Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.7045
Peer-review started: November 30, 2021
First decision: January 12, 2022
Revised: January 24, 2022
Accepted: May 16, 2022
Article in press: May 16, 2022
Published online: July 16, 2022
Processing time: 216 Days and 12.2 Hours
Atherosclerosis is one of the main causes of coronary artery ostial lesions seen clinically. Secondary coronary artery ostial lesions are rare, and cases reported previously were associated with syphilitic vasculitis and aortic dissection. Here, we report three rare cases of secondary coronary ostial lesions. Due to their rareness, these lesions can easily be neglected, which may lead to misdiagnosis and missed diagnosis.
We present three patients with acute myocardial infarction and unstable angina caused by secondary coronary artery ostial lesions. In Case 1, coronary angiogra
The cases reported here suggest that we should consider other causes of coronary ostial lesions other than atherosclerosis.
Core Tip: We present three cases of secondary coronary artery ostial lesions. These lesions are rare and therefore easily neglected. A lack of awareness of secondary causes of coronary artery ostial lesions may lead to misdiagnosis and missed diagnosis. This case series helps clinicians by bringing attention to the possibility of secondary causes of coronary ostial lesions in clinic.
- Citation: Liu XP, Wang HJ, Gao JL, Ma GL, Xu XY, Ji LN, He RX, Qi BYE, Wang LC, Li CQ, Zhang YJ, Feng YB. Secondary coronary artery ostial lesions: Three case reports. World J Clin Cases 2022; 10(20): 7045-7053
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/7045.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.7045
Atherosclerosis is one of the principal causes of coronary artery ostial lesions seen clinically. Rarely, coronary ostial stenosis or occlusion can be secondary to other pathological conditions. Because these secondary conditions are rare, this phenomenon can easily be neglected, which may lead to misdiagnosis and missed diagnosis. We report three unique and rare cases of coronary artery ostial lesions caused by thymic carcinoma, sinus of Valsalva aneurysm (SVA), or anomalous origin of the right coronary artery (AORCA). The aim of our case series is to increase awareness of the possibility of coronary ostial lesions caused by conditions other than atherosclerosis. We summarize and review the clinical characteristics of secondary coronary artery ostial lesions to improve the clinical guidance in the diagnosis and treatment of the condition.
Case 1: A 66-year-old man patient with a 1-year history of palpitation episodes and shortness of breath, was admitted to our hospital because of the recurrence of these symptoms 4 h before admittance.
Case 2: A 67-year-old man patient was admitted to the hospital with complaints of intermittent chest pain for 3 d, with worsening symptoms during the last 10 h.
Case 3: A 68-year-old woman was admitted to the hospital with a history of chest pain on exertion for 3 years with aggravation of symptoms 1 wk prior to admission.
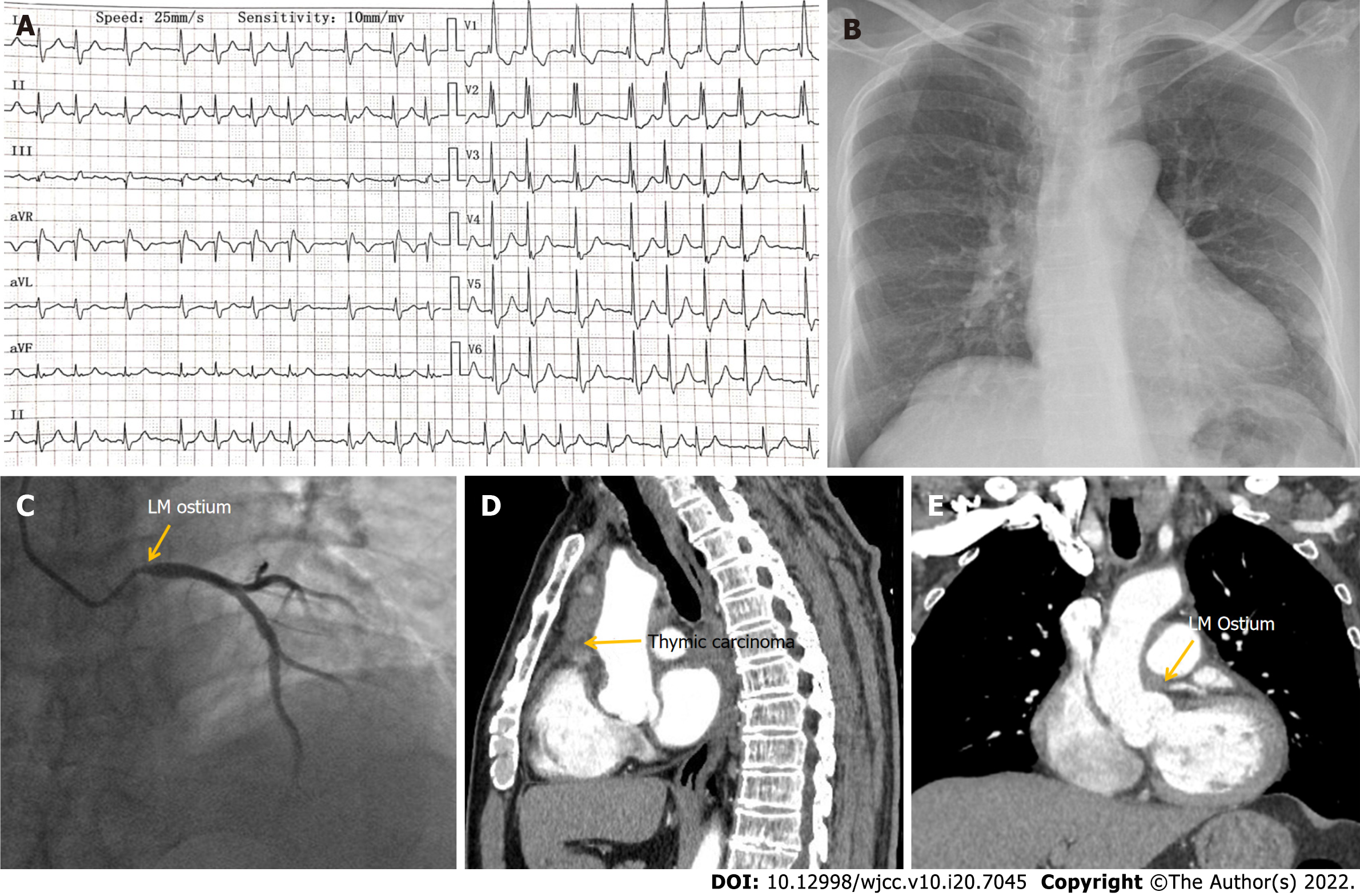
Case 1: The patient was admitted on April 15, 2020 with non-ST segment elevation myocardial infarction (NSTEMI). Initial electrocardiography (ECG) showed atrial fibrillation, complete right bundle branch block, and depressed ST segment of 0.1–0.3 mV in leads V1–V6 (Figure 1A). The values of myocardial enzymes were significantly increased and chest X-ray was normal (Figure 1B). Coronary angiography (CAG) revealed a 90% stenosis in the left main coronary ostium but no stenosis in other vessels (Figure 1C). Because the stenosis was detected only in the left main coronary ostium, whereas no stenosis or atherosclerosis was observed in other vessels, we presumed it might be secondary to other diseases. Therefore, implantation of a stent was not performed. Subsequent contrast chest CT suggested thymic carcinoma involving the middle mediastinum, which also invaded the left main coronary ostium (Figure 1D, E). The patient underwent resection of the tumor combined with coronary artery bypass grafting (CABG) on April 30, 2020.
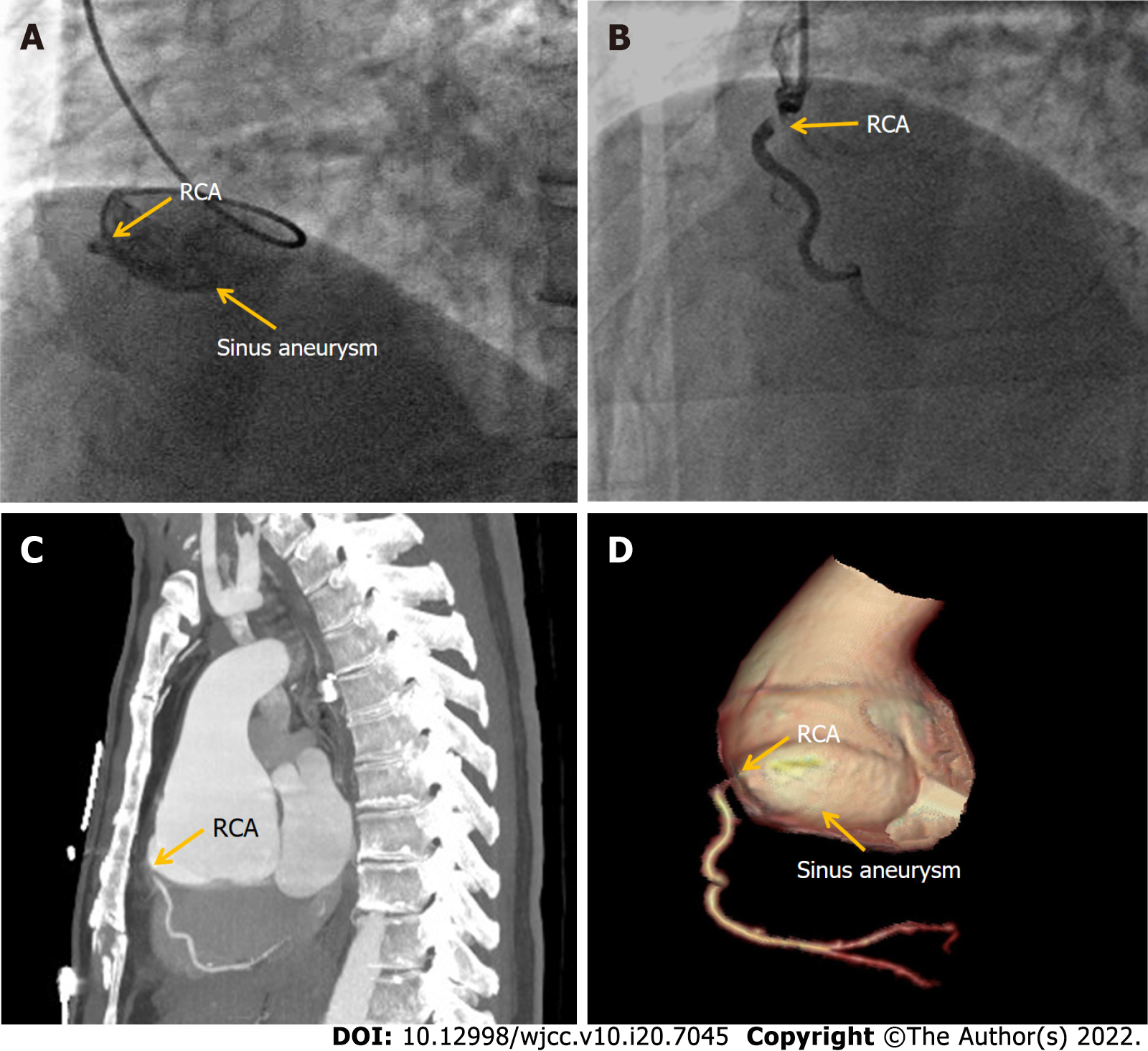
Case 2: The patient was admitted on September 26, 2018 with acute inferior myocardial infarction. Initial ECG showed an arched upward ST segment of 0.3–0.5 mV in leads II and III, and AVF. Laboratory tests showed significantly elevated cardiac enzymes and no antibodies for syphilis. Echocardiography revealed SVA. CAG showed a giant aneurysm in the right coronary sinus and complete obstruction of the RCA ostium (Figure 2A). Implantation of a coronary artery stent was attempted with several different guide catheters but the guide wire could not reach the distal end of the obstruction. The operation was stopped until distal thrombolysis in myocardial infarction grade 2 flow was obtained (Figure 2B). The chest pain disappeared after the operation and ECG showed that the ST segment dropped by 50% in leads II and III, and AVF. Subsequent aortic contrast CT confirmed an aneurysm arising from the right coronary sinus causing severe stenosis of the RCA ostium (Figure 2C, D). The Bentall procedure was carried out on November 15, 2018.
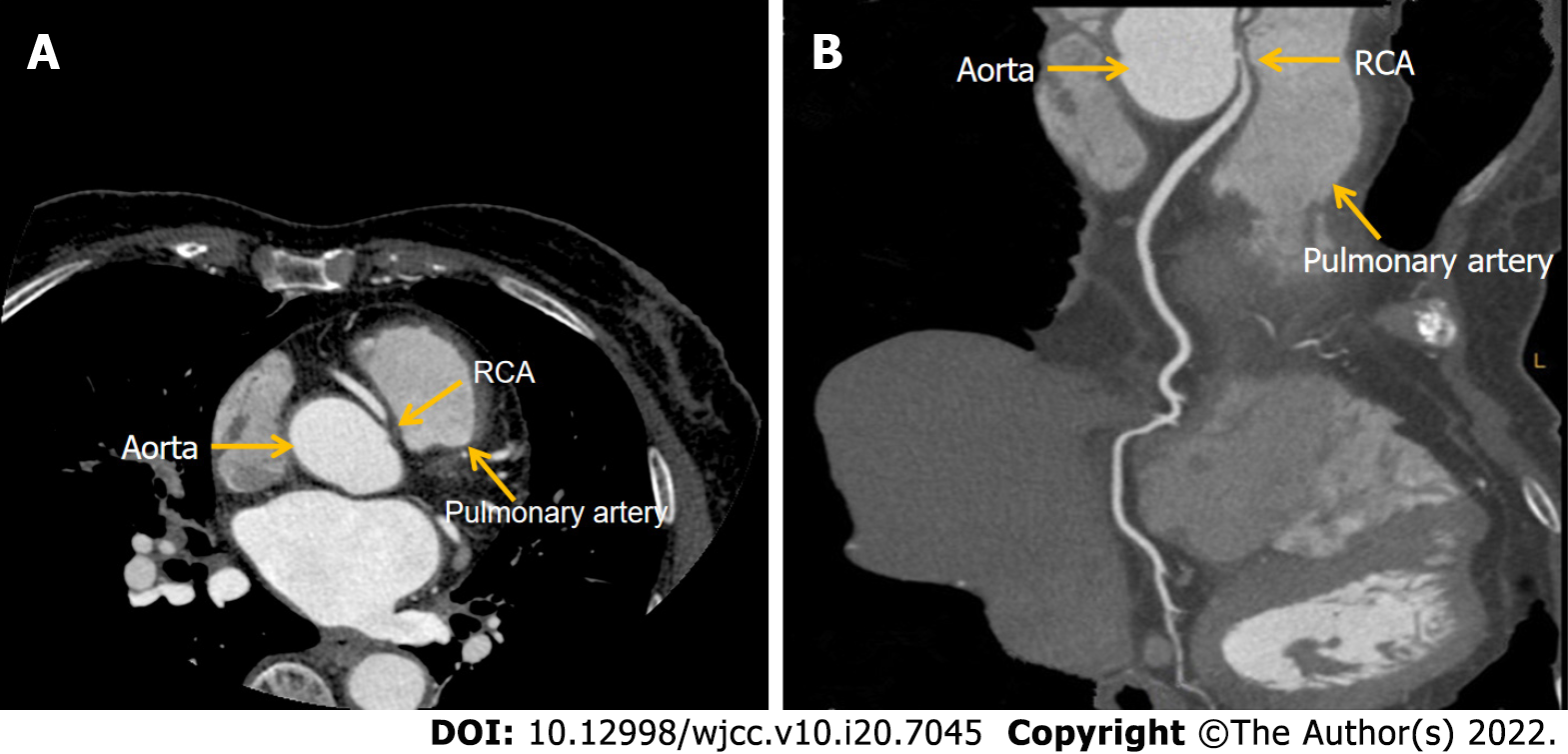
Case 3: The patient presented to our hospital due to unstable angina on November 24, 2020. The primary examination showed no abnormalities in cardiac enzymes, ECG, and echocardiography. Symptoms were significantly relieved after administration of aspirin and metoprolol tartrate. Coronary CT angiogram identified an AORCA originating from the left sinus of Valsalva and coursing between the aorta and pulmonary trunk. The ostium of the RCA was severely narrowed due to compression (Figure 3A, B). Surgical correction of the anomalous origin of the RCA was performed on March 10, 2021.
Case 1: The patient had a history of hypertension for > 10 years, with the highest blood pressure (BP) of 180/90 mmHg. BP could be kept around 140/90 mmHg with oral nifedipine tablets 30 mg once daily.
Case 2: The patient had a history of hypertension for > 10 years, with the highest BP of 180/100 mmHg. BP could be kept around 130/80 mmHg by long-term oral irbesartan hydrochlorothiazide tablets 150 mg once daily and amlodipine besylate tablets 5 mg once daily.
Case 3: The patient had no history of past illness.
For Cases 1, 2 and 3, no further details were available aside from those mentioned above in the history of past illness section.
Case 1: The patient’s heart rate (HR) upon admission was 99 bpm, BP was 130/75 mmHg, respiratory rate was 20 breaths/min, and O2 saturation of 96% was measured by pulse oximeter. There were no obvious abnormalities in the cardiorespiratory examination.
Case 2: The patient’s HR upon admission was 92 bpm, BP was 130/60 mmHg, respiratory rate was 22 breaths/min, and O2 saturation of 95% was measured by pulse oximeter.
Case 3: The patient’s HR upon admission was 85 bpm, BP 120/80 mmHg, respiratory rate 20 breaths/min, and O2 saturation of 95% was measured by pulse oximeter. There were no obvious abnormalities found in the cardiorespiratory examination.
Case 1: Laboratory examinations showed myoglobin (MYO) 664.6 ng/mL (normal < 72 ng/mL), cardiac troponin T (cTnT) 0.296 μg/L (normal < 0.014 μg/L), creatine kinase isoenzyme (CK-MB) 36.41 ng/mL (normal < 4.87 ng/mL) and pro-brain natriuretic peptide (NT-proBNP) 285.3 pg/mL (normal < 125 pg/mL).
Case 2: Laboratory examination showed MYO 1135 ng/mL (normal < 72 ng/mL), cTnT 2.1 μg/L (normal < 0.014 μg/L), CK-MB 106.1 ng/mL (normal < 4.87 ng/mL), D-dimer 0.78 μg/mL (normal < 0.5 μg/mL) and NT-proBNP 5072 pg/mL (normal < 125 pg/mL).
Case 3: Laboratory examination showed MYO 25 ng/mL (normal < 72 ng/mL), cTnT 0.001 μg/L (normal < 0.014 μg/L), CK-MB 2.3 ng/mL (normal < 4.87 ng/mL), D-dimer 0.32 μg/mL (Normal < 0.5 ug/mL) and NT-proBNP 125 pg/mL (normal < 125 pg/mL).
Case 1: Echocardiography was normal. CAG revealed 90% stenosis in the left main coronary ostium but no stenosis in other vessels. Chest CT suggested thymic carcinoma involving the middle mediastinum, which also invaded the left main coronary ostium.
Case 2: Echocardiography revealed an SVA-like dilatation with a diameter of 64 mm, the left ventricular end-diastolic dimension was 55 mm, the inferior wall was weakened, and the left ventricular ejection fractions was 47%. CAG showed a giant aneurysm in the right coronary sinus, complete obstruction in the RCA ostium and no stenosis in other vessels. Aortic contrast CT confirmed an aneurysm arising from the right coronary sinus, which caused severe stenosis of the RCA ostium.
Case 3: Coronary CT angiogram identified an anomalous RCA originating from the left sinus of Valsalva and coursing between the aorta and pulmonary trunk. The ostium of the RCA was severely narrowed due to compression and no distinct narrowing of other vessels was observed.
The patient was diagnosed with NSTEMI with thymic carcinoma.
The patient was diagnosed with acute inferior STEMI with right coronary Valsalva aneurysm.
The patient was diagnosed with unstable angina and AORCA.
The patient underwent resection of the tumor combined with CABG.
The patient underwent the Bentall procedure.
The patient underwent surgery for correction of AORCA.
The patient had recovered well at 1-year follow-up.
The 3-year follow-up after surgery continued to be satisfactory.
The 6-month follow-up after surgery demonstrated the patient recovered well.
Coronary artery ostial lesions are mainly caused by atherosclerosis and are commonly seen clinically, while secondary coronary artery ostial lesions are rare. Secondary coronary artery ostial lesions reported previously have often involved syphilitic vasculitis, aortic dissection, and other causes[1-5]. Here, we reported three rare cases of secondary coronary ostial lesions.
Case 1 was a patient whose left main ostium was involved by thymic carcinoma. Previous studies have demonstrated that thymic carcinoma is an anterior mediastinal malignancy originating from epithelial cells of the thymus with a low incidence but high invasiveness and poor prognosis[6]. The incidence of thymic carcinoma is estimated to be approximately 0.3-0.6 per million per year. It accounts for < 20% of all thymic neoplasms[7] but its 5-year survival rate is only 36%[8]. In the early stages of thymic carcinoma, patients may experience no specific symptoms until the tumor grows large enough to compress or invade the nearby surrounding tissues and organs, leading to chest tightness, chest pain, and myasthenia graves in a few patients[9]. Because there are no obvious clinical symptoms in the early stages of the disease, invasion of local tissues or metastasis to the pleura, pericardium, and even distant organs is commonly found in patients when they are diagnosed[10]. Currently, thymic carcinoma has a 5-year overall survival rate of approximately 70% when completely resected in its early-stages[11]. If it coexists with significant triple coronary vessel disease, then complete excision of the tumor, resection of locally involved structures, and subsequent chemoradiotherapy, is associated with the most favorable outcome[12]. Previously, Juan and colleagues presented two cases of a mediastinal tumor encasing the RCA, one with recurrent metastatic thymoma and another with primary poorly differentiated neoplasm[13]. However, to the best of our knowledge, a single invasion of the left main coronary ostium by thymic carcinoma has not been reported until now. Here, we report a unique case of thymic carcinoma involving the middle mediastinum, which also invaded the left main coronary ostium resulting in NSTEMI. The patient underwent tumor resection and CABG and recovered well after surgery.
Case 2 was a patient with an SVA involving the right coronary ostium and causing complete occlusion. SVA is caused by the absence or weakening of the muscular and elastic lamina at the junction of the aortic media and the annulus fibrosus. An isolated aneurysm of just one of the three sinuses of Valsalva is a rare condition reported in the literature with an incidence of 0.09%-1.5%[14]. The aneurysm originates predominantly from the right coronary sinus, approximately 70%, whereas approximately 29% originate from the noncoronary sinus; those originating from the left sinus are extremely rare[15]. SVAs may be congenital or acquired: In congenital SVAs, the condition is frequently associated with Marfans syndrome or other connective tissue disorders; whereas acquired forms of SVA are associated with infections (syphilis, bacterial endocarditis, and tuberculosis), atherosclerosis, hypertension, medial cystic necrosis, drug of alcohol abuse, and traumatic and degenerative diseases[16]. Upon expansion, SVAs can cause obstruction of the right ventricular outflow tract, aortic regurgitation, conduction disorders, and more rarely, myocardial ischemia or infarction due to compression of the coronary arteries[17]. Because aneurysmal rupture or involvement of the coronary arteries can have poor prognosis and possibly lethal consequences for patients, early detection and acceptance of timely surgery are the main treatments for SVA at present. Coronary artery stenosis or occlusion due to SVA is rare, while SVA leading to RCA occlusion is extremely rare[18]. In our case the giant right SVA combined with proximal RCA obstruction resulted in an AMI. The patient recovered well after Bentall procedure was performed.
Case 3 was a patient with abnormal origin of the RCA from the left coronary artery sinus. The anomalous aortic origin of a coronary artery occurs rarely and has a reported incidence of 0.64% of live births, with the prevalence of an RCA arising from the left sinus of Valsalva being estimated at 0.17%[19]. Congenital coronary artery anomalies are the third cause of sudden cardiac death, with approximately 20% of AORCA[20]. When the course of the RCA runs between the aorta and pulmonary trunk, the mortality rate is as high as 25%-40%[21] and is described as a “malignant course”. AORCA with a malignant course can be associated with angina, exercise-induced syncope, cardiac arrhythmia, myocardial ischemia, or cardiac arrest[22]. Current research shows that AORCA has a large angle, and the proximal end of the coronary artery is tangent or acute to the aortic wall, so that most of the ostia are in the shape of cracks. The proximal segment of the abnormal RCA is embedded in the aortic wall, which is in the same layer as the aorta without its own adventitia. When the arterial pressure, especially the diastolic pressure, increases during strenuous exercise, the ascending aorta expands and lengthens outward, which can cause partial flattening and obstruction of the RCA in the aortic wall. Additionally, the dilated aorta can form a valve in the fissure-like opening of the RCA and block blood flow, leading to angina, myocardial infarction, malignant arrhythmia, and even sudden death. Sometimes, even a small amount of physical exertion or stress may impact the conformation of these anatomical anomalies and cause adverse cardiovascular events[23]. This kind of anomaly can be detected early by coronary CT angiogram or CAG in clinical practice. Once diagnosed as AORCA, emergency corrective surgery should be performed to prevent myocardial ischemia, arrhythmia, or sudden cardiac death[24]. Here, we report the case of a patient with chest pains on exertion who was diagnosed with AORCA with a malignant course. Subsequently, correction of the coronary artery ostia was performed. The 6-month follow-up after the surgery demonstrated that the patient recovered well.
The cases we reported here suggest that we should consider the possibility of coronary ostial lesions caused by conditions other than atherosclerosis when the following manifestations occur in clinical practice. First, lesions occur only at the coronary ostium and no stenosis or atherosclerosis is observed in other coronary vessels; second, echocardiography or chest CT shows sinus of Valsalva aneurysm-like dilatation or mediastinum-occupying coronary artery ostial lesions; third, when coronary CT angiogram or CAG shows an anomalous origin of a coronary artery; and last, if clinical manifestations cannot be explained by coronary ostial lesions alone. In summary, as coronary ostial lesions caused by conditions other than atherosclerosis are relatively rare, they are easily neglected, which may lead to misdiagnosis and missed diagnosis. Therefore, more attention should be paid to these kinds of secondary coronary ostial lesions in clinical practice.
The cases we reported here suggest that we must consider the possibility of coronary ostial lesions caused by reasons other than atherosclerosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Astuti A, Indonesia; Xiang T, China S-Editor: Guo XR L-Editor: Kerr C P-Editor: Qi WW
| 1. | Nomura R, Yamazaki F, Egawa Y. Syphilitic aortitis: chronic left coronary ostial occlusion and aortic regurgitation with aortitis. Gen Thorac Cardiovasc Surg. 2021;69:736-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Jadeed R, Paarmann R, Harringer W, El-Essawi A. Syphilitic Aortitis Presenting with Coronary Ostial Stenosis and Aortic Regurgitation. J Heart Valve Dis. 2016;25:18-20. [PubMed] |
| 3. | Nakai M, Yamasaki F, Mitsuoka H, Miura Y, Terai Y, Goto S, Mizuno Y, Miyano Y, Tanaka H, Kawaguchi Y, Kawaguchi S. [Myocardial Ischemia in Acute Type A Aortic Dissection; Coronary Artery Dissection and Functional Ischemia]. Kyobu Geka. 2016;69:292-297. [PubMed] |
| 4. | Wang Y, Zhu Z, Xu R, Li D, Wang T, Liu K. A Complete Occlusion of Right Coronary Artery Due to Stanford Type A Aortic Dissection - Successful Treatment with Extracorporeal Membrane Oxygenation (ECMO). Braz J Cardiovasc Surg. 2019;34:491-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Mainwaring RD, Collins RT 2nd, Patrick WL, Martin E, MacMillen KL, Hanley FL. Surgical repair of coronary artery ostial stenosis in patients with Williams and elastin arteriopathy syndromes. J Thorac Cardiovasc Surg. 2021;162:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Yang X, Zhao K, Li C, Yang Y, Guo C, Pu Y, Liu L. Thymic Squamous Cell Carcinoma: A Population-Based Surveillance, Epidemiology, and End Result Analysis. Front Oncol. 2020;10:592023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | de Jong WK, Blaauwgeers JL, Schaapveld M, Timens W, Klinkenberg TJ, Groen HJ. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer. 2008;44:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Scorsetti M, Leo F, Trama A, D'Angelillo R, Serpico D, Macerelli M, Zucali P, Gatta G, Garassino MC. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol. 2016;99:332-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 9. | Robinson SP, Akhondi H. Thymoma. 2022 Jan 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 10. | Yang Y, Fan XW, Wang HB, Xu Y, Li DD, Wu KL. Stage IVb thymic carcinoma: patients with lymph node metastases have better prognoses than those with hematogenous metastases. BMC Cancer. 2017;17:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Ahmad U, Yao X, Detterbeck F, Huang J, Antonicelli A, Filosso PL, Ruffini E, Travis W, Jones DR, Zhan Y, Lucchi M, Rimner A. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg. 2015;149:95-100, 101.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Ried M, Neu R, Schalke B, von Süßkind-Schwendi M, Sziklavari Z, Hofmann HS. Radical surgical resection of advanced thymoma and thymic carcinoma infiltrating the heart or great vessels with cardiopulmonary bypass support. J Cardiothorac Surg. 2015;10:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Juan YH, Chatzizisis YS, Saboo SS, Rocha T, Steigner ML. Tumor encasement of the right coronary artery: role of anatomic and functional imaging in diagnosis and therapeutic management. Open Cardiovasc Med J. 2014;8:110-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Bricker AO, Avutu B, Mohammed TL, Williamson EE, Syed IS, Julsrud PR, Schoenhagen P, Kirsch J. Valsalva sinus aneurysms: findings at CT and MR imaging. Radiographics. 2010;30:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Fang ZF, Huang YY, Tang L, Hu XQ, Shen XQ, Tang JJ, Zhou SH. Long-term outcomes of transcatheter closure of ruptured sinus valsalva aneurysms using patent ductus arteriosus occluders. Circ J. 2014;78:2197-2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Weinreich M, Yu PJ, Trost B. Sinus of valsalva aneurysms: review of the literature and an update on management. Clin Cardiol. 2015;38:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 17. | Wei K, Guo H, Fang F, Qian XY. Giant right sinus of Valsalva aneurysm led to proximal right coronary artery occlusion. Anatol J Cardiol. 2020;23:350-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Sumiyoshi A, Fujii K, Hao H, Shibuya M, Imanaka T, Miki K, Tamaru H, Horimatsu T, Saita T, Nishimura M, Ryomoto M, Miyamoto Y, Masuyama T, Ishihara M. Right Sinus of Valsalva Aneurysm Causing Acute Myocardial Infarction. Circ J. 2015;79:2720-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Narayanan SR, Al Shamkhani W, Rajappan AK. Anomalous origin of RCA from left coronary sinus presenting as PSVT and recurrent acute coronary syndromes. Indian Heart J. 2016;68:208-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Cheezum MK, Liberthson RR, Shah NR, Villines TC, O'Gara PT, Landzberg MJ, Blankstein R. Anomalous Aortic Origin of a Coronary Artery From the Inappropriate Sinus of Valsalva. J Am Coll Cardiol. 2017;69:1592-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 245] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 21. | Cho SH, Joo HC, Yoo KJ, Youn YN. Anomalous Origin of Right Coronary Artery from Left Coronary Sinus: Surgical Management and Clinical Result. Thorac Cardiovasc Surg. 2015;63:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:1494-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 399] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 23. | Youniss MA, Ghoshhajra B, Bernard S, Bhatt AB, Aranki SF, MacGillivray TE, Defaria Yeh D. Familial Anomalous Origin of Right Coronary Artery from the Left Coronary Sinus. Am J Cardiol. 2018;122:1800-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Molossi S, Agrawal H, Mery CM, Krishnamurthy R, Masand P, Sexson Tejtel SK, Noel CV, Qureshi AM, Jadhav SP, McKenzie ED, Fraser CD Jr. Outcomes in Anomalous Aortic Origin of a Coronary Artery Following a Prospective Standardized Approach. Circ Cardiovasc Interv. 2020;13:e008445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |