Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.7060
Peer-review started: December 15, 2021
First decision: March 23, 2022
Revised: April 4, 2022
Accepted: May 21, 2022
Article in press: May 21, 2022
Published online: July 16, 2022
Processing time: 201 Days and 18.5 Hours
Myotonic dystrophy type 1 (DM1) is a genetic neuromuscular disease involving multiple systems, especially the cardiopulmonary system. The clinical phenotype of DM1 patients is highly variable, which limits early diagnosis and treatment. In the present study, we reported a 35-year-old female DM1 patient with dyspnea as the primary onset clinical manifestation, analyzed her family's medical history, and reviewed related literature.
A 35-year-old woman was admitted to the hospital with dyspnea of 1 mo duration, and sleep apnea for 3 d. Her respiratory pattern and effort were normal, but limb muscle tension was low. Investigation into the patient's medical history revealed that she might have hereditary neuromuscular disease. Electro
DM1 is a genetic neuromuscular disease involving multiple systems, and the clinical phenotype in DM1 is extremely variable. Some patients with DM1 may be presented at the respiratory department because of dyspnea, which should be cautioned by the pulmonologists. There may be no obvious or specific symptoms in the early stage of disease, and clinicians should improve their understanding of DM1 and make an early diagnosis, which will improve patients’ quality of life.
Core Tip: Myotonic dystrophy type 1 (DM1) is a genetic neuromuscular disease involving multiple systems, especially the cardiopulmonary system. The clinical phenotype of DM1 patients is highly variable, which limits early diagnosis and treatment. The gold standard for the diagnosis of DM1 is genetic testing. Moreover, the management of DM1 patients involves genetic counseling, providing symptomatic treatment for myotonia and lethargy, and preventing cardiopulmonary complications. In the present study, we reported a rare case of a DM1 patient with dyspnea, analyzed her family's medical history, and reviewed related literature.
- Citation: Jia YX, Dong CL, Xue JW, Duan XQ, Xu MY, Su XM, Li P. Myotonic dystrophy type 1 presenting with dyspnea: A case report. World J Clin Cases 2022; 10(20): 7060-7067
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/7060.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.7060
Myotonic dystrophy type 1 (DM1) is an inherited neuromuscular disease characterized by muscle rigidity, muscle weakness, and atrophy[1-3], with a prevalence of 0.5-18.1 per 100000 population[4]. DM1 patients may also have concurrent multiple systemic disorders, such as lens opacity[5], abnormal cardiac conduction systems[6], insulin resistance[7], gastrointestinal dysfunction[8], and cognitive dysfunction[9].
Symptoms related to respiratory dysfunction in DM1 have a significant negative impact on quality of life[10]. Moreover, respiratory dysfunction is the most common cause of death in patients with DM1, usually resulting from respiratory failure or aspiration[11]. Also, sleep disturbances, including excessive daytime sleepiness, central and obstructive sleep apnea, restless legs syndrome, and rapid eye movement sleep dysregulation, are prominent in patients with DM1[12,13]. Overall, the clinical phenotype of DM1 patients is highly variable[1,6,9,14].
Several studies on DM1 patients with different disorders have been reported[6,14-16]. However, the proband diagnosed DM1 with sleep apnea, who complained of dyspnea as the primary onset symptom and underwent genetic testing, has rarely been reported. Herein, this study reported a case of DM1 with sleep apnea, analyzed the patient's family medical history, and reviewed related literatures. We aimed to arouse clinicians' awareness of the DM1 with dyspnea and sleep apnea and collected as much clinical data as possible to make a correct diagnosis.
The study was reviewed and approved by the Second Hospital of Jilin University Ethics Committee Review Board.
A 35-year-old woman was admitted to Department of Respiratory and Critical Care Medicine of our hospital for shortness of breath for 1 mo and sleep apnea for 3 d.
The patient had shortness of breath without any obvious reason approximately 1 mo ago. The antibiotic treatment and oxygen therapy by local hospital did not improve her symptoms. She presented with orthopnea and was forced to sleep in sitting position 3 d before her admission.
The patient had mild limb weakness for almost 4 years. The patient denied a history of speech disorders, hypertension, diabetes, coronary heart disease, blood transfusion, or drug and food allergy.
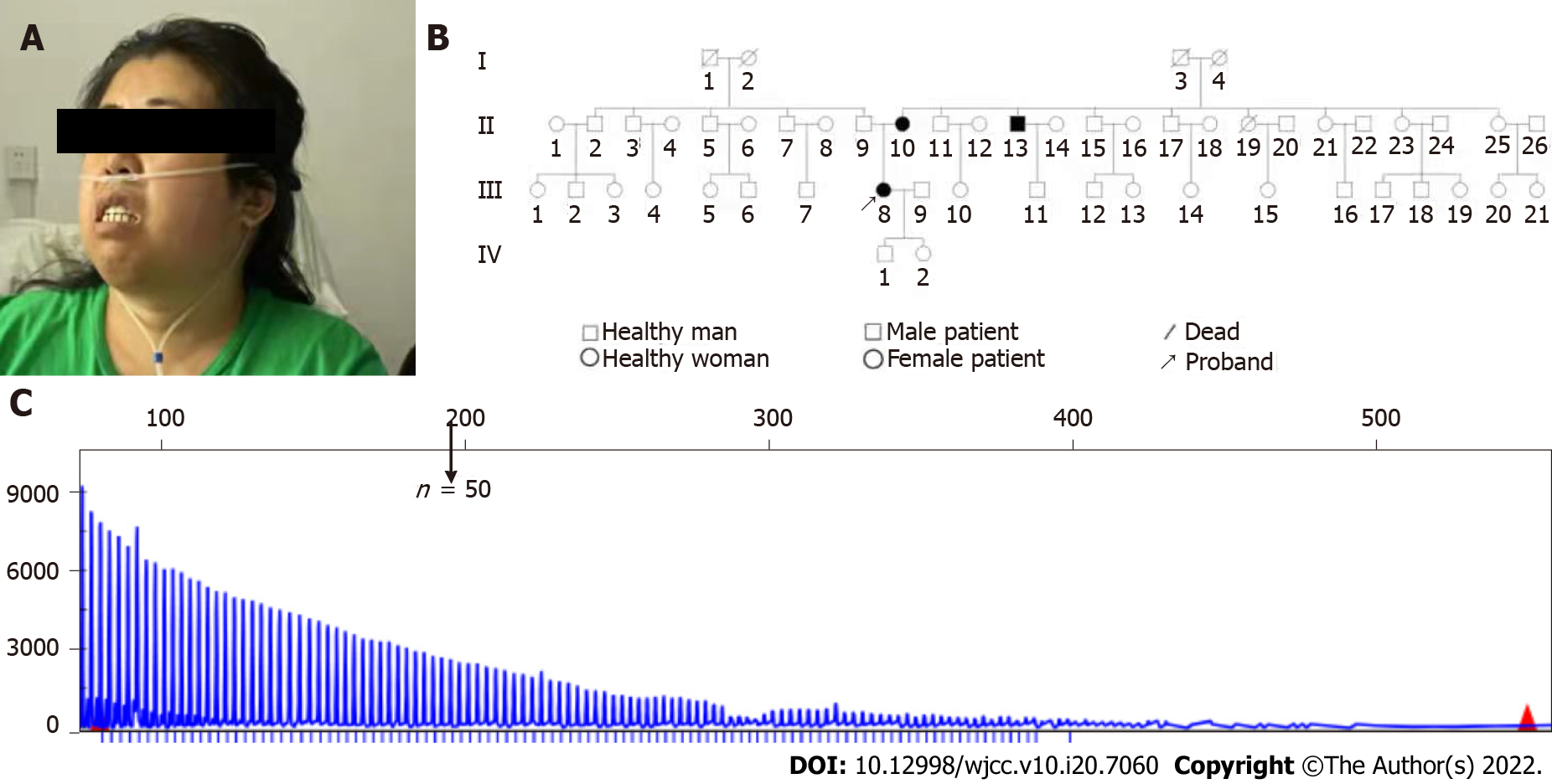
The pedigree of this patient (Ⅲ8) showed her father had 5 siblings; and her mother had 9 siblings (Figure 1). Her mother (Ⅱ10) had a history of “leg weakness” and slurred speech for 10 years. Her third-oldest uncle (Ⅱ13) on her mother’s side had a history of limb tremor and slurred speech for 8 years, and he was suspected of having “Parkinson’s disease”.
The patient looked tired and haggard. Her vital signs were as follows: blood pressure 104/68 mmHg, apical heart rate 78 per minute and regular, respiratory rate 24 per minute and somewhat labored, temperature 36.6 °C. The patient had clear consciousness, mild dysarthria, and a slightly shallow right nasolabial fold. Her respiratory pattern and effort were normal, with symmetrical respiratory movements and a bilateral speech tremor. Percussion and auscultation showed normal speech conduction and breath sounds in both lungs, and without dry or wet rales or pleural friction sounds. Additionally, her limb muscle strength was low, with grade 4 right limb muscle strength and grade 3–4 Left limb muscle strength using the Oxford Scale (AKA Medical Research Council Manual Muscle Testing scale). Her left-hand grip was weaker than that of the right side, and the right foot was hyperalgesic. Her plantar dorsiflexion was weak, and limb tendon reflexes could not be elicited.
Blood gas analysis results on admission (without inhaled oxygen) were pH: 7.32 (7.35-7.45), PaCO2: 69 (35-45) mmHg, PaO2: 55 (80-100) mmHg, HCO3−: 35.6 (22-27) mmol/L, and BE: 7.8 ± 3 mmol/L). The blood oxygen saturation was 55% without oxygen inhalation and reached 89% with oxygen inhalation during sleep. In addition, the b-type natriuretic peptide was 175 (0-100) pg/mL, creatine kinase 253 (40-200) U/L, lactate dehydrogenase 270 (120-250) U/L, a-hydroxybutyrate dehydrogenase 223 (72-182) U/L, and D-dimer was 2.9 (0-1.00) μg/mL. Erythrocyte sedimentation rate, C-reactive protein, rheumatoid factor, and thyroid function were within normal range.
Cardiac ultrasound reported a normal heart with a normal ejection fraction, and mild pericardial effusion. The electrocardiogram (ECG) results showed a normal sinus rhythm. Lung computed tomography (CT) showed normal mediastinal structures with no evidence of any mediastinal mass. No evidence of pleural lesions or pulmonary artery was demonstrated. A CT scan of her brain did not report abnormal sign.
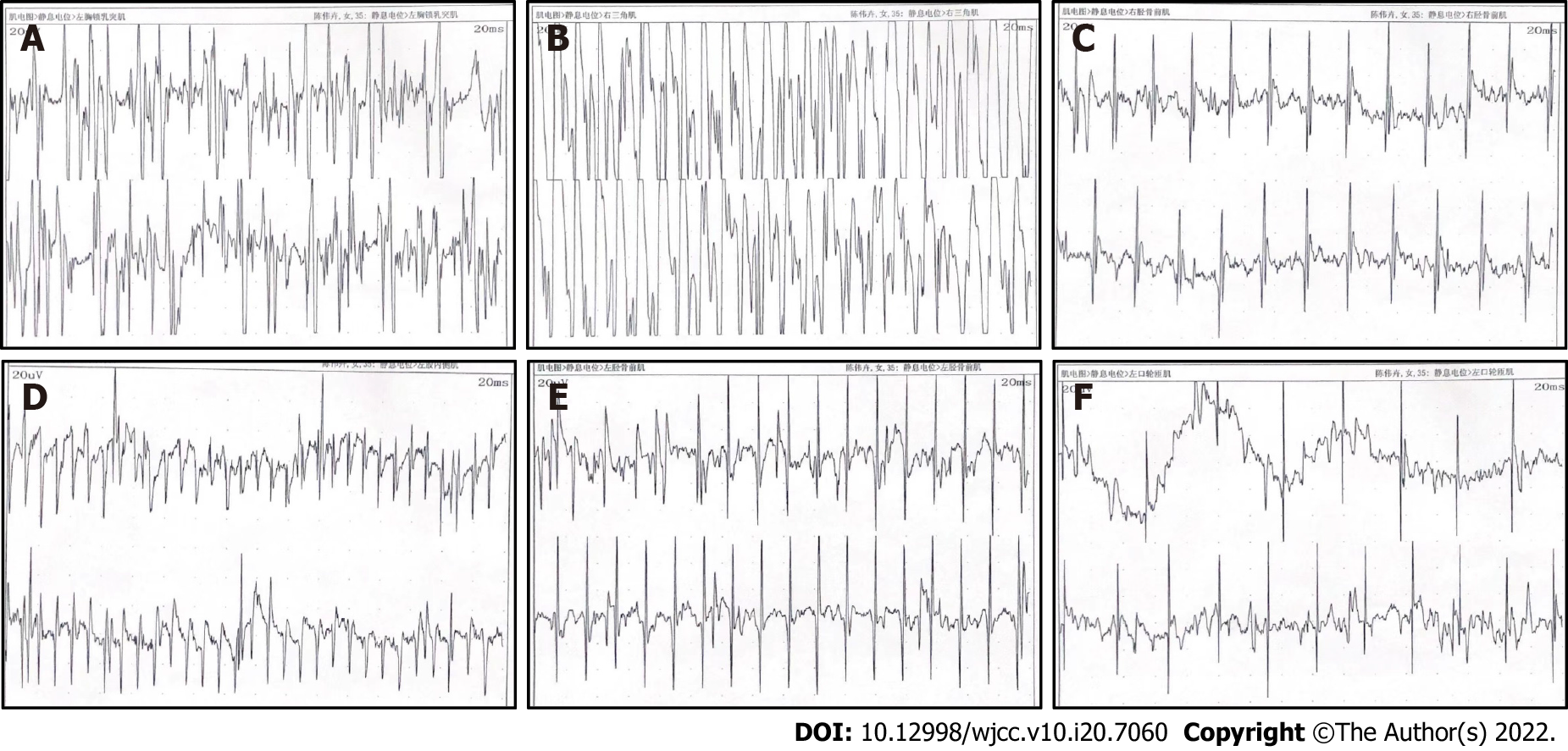
Considering that the patient may have neuromuscular diseases, we performed electromyography (EMG). The results (Figure 2) showed that myotonia potentials were visible in the resting state of the examined extremity muscles and the orbicularis oris muscles, with shortened motor unit potential (MUP) time limit, and the decreased amplitude. The results of motor nerve conduction velocity and sural nerve conduction velocity studies showed that peripheral sensory nerve conduction in both lower extremities was not elicited, and that peripheral motor nerve conduction in both lower extremities was difficult to elicit. There was no abnormality in the peripheral motor and peripheral sensory nerve conduction of the examined nerves in both upper extremities. There was also no abnormality in the F waves in bilateral median nerves and the H reflex of the tibial nerves. The results of repeat attenuation testing showed that the high and low frequency results of bilateral median nerves showed a decreasing trend, and the high frequency results of the right tibial nerve were decreased. There was no abnormality in the low frequency results. The patient's general condition was poor, and some examinations could not be performed. The above results indicated that the patient may have had myotonic myopathy, and further evaluation was recommended.
Considering the almost normal cardiac ultrasound and D-dimer, the cardiologists excluded the cardiac system or pulmonary vascular diseases as the causes that led to the dyspnea. As the patient had bilateral limb weakness with no fluctuation, combined with the family history of muscle weakness and EMG report of myotonic dystrophy, neurologist thought this patient might have an inherited neuromuscular disease and suggested the genetic test of myotonic dystrophy.
Blood samples were collected from this patient and her family for genomic DNA extraction using the CWBIO Blood Genomic DNA Mini Kit (CWBIO, Beijing, China). Capillary electrophoresis was performed by Chigene (Beijing, China) Translational Medical Research Center Co. Ltd (Beijing, China). Capillary electrophoresis for DM revealed that this patient’s cytosine-thymine-guanine (CTG) repeats of the DMPK gene exceeded 100 (Figure 1C). The number of cytosine-CTG repeats of the ZNF9 gene was < 30, which was within the normal range.
The final diagnosis of the presented case was DM1 and type 2 respiratory failure.
The patient underwent supportive and symptomatic treatment, including non-invasive ventilation (NIV), oxygen inhalation, and rehabilitation treatment. On hospital day 3, her respiratory failure resolved, and she was transferred to local hospital by her family.
The genetic test results were notified to the patient and her family with detailed clinical genetic counseling. The DM1 genetic test was recommended for her family. For Ⅱ10 and Ⅱ13 of the pedigree (Figure 1B), capillary electrophoresis for DM revealed that the copy of CTG repeats of the DMPK gene exceeded 100 (Figure 1C). Ⅱ10 and Ⅱ13 were also diagnosed with DM1. Other members of the family did not report muscle weakness or respiratory disorders. As of the submission date, the patient does not need oxygen inhalation during daytime and inhales oxygen at night with 93%-95% blood oxygen saturation. No cataract was found for these three cases of DM1 by ophthalmologist.
Annual ECG, biennial ophthalmology examination, and polysomnography for sleep disorders are suggested for these three patients with DM1. Moreover, agents and circumstances which can cause muscle pain and weakness, such as the anesthetic agent vecuronium and excessive alcohol intake, should be avoided for those with DM1.
The present findings indicated that the DM1 patient may present with dyspnea as the chief complaint. The most common initial symptom of DM1 is myotonia, which usually selectively affects specific muscle groups. However, the case in the present study demonstrated the respiratory system symptoms in DM1 that have been ignored previously. Moreover, an essential finding in the current study is that the EMG and genetic testing can promote diagnosis of DM1. With abnormal EMG results and more than 50 CTG repeats of the DMPK gene, she was diagnosed with DM1. Therefore, we reported this rare case of DM1 with respiratory symptoms as the chief complaint and analyzed the genetic features of 3 generations of her pedigree.
In 1992, Fu et al[17] reported for the first time that unstable and abnormally amplified CTG trinucleotide repeats were found in the DMPK genes of DM1 patients. This repetitive CTG sequence is located in the 3'-noncoding region of the DMPK gene on chromosome 19. Normal individuals have 5-37 repeated alleles that can spread stably, and 38-50 repeated alleles are called pre-mutation alleles; 51-100 repetitions are primitive mutations, all showing genetic instability[18]. In DM1 patients, the number of CTG copies of this repeated sequence ranges from 51 to 3000. In the current study, as the pedigree of the patient's family with DM1 indicated, DNA analysis suggested that amplified CTG trinucleotide repeated over 100 times present in the 3′ untranslated region of the DMPK gene of the proband (Ⅱ8), her mother (Ⅱ10), and her uncle (Ⅱ13). Thus, in this case, DM1 was preliminarily diagnosed based on the characteristics of genetic test results.
CTG repeat number increases with each generation, and the overall severity of the disease also worsens with each generation. Adult-onset patients usually carry 100-1000 CTG allele repeats, and the number of CTG repeats in patients with DM1 onset immediately after birth can be greater than 1000[18]. However, the research conclusions regarding the relationship between the number of CTG repeats and a single clinical manifestation are inconsistent. Arsenault et al[19] conducted a genotype–phenotype correlation study of 102 DM1 patients, and the results showed that muscle weakness, muscle rigidity, and decreased activity endurance in DM1 patients were positively correlated with the number of CTG repeats. A retrospective study by Monteiro et al[20] showed a relationship between the number of CTG repeats and hypoxemia and MEP decline. Rossi et al[3] confirmed that the number of CTG allele repeats was an independent predictor of impaired respiratory function in patients with DM1. The current study found that the proband has CTG trinucleotide repeated over 100 times in the DMPK gene and presented respiratory dysfunction. Our findings are consistent with that of Rossi et al[3]. On the contrary, Panaite et al[21] showed no significant correlation between the severity of the respiratory failure and the number of CTG repeats in a mice animal model.
The clinical symptoms of DM1 range from fatal effects in infants to mild and delayed onset symptoms in older individuals. The most common initial symptom of DM1 is myotonia, which usually selectively affects specific muscle groups, such as the forearm, hands, tongue, and jaw. Interestingly, the patient in this study presented with dyspnea, sleep apnea, and cyanosis when she lied down. According to previous authors, physical examination mainly manifests as motor and percussive muscle rigidity[1,3]. The muscle atrophy of DM1 usually presents as a characteristic distribution, first involving the head, trunk, and distal muscles of the extremities. All craniofacial muscles may be affected, with ptosis, atrophy of temporal and masseter muscles, and hair loss, resulting in a characteristic appearance of facial narrowing. Diaphragmatic weakness may also appear early. Among the limb muscles, the long finger flexors and dorsal flexors are the first to be affected, resulting in inflexible hand and foot movements. Symptoms of muscle weakness progress slowly, and most patients are not aware of weakness early in the disease. The present case had a slight decrease in muscle strength for 4 years which did not attract the patient's attention previously. Thus, pulmonologists should consider DM1 during the physical examination if they find a patient with any of the aforementioned symptoms, thus improving the probability of accurate diagnosis.
Respiratory system symptoms are common in DM1, and respiratory failure is one of the main causes of death[1,7]. One study reported that adult patients with respiratory symptoms accounted for approximately 31% of the total number of patients[22]. Among congenital patients, only 50% live to their 30s, and approximately 66% of the deaths in surviving patients are caused by respiratory disorders[23]. Respiratory muscle weakness and changes in the respiratory center cause alveolar hypoventilation, ineffective coughing, micro-atelectasis, and reduced alveolar compliance, leading to restricted lung ventilation, hypercapnia, and long-term pulmonary hypertension, pulmonary heart disease, and respiratory failure. Studies have shown that respiratory failure is the main cause of death, which can be caused by infection (approximately 90% of patients) or occurs during recovery from general anesthesia, rather than from carbon dioxide anesthesia[22]. Our patient is characterized by dyspnea as the main clinical symptom, especially the obvious aggravation of dyspnea in the lying position. Possible causes of dyspnea symptoms include respiratory disease, cardiogenic, blood system disease, poisoning, and nervous system disease. Through the improvement of related examinations, we ruled out the respiratory system, cardiovascular system, blood system, and causes of poisoning, and finally targeted the neuromuscular system. Considering the patient's young age, long medical history, combined with family history, we highly suspect that she has a genetic related disease, so the genetic testing was performed and provided essential evidence for the final diagnosis.
EMG in DM1 patients show myogenic damage and tonic potentials, slow motor unit activation, and an increase in polyphasic waves. In the present case, EMG results showed myotonia potentials, shortened MUP time, and decreased amplitude in the extremity muscles and the orbicularis oris muscles. Currently, the gold standard for the clinical diagnosis of DM1 is genetic testing. DM1 can be diagnosed if the number of CTG repeat amplifications in the DMPK gene exceeds 50. In this case, we combined the patient's clinical symptoms, family history, EMG results, and genetic testing results to make a definitive diagnosis for the patient.
Management guidelines have been developed for DM1 patients[7]. To date, no specific treatment fundamentally changes the progressive weakness course of the disease. Patients with DM1 may benefit from moderate-intensity aerobic exercise and may need assistance comprising orthotics and auxiliary equipment in the later stage of the disease. The use of various anticonvulsants or antiarrhythmic drugs can improve muscle rigidity. DM1 patients had a 50% reduction in grip stiffness after 7 wk of treatment with mexiletine[24]. When the patient has symptoms of sleep disturbance or excessive daytime sleepiness and vital capacity < 1.5 L, monitoring nighttime blood oxygen saturation during sleep is necessary. When there are symptoms and signs of hypercapnia during the day or hypoventilation at night, long-term NIV must be considered. Patients with DM1 should undergo an ECG examination once a year. If the PR interval or QRS complex is significantly prolonged, or any form of conduction block is present, or the patient has cardiac symptoms, further cardiac evaluation should be performed. Excessive daytime sleepiness in DM1 patients can be treated with stimulant drugs, such as methylphenidate and modafinil. In our study, the patient had type 2 respiratory failure, received NIV, oxygen inhalation, and rehabilitation treatment, which achieved satisfying clinical outcomes. We attribute this positive outcome to the appropriate treatment options.
Some therapeutic options targeting the genetic cause of DM1 have been developed in animals. One study showed that Cas9-targeting RNA was a feasible strategy for DM1 treatment, using a mouse model[2]. Injecting antisense oligonucleotides resulted in rapid knockdown of CUG (exp) RNA in animal skeletal muscle, which corrected the physiological, histopathological, and transcriptomic characteristics of DM1[25]. However, there is still a long way to go from animal experiments to human experiments to final clinical applications. Also, some studies reveal the efficacy of metformin delaying and/or limiting DM1, not only in diabetes, but also in additional characteristics of its pathobiology[26]. Besides, in our opinion, regular follow-up to assess lung function, oxygen saturation, ECG, and cardiac function is also critical.
Although the patient in this study achieved timely diagnosis and satisfactory clinical outcomes, there are still some limitations. Firstly, the duration of follow-up for this case is relatively short. Thus, long-term follow-up is needed. Secondly, our data are still based on small sample size. Thus, more samples, multiple centers, and randomized controlled clinical trials should be carried out in future studies.
DM1 is a genetic neuromuscular disease involving multiple systems, and the clinical phenotype of DM1 patients is highly variable. Some patients with DM1 may be presented at the respiratory department because of dyspnea, which should be cautioned by the pulmonologists. There may be no obvious or specific symptoms in the early stage of disease, and clinicians should improve their understanding of DM1 and make an early diagnosis, which will improve patients’ quality of life.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdelkreem E, Egypt; Cristaldi PMF, Italy; Rodrigues AT, Brazil A-Editor: Liu X, China S-Editor: Zhang H L-Editor: Filipodia CL P-Editor: Zhang H
| 1. | Mazzoli M, Ariatti A, Garuti G, Agnoletto V, Fantini R, Marchioni A, Galassi G. Predictors of respiratory decline in myotonic dystrophy type 1 (DM1): a longitudinal cohort study. Acta Neurol Belg. 2021;121:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Batra R, Nelles DA, Roth DM, Krach F, Nutter CA, Tadokoro T, Thomas JD, Sznajder ŁJ, Blue SM, Gutierrez HL, Liu P, Aigner S, Platoshyn O, Miyanohara A, Marsala M, Swanson MS, Yeo GW. The sustained expression of Cas9 targeting toxic RNAs reverses disease phenotypes in mouse models of myotonic dystrophy type 1. Nat Biomed Eng. 2021;5:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Rossi S, Della Marca G, Ricci M, Perna A, Nicoletti TF, Brunetti V, Meleo E, Calvello M, Petrucci A, Antonini G, Bucci E, Licchelli L, Sancricca C, Massa R, Rastelli E, Botta A, Di Muzio A, Romano S, Garibaldi M, Silvestri G. Prevalence and predictor factors of respiratory impairment in a large cohort of patients with Myotonic Dystrophy type 1 (DM1): A retrospective, cross sectional study. J Neurol Sci. 2019;399:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Theadom A, Rodrigues M, Roxburgh R, Balalla S, Higgins C, Bhattacharjee R, Jones K, Krishnamurthi R, Feigin V. Prevalence of muscular dystrophies: a systematic literature review. Neuroepidemiology. 2014;43:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 5. | Coleman SM, Prescott AR, Sleeman JE. Transcriptionally correlated subcellular dynamics of MBNL1 during lens development and their implication for the molecular pathology of myotonic dystrophy type 1. Biochem J. 2014;458:267-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Ahmad S, Kabunga P. Paradoxical cardiac conduction during exercise stress testing in myotonic dystrophy type 1: a case report. Eur Heart J Case Rep. 2021;5:ytab409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Nguyen CE, Campbell C. Myotonic dystrophy type 1. CMAJ. 2016;188:1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hilbert JE, Barohn RJ, Clemens PR, Luebbe EA, Martens WB, McDermott MP, Parkhill AL, Tawil R, Thornton CA, Moxley RT 3rd; National Registry Scientific Advisory Committee/Investigators. High frequency of gastrointestinal manifestations in myotonic dystrophy type 1 and type 2. Neurology. 2017;89:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Miller JN, Kruger A, Moser DJ, Gutmann L, van der Plas E, Koscik TR, Cumming SA, Monckton DG, Nopoulos PC. Cognitive Deficits, Apathy, and Hypersomnolence Represent the Core Brain Symptoms of Adult-Onset Myotonic Dystrophy Type 1. Front Neurol. 2021;12:700796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Araújo TL, Resqueti VR, Bruno S, Azevedo IG, Dourado ME Jr, Fregonezi G. Respiratory muscle strength and quality of life in myotonic dystrophy patients. Rev Port Pneumol. 2010;16:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Mathieu J, Allard P, Potvin L, Prévost C, Bégin P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology. 1999;52:1658-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 264] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Dauvilliers YA, Laberge L. Myotonic dystrophy type 1, daytime sleepiness and REM sleep dysregulation. Sleep Med Rev. 2012;16:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Yu H, Laberge L, Jaussent I, Bayard S, Scholtz S, Raoul M, Pages M, Dauvilliers Y. Daytime sleepiness and REM sleep characteristics in myotonic dystrophy: a case-control study. Sleep. 2011;34:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Spiesshoefer J, Runte M, Heidbreder A, Dreher M, Young P, Brix T, Boentert M. Sleep-disordered breathing and effects of non-invasive ventilation on objective sleep and nocturnal respiration in patients with myotonic dystrophy type I. Neuromuscul Disord. 2019;29:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Guedes H, Moreno N, Dos Santos RP, Marques L, Seabra D, Pereira A, Andrade A, Pinto P. Importance of three-dimensional speckle tracking in the assessment of left atrial and ventricular dysfunction in patients with myotonic dystrophy type 1. Rev Port Cardiol (Engl Ed). 2018;37:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Park JS, Kim N, Park D. Diastolic heart dysfunction is correlated with CTG repeat length in myotonic dystrophy type 1. Neurol Sci. 2018;39:1935-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Fu YH, Pizzuti A, Fenwick RG Jr, King J, Rajnarayan S, Dunne PW, Dubel J, Nasser GA, Ashizawa T, de Jong P. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1035] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 18. | Udd B, Krahe R. The myotonic dystrophies: molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012;11:891-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 335] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 19. | Arsenault ME, Prévost C, Lescault A, Laberge C, Puymirat J, Mathieu J. Clinical characteristics of myotonic dystrophy type 1 patients with small CTG expansions. Neurology. 2006;66:1248-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Monteiro R, Bento J, Gonçalves MR, Pinto T, Winck JC. Genetics correlates with lung function and nocturnal ventilation in myotonic dystrophy. Sleep Breath. 2013;17:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Panaite PA, Kuntzer T, Gourdon G, Lobrinus JA, Barakat-Walter I. Functional and histopathological identification of the respiratory failure in a DMSXL transgenic mouse model of myotonic dystrophy. Dis Model Mech. 2013;6:622-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Tzeng AC, Bach JR. Prevention of pulmonary morbidity for patients with neuromuscular disease. Chest. 2000;118:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Reardon W, Newcombe R, Fenton I, Sibert J, Harper PS. The natural history of congenital myotonic dystrophy: mortality and long term clinical aspects. Arch Dis Child. 1993;68:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Logigian EL, Martens WB, Moxley RT 4th, McDermott MP, Dilek N, Wiegner AW, Pearson AT, Barbieri CA, Annis CL, Thornton CA, Moxley RT 3rd. Mexiletine is an effective antimyotonia treatment in myotonic dystrophy type 1. Neurology. 2010;74:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Wheeler TM, Leger AJ, Pandey SK, MacLeod AR, Nakamori M, Cheng SH, Wentworth BM, Bennett CF, Thornton CA. Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature. 2012;488:111-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 389] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 26. | García-Puga M, Saenz-Antoñanzas A, Matheu A, López de Munain A. Targeting Myotonic Dystrophy Type 1 with Metformin. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |