Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.7029
Peer-review started: November 14, 2021
First decision: March 24, 2022
Revised: April 1, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: July 16, 2022
Processing time: 232 Days and 12.7 Hours
Shock is among the most common conditions that clinicians face in intensive care unit (ICU), of which hypovolemic shock is encountered most frequently; some patients instead suffer from neurogenic, cardiogenic, or infectious forms of shock. However, there are additional types of shock from unusual causes that are often undiagnosed. Here, we report the case of a patient who was initially misdiagnosed with hypovolemic shock, but exhibited persistent hypotension because of continuous fluid replacement and vasoactive drug administration, and was eventually diagnosed with hypopituitarism with crisis.
A 73-year-old Chinese man was admitted to the neurosurgery department following injury caused by a heavy object with symptoms of anemia and high fever. He was transferred to the ICU on the fourth day after hospitalization because of hypotension and unconsciousness. Blood analysis indicated that the patient was suffering from anemia and thrombocytopenia. Ultrasonography showed that there was no apparent abnormality in the cardiac structure but there was mild tricuspid regurgitation. Computed tomography revealed that there were signs of hemorrhage at the right basal ganglia; accordingly, hypovolemic shock, possibly septic shock, was initially considered. Even after routine treatment for shock, the hypotension remained severe. The patient was again thoroughly examined to investigate the underlying cause. The antishock therapy was supplemented with corticosteroids to counter potential hypopituitarism. The patient made a full recovery, and the blood pressure returned to normal.
A case of pituitary adenoma with multiple injuries was identified. Because of hypopituitarism, functionality of the corresponding endocrine system was restricted, with the most pronounced manifestation being unstable blood circulation requiring hormone replacement therapy. Such cases are relatively rare but may occur if multiple injuries are sustained. The present case represents a reference for the clinical treatment of patients with multiple injuries.
Core Tip: Here, we report that a patient was initially misdiagnosed with hypovolemic shock and later developed persistent hypotension due to continuous fluid replacement along with vasoactive drug administration. The patient was eventually diagnosed with hypopituitarism with a pituitary crisis, and the case was identified as pituitary adenoma with multiple lesions, which limited the corresponding endocrine system function. The most apparent manifestation was unstable blood circulation and hormone replacement therapy requirement. The presentation was relatively rare but could happen if multiple injuries persisted. This case can be a reference for the clinical treatment of patients with multiple injuries, and is different from a hypopituitary-pituitary crisis secondary to craniocerebral trauma. Hypopituitary-pituitary crisis in trauma patients is rarely reported in the literature.
- Citation: Zhang XC, Sun Y. Hypopituitary syndrome with pituitary crisis in a patient with traumatic shock: A case report. World J Clin Cases 2022; 10(20): 7029-7036
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/7029.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.7029
Hypopituitarism refers to one or more pituitary hormone deficiency disorders caused by a decline in the function of the adenohypophysis and/or hypothalamus. It can reduce the quality of life and increase morbidity and mortality risks[1]. The most common cause of hypopituitarism is the presence of suprasellar lesions. Usually in adenoma, changes to the hypophysis are caused by the effect of tumor mass, surgery, radiation, etc. Other clinical conditions, such as traumatic brain injury, genetics, or autoimmune or inflammatory diseases, can also result in reduced pituitary function. Clinical symptoms of hypopituitarism are often non-specific, including physical discomfort, fatigue, decreased energy, weight loss, and decreased appetite[2]. The prevalence and incidence of hypopituitarism reported in the literature are 45.5 and 4.2 cases per 100000 adults in a population, respectively, with the mean age at diagnosis being 50 among a population aged 18-79 years, with no significant difference in gender[3].
Traumatic shock caused by multiple injuries is complex, with hypovolemic shock being the most commonly observed. The condition of some patients may be complicated by neurogenic, cardiogenic, or infectious shock[4]. However, other types of shocks from rare causes are often missed. Here a case of hypovolemic shock was misdiagnosed, with persistent hypotension remaining after continuous fluid replenishment and application of vasoactive drugs. Finally, the patient was diagnosed with hypopituitarism with a pituitary crisis. In combination with evidence from the literature, the aim of this case report is to set out the causes of shock so that clinicians may gain a more comprehensive understanding of the condition.
The primary manifestations were multiple bodily injuries caused after being knocked over by a heavy object 5 h ago. The patient was not in a coma after injury and he experienced no nausea or vomiting, but was exhibiting head and chest pain combined with overall discomfort.
A 73-year-old male patient was admitted to The Second Affiliated Hospital of Anhui Medical University on March 3, 2021 because of "multiple bodily injuries caused by being knocked down by a heavy object 5 h ago". The patient was at work near the bus station and was struck to the chest by a tire from a lorry, after which he fell on the ground. He was not in a coma after the injury and there was no nausea or vomiting, but the patient suffered head and chest pain and discomfort, and was therefore taken to a local hospital; additionally, the patient had a tip fracture of the lower limb and left ear bleeding. To make a further diagnosis and initiate treatment, computed tomography (CT) of the head, neck, and chest was performed, and multiple intracranial contusions and multiple fractures were identified. Neurosurgery was recommended as an emergency plan for "brain contusions", for which the patient provided consent. After admission, the patient was treated for dehydration, hemostasis, protection of the gastric mucosa, reduction of intracranial pressure, prevention of epilepsy, and correction of anemia, among other treatments. During the course of treatment, a high fever was repeatedly observed. The highest recorded temperature was 39.0 °C. On March 7, the patient developed disturbed consciousness, was unable to breathe, and had a pale face, cold and clammy skin, shortness of breath, and a rapid heart rate. His blood pressure was 79/28 mmHg with no detectable oxygen saturation, and was therefore transferred from the neurosurgery department to intensive care unit (ICU).
The patient stated that he did not suffer from hypertension or diabetes, and had no other medical history.
The patient had no history of surgical procedures, did not smoke or drink alcohol, had no history of food or drug allergies, and had no family history of hereditary diseases.
Physical examination in the ICU demonstrated a temperature of 37.6 °C, pulse rate of 127 beats/min, respiratory rate of 30 breaths/min, and blood pressure of 82/55 mmHg. The patient was in a shallow coma with oxygen provided via an inhalation mask. SPO2 was 95%, FiO2 was 220 mmHg, and the patient had a pale face, wet and cold skin, and bilateral pupils that were large and round, 2 mm in diameter, with no response to light. He had a soft neck, fixed chest band, noisy breathing from both lungs but without wet or dry rales, and a soft abdomen with no tenderness or rebound pain, but there were audible bowel sounds. The right lower limb had a plaster fixation with negative pathological signs.
The laboratory examinations are shown in Tables 1 and 2.
| Parameter | Value | Reference range |
| WBC | 5.16 × 109 /L | 3.50-9.50 × 109 /L |
| HB | 79 g/L↓ | 130-175 g/L |
| PLT | 59 × 109/L↓ | 125-350 × 109/L |
| PCT | 1.080 ng/mL↑ | 0-0.046 ng/mL |
| IL-6 | 138.2 pg/mL↑ | 0-7.0 pg/mL |
| Total protein | 57.7 g/L↓ | 65.0-85.0 g/L |
| Albumin | 29.9 g/L↓ | 40.0-55.0 g/L |
| Alanine aminotransferase | 25 U/L | 9–50 U/L |
| Aspartate aminotransferase | 47 U/L↑ | 15-40 U/L |
| Total bilirubin | 29.7 μmol/L↑ | 0.0-26.0 μmol/L |
| Direct bilirubin | 6.9 μmol/L↑ | 0.0-4.0 μmol/L |
| Indirect bilirubin | 22.8 μmol/L↑ | 0.0-22.0 μmol/L |
| BUN | 5.54 mmol/L | 3.60-9.50 mmol/L |
| CR | 85 μmol/L | 57-111 μmol/L |
| Uric acid | 290 μmol/L | 208-428 μmol/L |
| GLU | 7.88 mmol/L↑ | 3.90-6.10 mmol/L |
| K+ | 3.62 mmol/L | 3.50-5.30 mmol/L |
| Na+ | 151.9 mmol/L↑ | 137.0-147.0 mmol/L |
| Creatine kinase | 612 U/L↑ | 50-310 U/L |
| Lactate dehydrogenase | 292 U/L↑ | 120-250 U/L |
| Troponin I | 0.26 ng/mL↑ | 0.00-0.05 ng/mL |
| BNP | 278 ng/L↑ | 0-100 ng/L |
| D-dimer | 11.24 μg/mL | 0.00-0.55 μg/mL |
| Parameter | Value | Reference range |
| Triiodothyronine | 0.700 nmol/L↓ | 1.300-3.100 nmol/L |
| Free triiodothyronine | 1.890 pmol/L↓ | 3.100-6.800 pmol/L |
| Thyroid hormone | 38.460 nmol/L↓ | 166.000-181.000 nmol/L |
| Free thyroxine | 5.210 pmol/L↓ | 12.000-22.000 pmol/L |
| Thyrotropin | 0.130 mIU/L↓ | 0.270-4.200 mIU/L |
| Cortisol | 532.0 nmol/L | 138.0-690.0 nmol/L |
| Adrenocorticotropic hormone | 5.77 pg/mL | 0-46.00 pg/mL |
| Follicle-stimulating hormone | 0.82 mIU/mL↓ | 1.50-12.40 mIU/mL |
| Luteinizing hormone | 0.13 mIU/mL | 1.70-8.60 mIU/mL |
| Testosterone | 18.8 ng/dL↓ | 193-740 ng/dL |
| Pituitary prolactin | 14.33 ng/mL | 4.10-18.40 ng/mL |
| Osmotic pressure (blood) | 301.4 mOsm/kg | 275-305 mOsm/kg |
| Growth hormone | 0.14 ng/mL | 0.06-5.00 ng/mL |
| Insulin-like growth factor-1 | 54.30 ng/mL↓ | 64.00-188.00 ng/mL |
| Insulin-like growth factor binding protein | 30.70 μg/mL↓ | 2.80-5.70 μg/mL |
| Osmotic pressure (urine) | 712.4 mOsm/kg | 600-1000 mOsm/kg |
Electrocardiogram (ECG) demonstrated sinus tachycardia with T wave changes. Ultrasonography of the heart + liver, gallbladder, pancreas, spleen + abdominal cavity + pelvic cavity indicated that there was no apparent abnormality in the structure of the heart, but there was mild tricuspid regurgitation, bile deposition in the gallbladder, and bilateral pleural effusion.
CT of the head, neck, chest, abdomen, and pelvis indicated the following: (1) Bleeding in the right basal ganglia area, right frontoparietal temporal subdural hematoma, and subarachnoid hemorrhage; (2) left temporal occipital fracture involving the mastoid process; (3) a small degree of pneumothorax on both sides; (4) traumatic wet lung; (5) bilateral pleural effusion; (6) bilateral partial rib, left scapula, and sternum fractures; (7) multiple liver cysts; and (8) hyperplasia of the prostate.
CT scan of the right knee indicated a tibial fracture of the right lower limb.
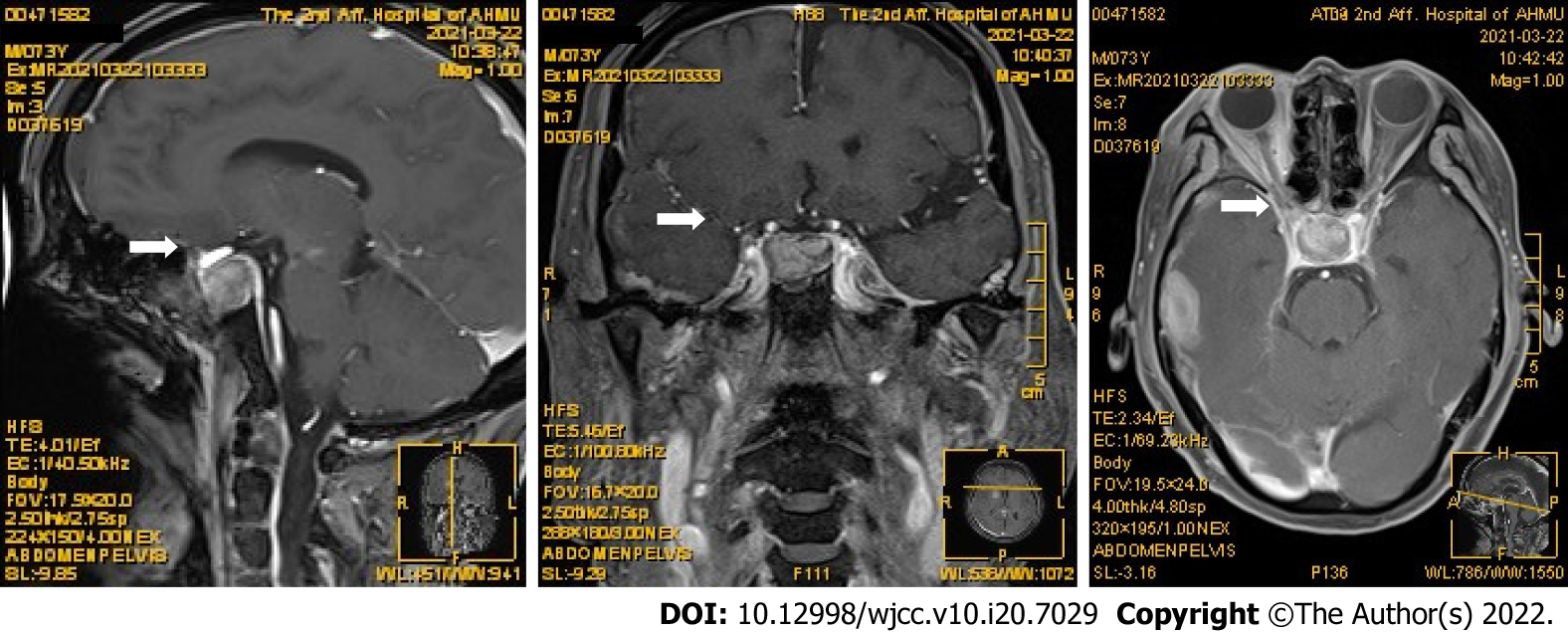
Magnetic resonance imaging (MRI) of the pituitary showed that the pituitary fossa was apparently filled, so the possibility of adenoma was considered, as shown in Figure 1.
Shock (hypovolemic, possibly infectious); multiple injuries; craniocerebral injury: Right basal ganglia hemorrhage, subdural hematoma, subarachnoid hemorrhage, left temporo-occipital fracture involving the mastoid process; multiple chest injuries: Pneumothorax, traumatic wet lung, and bilateral pleural effusion; multiple systemic fractures: Bilateral partial rib fracture, left scapula fracture and sternum fracture, and right lower limb fracture of the tibia; multiple liver cysts; and prostatic hyperplasia.
Based on the clinical signs, laboratory examination, ultrasound findings, and re-examination of brain CT scans, neurogenic and cardiogenic shock was excluded. Hypovolemic shock caused by trauma was considered, while infection could not be excluded. Therefore, fluid resuscitation, enhanced anti-infection treatment, sedation, and analgesia were provided, with protective mechanical ventilation, prevention and treatment of cerebral edema and stress ulcers, nutritional support, and enhanced comprehensive nursing and other treatments. The net fluid intake was approximately 4068 mL during the first 48 h after admission to the ICU, with a maximum dose of norepinephrine of 1.2 μg/kg/min to maintain blood pressure. After 48 h, the patient regained consciousness and urine volume recovered. As a continuous high dose of norepinephrine was required to maintain blood pressure, terisopressin at 0.02 μg/kg/min was introduced, allowing norepinephrine to be reduced to 0.4 μg/kg/min. Although bedside echocardiography demonstrated insufficient volume and low cardiac output, when combined with a central venous pressure of 10 cmH2O, shock was considered to have improved. Blood pressure was still fluctuating with positive fluid balance the following night, with a minimum blood pressure of 76/53 mmHg (0.48 μg/kg/min). Other possibilities such as bleeding and fluid loss were ruled out. The etiology of sputum/blood (-), combined with observations from the physical examination, namely, a pale face, sparse eyebrows, and the absence of armpit and pubic hair, prompted us to consider the possibility of secretory shock and hypopituitary crisis. Hydrocortisone was empirically used to further improve hormone levels.
Distributed shock (secreted); pituitary adenoma, central hypothyroidism, hypopituitary function, and pituitary crisis syndrome; and multiple injuries: Craniocranial trauma, multiple rib fractures, and tibial fracture of the right lower extremity.
On March 11, a hydrocortisone sodium succinate (100 mg q12 h) intravenous drip was provided. On March 14, levothyroxine sodium tablet 25 (μg qd) was additionally administered via nasal feeding, and hormone replacement therapy was provided. Post-shock was corrected and norepinephrine was withdrawn. One week after hormone therapy, the dosage of levothyroxine was increased to 50 μg qd. Blood cortisol was 1087.0 nmol/L (reference value: 138.0-690.0 nmol/L); accordingly, the hydro
The patient was transferred out of the ICU in generally good condition with stable hemodynamics. Hormone replacement therapy was continued, and elective neurosurgery was performed.
Shock is among the most common conditions observed in the ICU. Depending on hemodynamic changes, it can be categorized as hypovolemic shock, cardiogenic shock, distributed shock, or obstructive shock. Depending on its etiology, it is often classified as hypovolemic shock, septic shock, cardiogenic shock, anaphylactic shock, neurogenic shock, or secretory shock. Severe multiple injuries are a common cause of shock. The most common type is hypovolemic shock[5]. The mechanism by which it occurs is considered to be the result of severe visceral damage occurring in the aftermath of severe injury because of complex fractures, brain damage, etc., resulting in a large loss of blood and bodily fluids. In addition, it is also known that a proportion of patients can also suffer a combination of neurogenic shock and septic shock, because of the breakdown and absorption of toxins, combined with pain and mental impact caused by factors such as traumatic shock. Such patients may exhibit anemia, semi-consciousness, wet and cold skin on the limbs, and other symptoms. Treatments such as blood transfusion, fluid resuscitation, and vasoactive drug administration can quickly relieve the symptoms of shock. Because the current patient had clearly suffered major trauma, it could not be ruled out that infection was responsible for the recurrent high fever and shock many days after admission. Although the patient was delirious several days after trauma, no new lesions were found on brain CT reexamination. Combined with the bedside cardiac ultrasound results, neurogenic and cardiogenic shock were ruled out. Therefore, hypovolemic shock caused by trauma was first considered after admission, with early broad-spectrum antibiotic treatment provided for possible septic shock. However, after a series of treatments, the shock persisted, and at this point, hemoglobin levels were rising with all microbial cultures being negative. Therefore, refractory hypotensive shock caused by other causes should be considered. To investigate the potential causes, a comprehensive physical examination (indicating pale skin, sparse eyebrows, absence of armpit and pubic hair, systemic edema, etc.), laboratory tests [e.g., cortisol + adrenocorticotropic hormone (ACTH), five thyroid hormone tests, and three growth hormone tests], and pituitary MRI were performed on the patient. Based on the results of the physical examination, the possibility of hypopituitarism was considered. Subsequent endocrine function tests and MRI also confirmed the diagnosis.
Hypopituitarism is easily neglected, as it has various clinical manifestations, especially in the elderly. Many related symptoms, such as fatigue, poor appetite, and lethargy[6], may overlap with those caused by normal aging. This case differed from hypopituitarism with a pituitary crisis secondary to traumatic craniocerebral injury[7,8], because the patient with hypopituitarism developed a pituitary crisis as a result of trauma, which has been reported only infrequently in the literature. Hypopituitarism affects the heart, lungs, endocrine system, and kidneys, among other systems. In this case, hypotensive shock was the first and most prominent manifestation, and we believe that the main pathogenesis was as follows. First, pituitary hormone levels decreased in the sequence of growth hormone (GH) and luteinizing hormone (LH) to thyroid-stimulating hormone (TSH), and then ACTH, of which TSH and ACTH deficiency was the more important. Hypothyroidism leads to long-term lower levels of thyroid hormone, resulting in a further decline in gene transcription levels, myocardial protein, NA+/K+-activated ATPase activity, mucin deposition, myofibrillary degeneration, and necrosis, with decreased cardiac output[9]. Second, abnormal secretion of renin and aldosterone in patients with adrenal hypofunction led to poor regulation of blood pressure and water and salt metabolism dysfunction. Third, decreased glucocorticoid secretion led to the dysregulation of glucose lipid and protein metabolism, and a decline in immune function. In addition, the demand for glucocorticoids increased because of the post-traumatic stress reaction, leading to further hormone deficiency, finally inducing pituitary crisis. We believe that the patient's cortisol and ACTH were still within the normal range when admitted to hospital; this may be related to critical illness-related corticosteroid insufficiency.
Based on the pathogenesis described above, glucocorticoid replacement therapy was immediately provided. There is no uniform standard for the dosage or duration of glucocorticoid replacement therapy. Stress dose hydrocortisone (100 mg q12 h) was provided, followed by nasal feeding with low dose levothyroxine (25 μg qd). Treatment with glucocorticoid replacement therapy should be followed by thyroxine to avoid exacerbating the pituitary crisis[10]. Vasoactive drugs were withdrawn 7 d after hormone replacement therapy and blood pressure returned to normal. The patient received physiological doses of hydrocortisone (50 mg bid) and levothyroxine (50 μg qd) after transfer to the neurosurgery department[11]. The patient received only glucocorticoid and thyroxine supplementation because of the major role of glucocorticoid and thyroxine deficiency in all endocrine hormone deficiencies associated with refractory shock. Moreover, in patients of advanced age, where growth and development have concluded, low levels of sex hormones are not life-threatening; therefore, supplementation with growth and sex hormones is not required[12-14].
In this case, secretory factors were the underlying cause of shock. The patient was admitted to ICU for treatment of trauma, loss of blood, and coma. The early symptoms of shock masked the clinical features of hypopituitarism, making the diagnosis more difficult. In addition, severe hypopituitarism may have further exacerbated the clinical manifestations of shock, making the shock more difficult to correct. However, after the second full physical examination, the patient's clinical manifestations may have reflected hypopituitarism to a certain extent, with very low levels of blood markers such as TSH, total triiodothyronine, and free thyroxine, and normal but low levels of ACTH, strongly suggesting hypopituitarism. The principal reasons for misdiagnosis were as follows: (1) The symptoms caused by gonadotropin deficiency in the early stages have little impact on the daily life of patients and are often not acknowledged by patients; (2) the most common cause of traumatic shock is hypovolemia, where secretory factors are easily omitted; and (3) clinicians in the ICU have an insufficient understanding of pituitary and target gland level detection during early shock. We conclude that if at the time of admission, adenohypophysis hormone tests were performed, the genuine nature of the shock would have been identified sooner.
In summary, trauma-induced pituitary crisis is rare in patients with hypopituitarism, with insidious manifestations and rapid development, and life may be endangered if hormone drugs are not supplemented in a timely manner[4]. Therefore, when trauma occurs with refractory shock, especially when combined with hypotension, hypoglycemia, and hyponatremia, it is necessary to consider whether the patient is also suffering from hypopituitarism by additionally conducting blood tests for cortisol levels, with pituitary MRI to confirm the diagnosis and thus prevent misdiagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Turkey; Jabbarpour Z, Iran; Lo Furno D, Italy A-Editor: Yao QG, China S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang H
| 1. | Yamanaka T, Tatsushima K, Kondoh C, Takemura K, Masuda J, Ozaki Y, Tanabe Y, Miura Y, Takano T. [Hypopituitarism]. Gan To Kagaku Ryoho. 2020;47:885-890. [PubMed] |
| 2. | Toogood AA, Stewart PM. Hypopituitarism: clinical features, diagnosis, and management. Endocrinol Metab Clin North Am. 2008;37:235-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Regal M, Páramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf). 2001;55:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 213] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Rochwerg B, Hylands M, Møller M, Asfar P, Cohen D, Khadaroo RG, Laake JH, Perner A, Tanguay T, Widder S, Vandvik P, Kristiansen A, Lamontagne F. CCCS-SSAI WikiRecs Clinical Practice Guideline: vasopressors in early traumatic shock. Can J Anaesth. 2017;64:766-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Standl T, Annecke T, Cascorbi I, Heller AR, Sabashnikov A, Teske W. The Nomenclature, Definition and Distinction of Types of Shock. Dtsch Arztebl Int. 2018;115:757-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Yeliosof O, Gangat M. Diagnosis and management of hypopituitarism. Curr Opin Pediatr. 2019;31:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Liu Y, Yao Y, Zhu HJ. [Research Advances in Hypothalamic-pituitary Dysfunction Related to Traumatic Brain Injury]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Tanriverdi F, De Bellis A, Ulutabanca H, Bizzarro A, Sinisi AA, Bellastella G, Amoresano Paglionico V, Dalla Mora L, Selcuklu A, Unluhizarci K, Casanueva FF, Kelestimur F. A five year prospective investigation of anterior pituitary function after traumatic brain injury: is hypopituitarism long-term after head trauma associated with autoimmunity? J Neurotrauma. 2013;30:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Hampton J. Thyroid gland disorder emergencies: thyroid storm and myxedema coma. AACN Adv Crit Care. 2013;24:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Glynn N, Agha A. Which patient requires neuroendocrine assessment following traumatic brain injury, when and how? Clin Endocrinol (Oxf). 2013;78:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Quinn M, Agha A. Post-Traumatic Hypopituitarism-Who Should Be Screened, When, and How? Front Endocrinol (Lausanne). 2018;9:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Tan CL, Alavi SA, Baldeweg SE, Belli A, Carson A, Feeney C, Goldstone AP, Greenwood R, Menon DK, Simpson HL, Toogood AA, Gurnell M, Hutchinson PJ. The screening and management of pituitary dysfunction following traumatic brain injury in adults: British Neurotrauma Group guidance. J Neurol Neurosurg Psychiatry. 2017;88:971-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 13. | Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB. American association of clinical endocrinologists/american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26:1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 613] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 14. | Hannon MJ, Crowley RK, Behan LA, O'Sullivan EP, O'Brien MM, Sherlock M, Rawluk D, O'Dwyer R, Tormey W, Thompson CJ. Acute glucocorticoid deficiency and diabetes insipidus are common after acute traumatic brain injury and predict mortality. J Clin Endocrinol Metab. 2013;98:3229-3237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |