence supporting its application in abdominal surgery. After consulting the literature and mastering the basic principles of PRP, we invited experienced physicians from the dermatology department of our hospital to prepare PRP under strictly aseptic conditions to ensure its quality. In this first application of this procedure, the surgeon and the physicians who prepared the PRP independently and reliably performed their duties. The PRP was prepared when needed to avoid prolonged storage and ensure quality. The patient recovered well and was discharged from the hospital 7 d after the operation, with no recurrence over 1 year of follow-up. However, this study only comprised one case. Whether PRP can accelerate recovery from PLAs must be further confirmed by studies with larger samples. Our treatment method involved traditional laparotomy for open drainage to allow complete flushing and accurate injection of the PRP. After this method has been mastered, we plan to adopt a minimally invasive endoscopic approach for the incision and flushing steps and to use a trocar channel for PRP injection to reduce the pain from the surgical incision and accelerate patient recovery.
Previously, PLAs have typically been treated with antibacterial drugs, percutaneous puncture and drainage, or surgical incision and drainage; treatment by surgical incision and drainage combined with PRP has not been reported. By reviewing the relevant literature and combining the previously reported benefits of PRP in terms of liver function improvement and liver tissue regeneration and repair, we treated a large abscess with complete liquefaction and a poor response to antibacterial drug treatment by surgical incision and drainage combined with PRP at our hospital. The patient was discharged after 7 d of treatment without pus cavity flushing and with no recurrence during follow-up. Therefore, we consider that PRP may have direct antimicrobial effects and promote liver tissue regeneration, but confirmation through a clinical case–control study with a larger sample is required.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mikulic D, Croatia; Muthu S, India A-Editor: Wang JL, United States S-Editor: Chang KL L-Editor: Wang TQ P-Editor: Chang KL
| 1. | Deng L, Jia R, Li W, Xue Q, Liu J, Miao Y, Wang J. A Klebsiella pneumoniae liver abscess presenting with myasthenia and tea-colored urine: A case report and review of 77 cases of bacterial rhabdomyolysis. Medicine (Baltimore). 2017;96:e9458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Khim G, Em S, Mo S, Townell N. Liver abscess: diagnostic and management issues found in the low resource setting. Br Med Bull. 2019;132:45-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Aydın O, Pehlivanlı F, Karaca G, Aydın G, Altunkaya C, Bulut H. May dexpanthenol, platelet-rich plasma, and thymoquinone provide new hope to maintain liver regeneration after partial hepatectomy? Turk J Gastroenterol. 2019;30:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Aydin O, Karaca G, Pehlivanli F, Altunkaya C, Uzun H, Özden H, Aydin G, Şahiner İT, Niyaz M, Güler O. Platelet-Rich Plasma May Offer a New Hope in Suppressed Wound Healing When Compared to Mesenchymal Stem Cells. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | El-Kholy W, Elgohary S, El Kholy A, El-Ashkar A. The efficacy of platelet rich plasma as adjuvant therapy in the treatment of cryptosporidiosis in experimentally infected immunosuppressed rats. Parasitologists United Journal. 2021;14:162-170. [DOI] [Full Text] |
| 6. | Salem NA, Hamza A, Alnahdi H, Ayaz N. Biochemical and Molecular Mechanisms of Platelet-Rich Plasma in Ameliorating Liver Fibrosis Induced by Dimethylnitrosurea. Cell Physiol Biochem. 2018;47:2331-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Ohkohchi N, Murata S, Takahashi K. Platelet and Liver Regeneration. Tissue Regeneration - From Basic Biology to Clinical Application, 2012: 109-144. [DOI] [Full Text] |
| 8. | Matsuo R, Ohkohchi N, Murata S, Ikeda O, Nakano Y, Watanabe M, Hisakura K, Myronovych A, Kubota T, Narimatsu H, Ozaki M. Platelets Strongly Induce Hepatocyte Proliferation with IGF-1 and HGF In Vitro. J Surg Res. 2008;145:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Yao XM, Zhao J, Li Y. Effects of bicyclol on liver regeneration after partial hepatectomy in rats. Dig Dis Sci. 2009;54:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Beyer TA, Xu W, Teupser D, auf dem Keller U, Bugnon P, Hildt E, Thiery J, Kan YW, Werner S. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Horimoto M, Fülöp P, Derdák Z, Wands JR, Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004;39:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Hosnuter M, Aslan C, Isik D, Caliskan G, Arslan B, Durgun M. Functional assessment of autologous platelet-rich plasma (PRP) after long-term storage at -20 °C without any preservation agent. J Plast Surg Hand Surg. 2017;51:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 808] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 14. | Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 15. | Ng FH, Wong WM, Wong BC, Kng C, Wong SY, Lai KC, Cheng CS, Yuen WC, Lam SK, Lai CL. Sequential intravenous/oral antibiotic vs. continuous intravenous antibiotic in the treatment of pyogenic liver abscess. Aliment Pharmacol Ther. 2002;16:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | El-Tahawy N, Rifaai1 RA, Saber EA, Saied SR, Ibrah RA. Effect of Platelet Rich Plasma (PRP) Injection on the Endocrine Pancreas of the Experimentally Induced Diabetes in Male Albino Rats: A Histological and Immunohistochemical Study. J Diabetes Metab. 2017;8:3. |
| 17. | Bos-Mikich A, de Oliveira R, Frantz N. Platelet-rich plasma therapy and reproductive medicine. J Assist Reprod Genet. 2018;35:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |