Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.7068
Peer-review started: December 21, 2021
First decision: April 8, 2022
Revised: April 15, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: July 16, 2022
Processing time: 195 Days and 20.2 Hours
Approximately 10% of adults and nearly all children who receive renal replacement therapy have inherited risk factors or are related to genetic factors. In the past, due to the limitations of detection technology and the nonspecific manifestations of uraemia, the etiological diagnosis is unclear. In addition to common monogenic diseases and complex disorders, advanced testing techniques have led to the recognition of more hereditary renal diseases. Here, we report a four-generation Chinese family in which four individuals had a novel SALL1 mutation and presented with uraemia or abnormal urine tests.
A 32-year-old man presented with end-stage renal disease with a 4-year history of dialysis. His father and paternal aunt both had a history of unexplained renal failure with haemodialysis, and his 10-year-old daughter presented with pro
We report a novel SALL1 exon 2 (c.3437delG) mutation and clinical syndrome with kidney disease, bilateral overlapping toes, unilateral dysplastic external ears, and sensorineural hearing loss in a four-generation Chinese family.
Core Tip: We report a novel SALL1 exon 2 (c.3437delG) mutation and clinical syndrome with kidney disease, bilateral overlapping toes, unilateral dysplastic external ears, and sensorineural hearing loss in a four-generation Chinese family. As patients with kidney diseases do not have specific clinical presentations, symptoms other than kidney disease were relatively hidden or easily ignored, thus resulting in missed diagnosis. Gene sequencing is recommended in patients with a family history and with extrarenal phenotypes to avoid blind use of immunosuppressive drugs, which may cause adverse effects.
- Citation: Fang JX, Zhang JS, Wang MM, Liu L. Novel mutation in the SALL1 gene in a four-generation Chinese family with uraemia: A case report. World J Clin Cases 2022; 10(20): 7068-7075
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/7068.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.7068
Hereditary kidney disease is one of the important causes of end-stage renal disease (ESRD) in patients requiring renal replacement therapy. Rapid advances in detection techniques have allowed more unexplained kidney diseases to be accurately diagnosed, including polycystic kidney disease, Alport syndrome, Fabry disease, Bartter syndromes, Gitelman syndromes, and other multifactorial disorders[1]. New genetic mutations causing kidney disease are constantly identified, along with other extrarenal phenotypes.
Townes-Brocks syndrome (TBS), first described by Townes and Brocks[2] in 1972, is a rare autosomal dominant disease resulting from mutations in the developmental gene SALL1[2]. Its main features are the triad of anorectal, hand, and external ear malformations. Another key characteristic of TBS is kidney involvement, which results in progression to ESRD early in life. A report of 154 patients with TBS identified renal anomalies in 43% of affected individuals[3]. Previous reports have shown that syndrome-related kidney and genitourinary defects include renal hypoplasia, unilateral renal agenesis, dysplastic kidneys, vesicoureteric reflux, meatal stenosis, and glandular hypoplasia[3]. Here, we report a four-generation family including four affected individuals suffering from kidney disease who carry a novel SALL1 mutation. Among them, three patients progressed to ESRD.
A 32-year-old man was admitted to our hospital because of ESRD characterized by severe hypertension and elevated serum creatinine (SCr) for 9 years and he had received renal dialysis for 4 years.
Nine years ago, the patient was admitted to a hospital because of syncope and was diagnosed with uraemia. After diagnosis, he was treated with traditional Chinese medicine. He started renal dialysis 4 years ago because of gradually deteriorating kidney function with a SCr level of 900 μmol/L. A kidney biopsy was not performed during the course of the kidney disease.
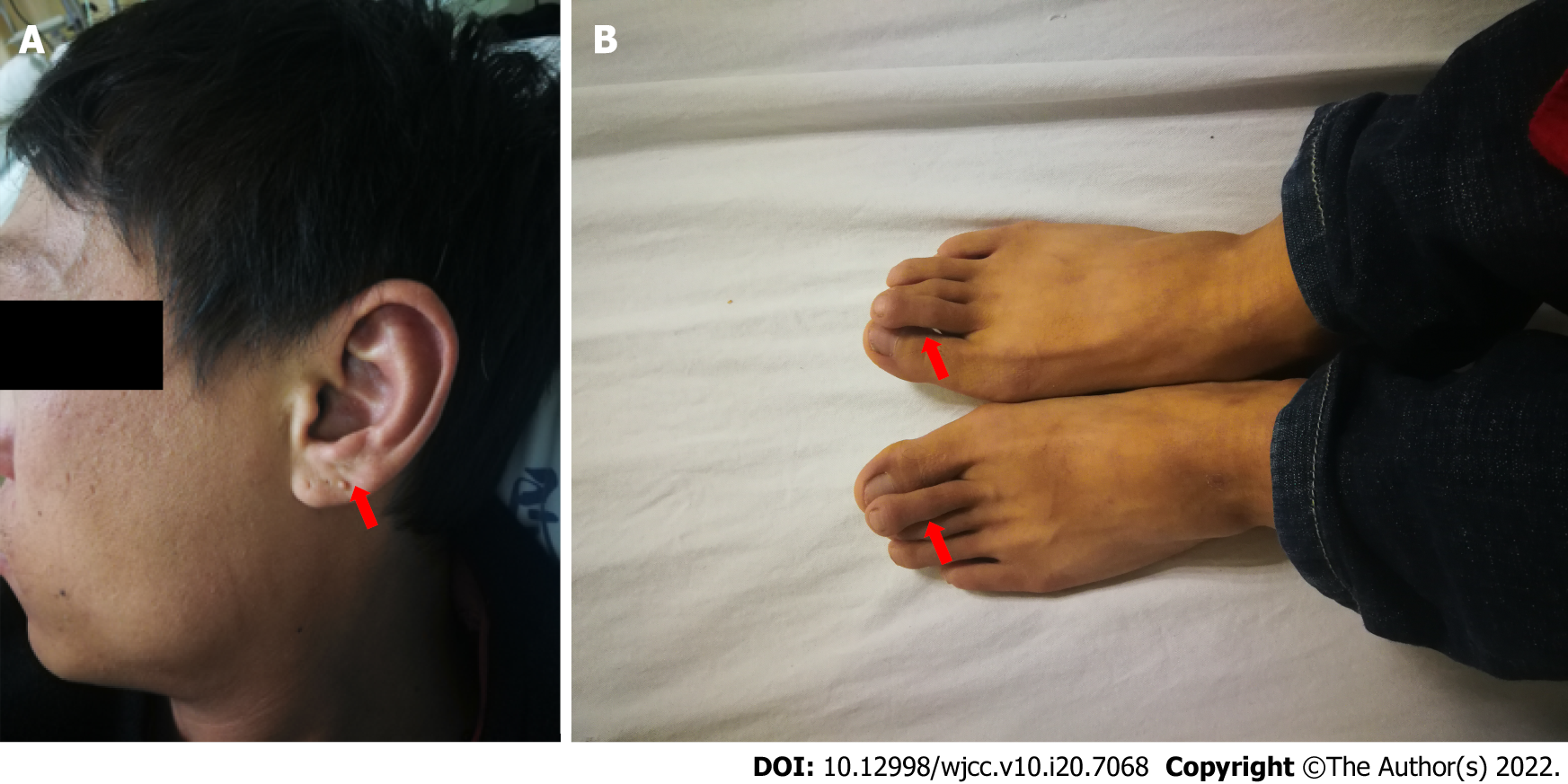
The patient was born with multiple congenital abnormalities, including limb malformation (bilateral overlapping toes) and unilateral dysplastic external ears (Figure 1). A unilateral moderate degree of sensorineural hearing loss was noted when he was 10 years old.
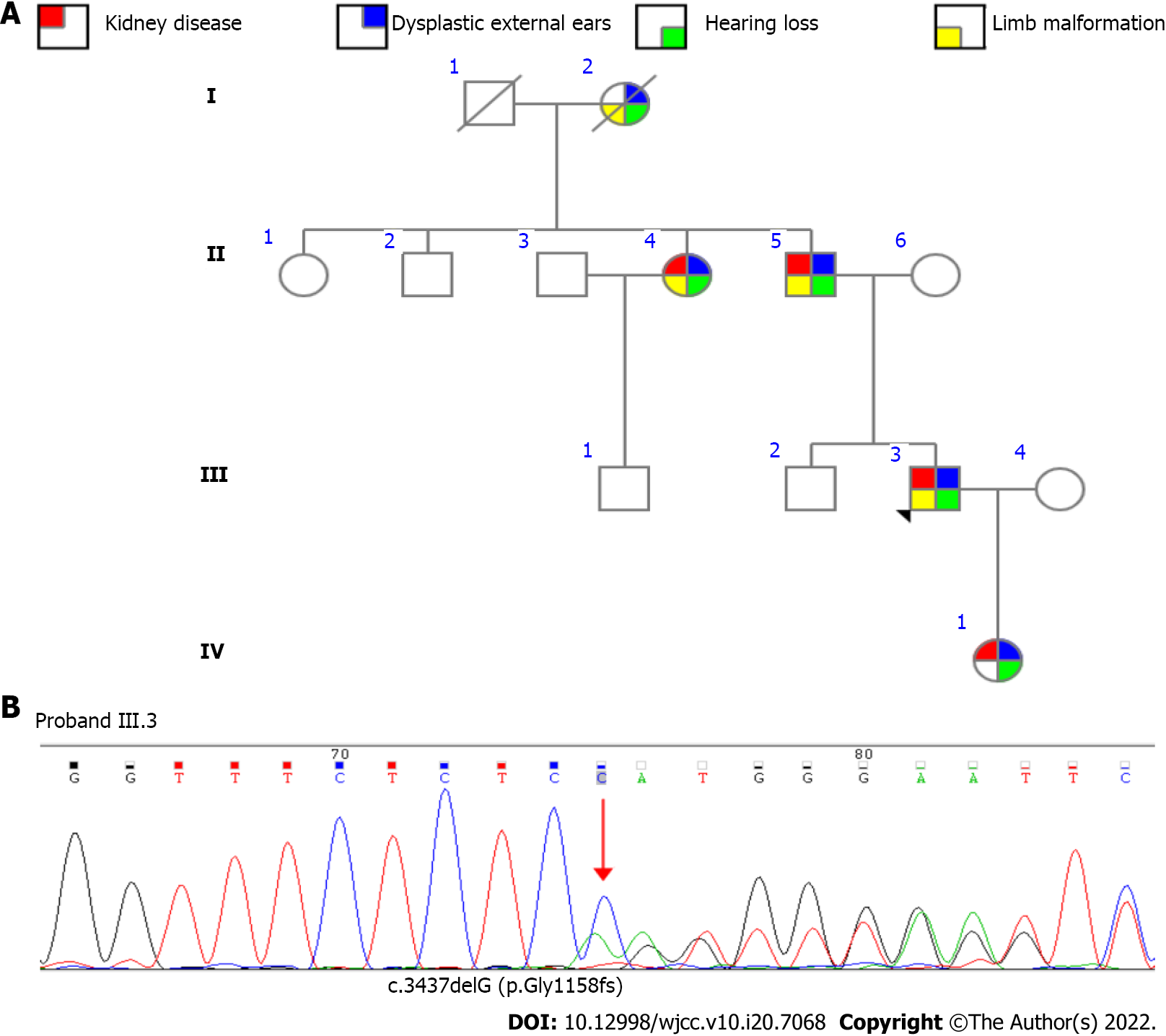
The family pedigree for the patient (proband, III.3) is shown in Figure 2A. The patient’s grandmother (I.2) died at an early age without a clear diagnosis due to the poor level of medical care. However, she presented with a sixth finger malformation, unilateral dysplastic external ears, and hearing loss. The patient’s father (II.5) had unilateral dysplastic external ears, hearing loss, and bilateral overlapping toes. At the age of 53 years, he began dialysis therapy. The patient’s aunt (II.4) was born with unilateral dysplastic external ears, hearing loss, and limb malformation (unilateral preaxial polydactyly of one hand). At the age of 53 years, she also began haemodialysis due to unexplained ESRD. The patient’s daughter (IV.1) was born with bilateral dysplastic external ears, without limb malformation, and was found to have unilateral hearing dysfunction misdiagnosed as otitis media.
Vital signs were in the normal ranges: Body temperature, 36.7 °C; respiratory rate, 18 breaths/min; pulse rate, 80 bpm; and blood pressure (under antihypertensive treatment), 134/85 mmHg. Limb malformation (bilateral overlapping toes) and unilateral dysplastic external ears existed. His ophthalmic examination results were normal, and no anorectal abnormalities were found.
Laboratory analysis revealed an increased SCr level (941.9 μmol/L), high potassium level (6.87 mmol/L), high phosphate level (2.73 mmol/L), normal haemoglobin level (145 g/L), and high parathyroid hormone level (663.6 pg/mL). The patient was anuria.
Ultrasound examination revealed bilaterally small kidneys (left kidney, 52 mm × 34 mm; right kidney, 50 mm × 25 mm) with multiple 5-7 mm medullary cysts. The urinary tract was normal, and no additional abnormalities of the liver, spleen, or pancreas were detected in the imaging studies.
After obtaining informed consent, we conducted high-throughput detection and analysis of approximately 20000 genes of the proband, focusing on the genes related to uraemia and nephropathy. One pathogenic mutation, a heterozygous shift mutation in SALL1_ exon 2 (c.3437delG), was found to be related to the patient’s symptoms (Figure 2B). It was verified that the three relatives mentioned above carried the same mutation (Figure 2B). The genetic pattern of SALL1 associated with TBS is autosomal dominant. It is speculated that this pathogenic mutation is the main cause of familial hereditary disease. Finally, based on clinical features and the presence of the SALL1 pathogenic variant, the suspected diagnosis of TBS without imperforate anus was confirmed.
The patient received maintenance haemodialysis three times a week, sevelamer carbonate tablets of 1.6 g three times per day, calcitriol soft capsules of 0.5 μg per day, and nifedipine controlled-release tablets of 60 mg per day.
The patient and two relatives were on dialysis and received regular follow-up. His daughter currently presents with minimal proteinuria and normal SCr. She was treated with losartan potassium tablets of 25 mg per day and was followed every 3 mo.
In this four-generation family diagnosed with TBS with renal manifestations, uraemia was the first symptom to prompt the affected members to go to the hospital. The diagnosis of TBS was confirmed by a genetic test in four family members, three of whom had already progressed to ESRF. This indicates that symptoms other than kidney disease were relatively hidden or easily ignored and resulted in missed diagnosis.
The transcoding mutation resulted in a change in the amino acid at position 1158 from glycine to glutamate, which changed the subsequent reading frame and caused advanced termination at the downstream codon 45. This mutation site has not been previously included in the ClinVar database. The frequency of mutation at this locus in the normal East Asian population has not been reported.
Since Townes and Brocks[2] first described TBS in 1972, hundreds of similar cases have been reported. It is characterized by ear anomalies, thumb anomalies (preaxial polydactyly, triphalangeal thumbs, and hypoplastic thumbs), and anal anomalies. The prevalence of TBS is estimated to be 1/250000[4]. The gene mutated in TBS is SALL1, which is located on chromosome 16q12.1 and encodes a zinc finger protein believed to act as a transcriptional repressor[5]. SALL1 is expressed in the metanephric mesenchyme surrounding the ureteric bud. Homozygous deletion of SALL1 produces incomplete ureteric bud outgrowth. Therefore, SALL1 is important for metanephros development[6]. Most mutations occur within a hotspot prior to the first C2H2 zinc finger domain encoded within exon 2 and result in the truncation of the protein upstream of the DNA binding domain[7].
In a murine experiment, all of the SALL1 knockout mice (SALL1-/-) died within 24 h after birth owing to renal agenesis or severe dysgenesis[6]. Mutant mice that produced a truncated SALL1 protein exhibited more severe defects than SALL1-null mice, including renal agenesis, exencephaly, and limb and anal deformities[8]. This finding may be explained by the fact that truncated proteins arising from certain SALL1 mutations can disrupt cilia formation and function. Therefore, TBS might be considered a ciliopathy-like disease, just as the proband presented with multiple renal cysts. A recent study showed that the Dishevelled Binding Antagonist Of Beta Catenin 1 (DACT1) c.1256G>A nonsense variant was causative of a specific genetic syndrome with features overlapping those of TBS[3]. Therefore, genetic testing to identify the mutated gene is critical for the diagnosis.
Newman first drew attention to symptomatic renal failure in TBS[9]. A previous study of TBS showed that 43% of affected individuals exhibited renal abnormalities. To determine genotype-phenotype correlations in the renal manifestations of TBS, we reviewed 76 affected individuals from 51 families, including 44 men (57.9%), 29 women (38.1%), and three (3.9%) for whom sex was not reported, giving a female-to-male ratio of 0.5:1. The details of the SALL1 gene variations and renal manifestations in these 76 patients with TBS are summarized in Table 1. Overall, 43 different variants were identified, including 29 frameshift mutations, 12 nonsense mutations, one intronic mutation, and one exonic deletion. The most common renal manifestations were renal hypodysplasia and unilateral renal agenesis (n = 25), followed by vesicoureteral reflux (n = 17). Almost half of the individuals had varying degrees of renal impairment, and 22% of the individuals progressed to renal failure. Some of the patients successfully underwent kidney transplantation, and two of them experienced graft rejection[3,7,9,10].
| Genetic finding | F/N | Gender | Phenotype | Ref. | |||||
| BRA | URA | PK | VR | RF | RFI | ||||
| c.313delA | FA/N1 | F | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 1/1 | [7] |
| c.419delC | FA/N1 | M | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 1 | [15] |
| c.764delT | FA/N1 | M | 0/1 | 0/1 | 1/1 | 0/1 | 1/1 | 0/1 | [3] |
| c.792delGC | FA/N2 | M/F | 0/2 | 1/2 | 0/2 | 2/2 | 0/2 | 0/2 | [16] |
| c.817delG | FA/N1 | M | 0/1 | 1/1 | 0/1 | 0/1 | 1/1 | 0/1 | [17] |
| c.981insTGGC | FA/N1 | M | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 1/1 | [18] |
| c.995delC | FA/N1 | M | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | [7] |
| c.1047dupC | FA/N1 | M | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | [7] |
| c.1119del79 | FA/N1 | F | 1/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | [7] |
| c.1134delT | FA/N1 | F | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | [7] |
| c.1145insTA | FA/N1 | M | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | 1/1 | [3] |
| FB/N1 | M | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | [7] | |
| c.1146delT | FA/N3 | 2F/M | 0/3 | 2/3 | 0/3 | 0/3 | 0/3 | 3/3 | [15] |
| c.1174delCT | FA/N1 | M | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | [7] |
| c.1200del7 | FA/N1 | M | 1/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | [15] |
| c.1263delC | FA/N1 | F | 1/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | [3] |
| c.1273delC | FA/N1 | M | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | [7] |
| c.1277del2bp | FA/N1 | F | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 1/1 | [15] |
| c.1291del10 | FA/N2 | 2M | 2/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 | [15] |
| c.1321dupA | FA/N1 | F | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 1/1 | [3] |
| c.1327delG | FA/N2 | M/? | 1/2 | 1/2 | 0/2 | 0/2 | 0/2 | 0/2 | [3] |
| c.1347delCA | FA/N1 | M | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | [19] |
| c.1404dupG | FA/N9 | 4F/5M | 3/9 | 3/9 | 0/9 | 2/9 | 2/9 | 1/9 | [3] |
| c.1451del7insT | FA/N1 | M | 0/1 | 1/1 | 0/1 | 0/1 | 1/1 | 0/1 | [18] |
| c.1470delG | FA/N1 | M | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | 1/1 | [20] |
| c.1487del562 | FA/N1 | M | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | [19] |
| c.1516dupAT | FA/N2 | 2M | 2/2 | 0/2 | 0/2 | 2/2 | 0/2 | 0/2 | [7] |
| c.1819delG | FA/N3 | 2F/M | 2/3 | 0/3 | 0/3 | 1/3 | 1/3 | 1/3 | [21] |
| c.3249del7 | FA/N1 | M | 1/1 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | [7] |
| c.3414delAT | FA/N1 | M | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | [7] |
| FB/N1 | M | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | [22] | |
| Total (%) | F31N47 | 15F/31M/1? | 16/47 (34) | 18/47 (38) | 2/47 (4) | 11/47 (23) | 9/47 (19) | 14/47 (30) | |
| p.Gln272X | FA/N1 | M | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | [7] |
| p.Leu275X | FA/N1 | M | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | [12] |
| p.Arg276X | FA/N2 | / | 1/2 | 0/2 | 0/2 | 0/2 | 1/2 | 0/2 | [19] |
| F B/N1 | F | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | [23] | |
| F C/N3 | 3M | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 2/3 | [24] | |
| FD/N2 | 2M | 0/2 | 1/2 | 1/2 | 0/2 | 0/2 | 1/2 | [15] | |
| FE/N1 | F | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 1/1 | [25] | |
| FF/N1 | F | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | [26] | |
| P.Gln243X | FA/N2 | F | 2/2 | 0/2 | 0/2 | 0/2 | 2/2 | 0/2 | [10] |
| p.Gln323X | FA/N2 | 2M | 1/2 | 0/2 | 0/2 | 1/2 | 2/2 | 0/2 | [3] |
| p.Ser371X | FA/N1 | M | 1/1 | 0/1 | 0/1 | 1/1 | 0/1 | 1/1 | [3] |
| p.Ser372X | FA/N2 | M/F | 0/2 | 1/2 | 0/2 | 1/2 | 0/2 | 0/2 | [17] |
| p.Lys419X | FA/N2 | 2F | 2/2 | 0/2 | 0/2 | 0/2 | 1/2 | 0/2 | [3] |
| FB/N2 | 2F | 0/2 | 2/2 | 0/2 | 0/2 | 0/2 | 0/2 | [27] | |
| p.Tyr503X | FA/N1 | F | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | [21] |
| p.Gln507X | FA/N1 | F | 1/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | [3] |
| p.Gln927X | FA/N1 | M | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | [3] |
| p.Arg1054X | FA/N1 | F | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | 1/1 | [28] |
| Total (%) | F17/N27 | 13F/12M/2? | 10/27 (37) | 7/27 (26) | 2/27 (7) | 5/27 (18) | 8/27 (30) | 6/27 (22) | |
| del 3384 dp | FA/N1 | F | 0/1 | 0/1 | 0/1 | 1/1 | 1/1 | 0/1 | [3] |
| IVS2/19T | FA/N1 | M | 1/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | [21] |
| Total (%) | F51/N76 | 29F/44M/3? | 25/76 (33) | 25/76 (33) | 4/76 (5) | 17/76 (22) | 17/76 (22) | 20/76 (26) | |
Because TBS is a polymorphic syndrome, it needs to be distinguished from the following syndromes: Vertebral, anal, cardiac, tracheal-esophageal, renal (VACTER) and limb anomalies association[11], Goldenhar syndrome (oculo-auriculo-vertebral; impaired development of structures such as the eyes, ears, lip, tongue, palate, mandible, and maxilla and deformations of the tooth structures)[12], Okihiro syndrome (forearm malformations with Duane syndrome of eye retraction)[13], branchio-oto-renal syndrome (hearing loss, auricular malformations, branchial arch remnants, and renal anomalies)[3], and syndactyly, telecanthus, anogenital, and renal malformations syndrome[14].
As patients with kidney diseases do not have specific clinical presentations, it is possible to find rare diseases in any dialysis patients. Doctors are advised to concern about the following issues: Ask patients for family history of kidney diseases, see whether the patient has ocular or hear pathologies, see whether there is poliglobulia or abdominal masses or a family history of cerebral aneurysms, and verify that if there is neuropathy or cardiopathy when they do not obey to uremia or hypertension alone. Gene sequencing is recommended in the following categories of patients with kidney disease: Individuals with a family history of kidney disease; individuals with an unexplained renal phenotype associated with extrarenal phenotypes, especially in the eyes and ears; and individuals with polycystic kidney disease. Patients and their families can avoid the blind use of immunosuppressive drugs, which may cause adverse effects.
We report a novel SALL1 exon 2 (c.3437delG) mutation and clinical syndrome with kidney disease, bilateral overlapping toes, unilateral dysplastic external ears, and sensorineural hearing loss in a four-generation Chinese family.
The authors are grateful to the family for their participation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gonzalez FM, Chile; Kaewput W, Thailand; Sureshkumar KK, United States; Taheri S, Iran; Yuksel S, Turkey A-Editor: Yao QG, China S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Mehta L, Jim B. Hereditary Renal Diseases. Semin Nephrol. 2017;37:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Townes PL, Brocks ER. Hereditary syndrome of imperforate anus with hand, foot, and ear anomalies. J Pediatr. 1972;81:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 97] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Miller EM, Hopkin R, Bao L, Ware SM. Implications for genotype-phenotype predictions in Townes-Brocks syndrome: case report of a novel SALL1 deletion and review of the literature. Am J Med Genet A. 2012;158A:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Martínez-Frías ML, Bermejo Sánchez E, Arroyo Carrera I, Pérez Fernández JL, Pardo Romero M, Burón Martínez E, Hernández Ramón F. [The Townes-Brocks syndrome in Spain: the epidemiological aspects in a consecutive series of cases]. An Esp Pediatr. 1999;50:57-60. [PubMed] |
| 5. | Netzer C, Rieger L, Brero A, Zhang CD, Hinzke M, Kohlhase J, Bohlander SK. SALL1, the gene mutated in Townes-Brocks syndrome, encodes a transcriptional repressor which interacts with TRF1/PIN2 and localizes to pericentromeric heterochromatin. Hum Mol Genet. 2001;10:3017-3024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Nishinakamura R, Takasato M. Essential roles of Sall1 in kidney development. Kidney Int. 2005;68:1948-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Botzenhart EM, Bartalini G, Blair E, Brady AF, Elmslie F, Chong KL, Christy K, Torres-Martinez W, Danesino C, Deardorff MA, Fryns JP, Marlin S, Garcia-Minaur S, Hellenbroich Y, Hay BN, Penttinen M, Shashi V, Terhal P, Van Maldergem L, Whiteford ML, Zackai E, Kohlhase J. Townes-Brocks syndrome: twenty novel SALL1 mutations in sporadic and familial cases and refinement of the SALL1 hot spot region. Hum Mutat. 2007;28:204-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Kiefer SM, Ohlemiller KK, Yang J, McDill BW, Kohlhase J, Rauchman M. Expression of a truncated Sall1 transcriptional repressor is responsible for Townes-Brocks syndrome birth defects. Hum Mol Genet. 2003;12:2221-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Newman WG, Brunet MD, Donnai D. Townes-Brocks syndrome presenting as end stage renal failure. Clin Dysmorphol. 1997;6:57-60. [PubMed] |
| 10. | Beaudoux O, Lebre AS, Doco Fenzy M, Spodenkiewicz M, Canivet E, Colosio C, Poirsier C. Adult diagnosis of Townes-Brocks syndrome with renal failure: Two related cases and review of literature. Am J Med Genet A. 2021;185:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | van de Putte R, Dworschak GC, Brosens E, Reutter HM, Marcelis CLM, Acuna-Hidalgo R, Kurtas NE, Steehouwer M, Dunwoodie SL, Schmiedeke E, Märzheuser S, Schwarzer N, Brooks AS, de Klein A, Sloots CEJ, Tibboel D, Brisighelli G, Morandi A, Bedeschi MF, Bates MD, Levitt MA, Peña A, de Blaauw I, Roeleveld N, Brunner HG, van Rooij IALM, Hoischen A. A Genetics-First Approach Revealed Monogenic Disorders in Patients With ARM and VACTERL Anomalies. Front Pediatr. 2020;8:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Liberalesso PBN, Cordeiro ML, Karuta SCV, Koladicz KRJ, Nitsche A, Zeigelboim BS, Raskin S, Rauchman M. Phenotypic and genotypic aspects of Townes-Brock syndrome: case report of patient in southern Brazil with a new SALL1 hotspot region nonsense mutation. BMC Med Genet. 2017;18:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Kohlhase J, Heinrich M, Schubert L, Liebers M, Kispert A, Laccone F, Turnpenny P, Winter RM, Reardon W. Okihiro syndrome is caused by SALL4 mutations. Hum Mol Genet. 2002;11:2979-2987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Li AL, Borooah S, Nudleman E. Multimodal imaging of retinal findings in syndactyly, telecanthus, anogenital, and renal malformations (STAR) syndrome. Am J Ophthalmol Case Rep. 2022;25:101284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Kohlhase J, Taschner PE, Burfeind P, Pasche B, Newman B, Blanck C, Breuning MH, ten Kate LP, Maaswinkel-Mooy P, Mitulla B, Seidel J, Kirkpatrick SJ, Pauli RM, Wargowski DS, Devriendt K, Proesmans W, Gabrielli O, Coppa GV, Wesby-van Swaay E, Trembath RC, Schinzel AA, Reardon W, Seemanova E, Engel W. Molecular analysis of SALL1 mutations in Townes-Brocks syndrome. Am J Hum Genet. 1999;64:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Surka WS, Kohlhase J, Neunert CE, Schneider DS, Proud VK. Unique family with Townes-Brocks syndrome, SALL1 mutation, and cardiac defects. Am J Med Genet. 2001;102:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Salerno A, Kohlhase J, Kaplan BS. Townes-Brocks syndrome and renal dysplasia: a novel mutation in the SALL1 gene. Pediatr Nephrol. 2000;14:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Faguer S, Pillet A, Chassaing N, Merhenberger M, Bernadet-Monrozies P, Guitard J, Chauveau D. Nephropathy in Townes-Brocks syndrome (SALL1 mutation): imaging and pathological findings in adulthood. Nephrol Dial Transplant. 2009;24:1341-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Marlin S, Blanchard S, Slim R, Lacombe D, Denoyelle F, Alessandri JL, Calzolari E, Drouin-Garraud V, Ferraz FG, Fourmaintraux A, Philip N, Toublanc JE, Petit C. Townes-Brocks syndrome: detection of a SALL1 mutation hot spot and evidence for a position effect in one patient. Hum Mutat. 1999;14:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Choi WI, Kim JH, Yoo HW, Oh SH. A family with Townes-Brocks syndrome with congenital hypothyroidism and a novel mutation of the SALL1 gene. Korean J Pediatr. 2010;53:1018-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Blanck C, Kohlhase J, Engels S, Burfeind P, Engel W, Bottani A, Patel MS, Kroes HY, Cobben JM. Three novel SALL1 mutations extend the mutational spectrum in Townes-Brocks syndrome. J Med Genet. 2000;37:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Lawrence C, Hong-McAtee I, Hall B, Hartsfield J, Rutherford A, Bonilla T, Bay C. Endocrine abnormalities in Townes-Brocks syndrome. Am J Med Genet A. 2013;161A:2266-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Keegan CE, Mulliken JB, Wu BL, Korf BR. Townes-Brocks syndrome vs expanded spectrum hemifacial microsomia: review of eight patients and further evidence of a "hot spot" for mutation in the SALL1 gene. Genet Med. 2001;3:310-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Kohlhase J, Liebers M, Backe J, Baumann-Müller A, Bembea M, Destrée A, Gattas M, Grüssner S, Müller T, Mortier G, Skrypnyk C, Yano S, Wirbelauer J, Michaelis RC. High incidence of the R276X SALL1 mutation in sporadic but not familial Townes-Brocks syndrome and report of the first familial case. J Med Genet. 2003;40:e127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | van Bever Y, Gischler SJ, Hoeve HL, Smit LS, Nauta J, Dooijes D. Obstructive apneas and severe dysphagia in a girl with Townes-Brocks syndrome and atypical feet involvement. Eur J Med Genet. 2009;52:426-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Barry JS, Reddy MA. The association of an epibulbar dermoid and Duane syndrome in a patient with a SALL1 mutation (Townes-Brocks Syndrome). Ophthalmic Genet. 2008;29:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Sudo Y, Numakura C, Abe A, Aiba S, Matsunaga A, Hayasaka K. Phenotypic variability in a family with Townes-Brocks syndrome. J Hum Genet. 2010;55:550-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Vodopiutz J, Zoller H, Fenwick AL, Arnhold R, Schmid M, Prayer D, Müller T, Repa A, Pollak A, Aufricht C, Wilkie AO, Janecke AR. Homozygous SALL1 mutation causes a novel multiple congenital anomaly-mental retardation syndrome. J Pediatr. 2013;162:612-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |