Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.7020
Peer-review started: November 15, 2021
First decision: April 16, 2022
Revised: April 21, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: July 16, 2022
Processing time: 231 Days and 19 Hours
Radiofrequency ablation (RFA) is an effective treatment for early-stage hepatocellular carcinoma (HCC). Although RFA is a relatively safe technique compared with surgery, several complications have been reported to be following/accom
An 80-year-old woman with segment VIII HCC was treated twice in 5 years with RFA; 28 mo after the second RFA, the patient complained of right hypochondriac pain. Computed tomography revealed that the small intestine was incarcerated in the right thorax. The patient was diagnosed with diaphragmatic hernia and underwent laparoscopic repair by non-absorbable running sutures. The patient’s postoperative course was favorable, and the patient was discharged on postoperative day 12. The diaphragmatic hernia has not recurred 24 mo after surgery.
Laparoscopic treatment of iatrogenic diaphragmatic hernia is effective and minimally invasive.
Core Tip: Radiofrequency ablation (RFA) is an effective treatment for hepatocellular carcinoma (HCC). Delayed diaphragmatic hernia caused by RFA is uncommon; however, the best surgical approach to its treatment has not been determined. Herein, we present a rare case of delayed-onset diaphragmatic hernia due to RFA and its treatment with laparoscopic repair. This case highlights the ultimate importance of that RFA for HCC located close to the diaphragm should be performed using artificial ascites under computed tomography guidance to prevent an injury to the diaphragm. Laparoscopic treatment of iatrogenic diaphragmatic hernia is effective and minimally invasive.
- Citation: Tsunoda J, Nishi T, Ito T, Inaguma G, Matsuzaki T, Seki H, Yasui N, Sakata M, Shimada A, Matsumoto H. Laparoscopic repair of diaphragmatic hernia associating with radiofrequency ablation for hepatocellular carcinoma: A case report. World J Clin Cases 2022; 10(20): 7020-7028
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/7020.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.7020
Hepatocellular Carcinoma (HCC) is ranked as the sixth most common neoplasm and the third leading cause of death to cancer[1]. Surgical resection, transplantation, ablation, transarterial chemoembolization and the use of tyrosine-kinase inhibitors are treatments with proven survival benefit. Radiofrequency ablation (RFA) is an effective treatment for early-stage HCC. Although RFA is a relatively safe technique compared with surgery, several complications have been identified[2-7]. In an analysis of 3670 patients who underwent RFA for HCC, Mulier et al[3] reported an overall complication rate of 8.9%. The major complications following RFA were abdominal bleeding, abdominal infection, and biliary tract damage; 5 cases (0.1%) of injury to the diaphragm were also reported. Delayed diaphragmatic hernia caused by RFA is uncommon; however, the best surgical approach to its treatment has not been determined. Here, we present a case of delayed-onset diaphragmatic hernia resulting from RFA and its treatment with laparoscopic repair, along with the review of the relevant literature.
An 80-year-old woman had been followed up for autoimmune hepatitis-related liver cirrhosis and recurrent HCC. Colonoscopy for chronic diarrhea revealed rectal cancer, and the patient was accordingly admitted to our hospital for resection of the tumor. High anterior resection was performed. On postoperative day 10, the patient complained of right hypochondriac pain.
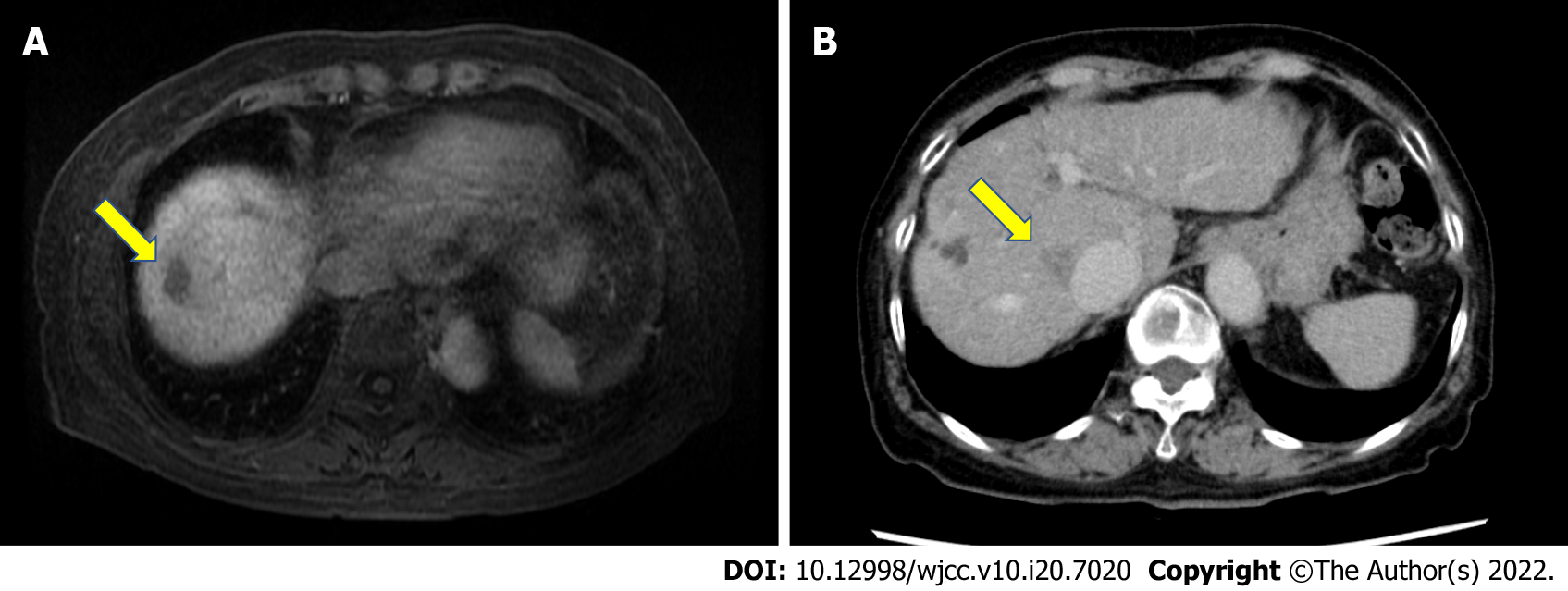
The patient had been followed up for autoimmune hepatitis-related liver cirrhosis and recurrent HCC. The patient’s condition was classified as Child-Pugh Class B (7 points) with hypoalbuminemia (2.1 g/dL) without encephalopathy or ascites. Gadoxetate sodium enhanced magnetic resonance imaging revealed masses that were highly suspicious for HCC located in the Segment VIII (S8) near the liver surface (Figure 1A). RFA was performed under ultra-sonographic guidance using an expandable needle (LeVeen™ Needle Electrode; Boston Scientific, Inc., Natick, MA, United States) 55 mo before hernia repair, with no early complications. No artificial pleural effusion or artificial ascites was used. Twenty-eight months before the hernia repair, the patient underwent repeat RFA for recurrent HCC located in S8 near the inferior vena cava (Figure 1B). Artificial pleural effusion was used during the second RFA.
The patient had medical histories of hypertension, hyperuricemia, heart failure, pneumonia, and laparoscopic cholecystectomy.
There was no family history of malignant tumors.
On her physical examination, the patient showed tenderness of the right hypochondrium without rebound tenderness, although the vital signs were normal.
A blood test revealed normal white cell count (4800/μL; normal range, 3500-8000/μL) and C-reactive protein level (0.22 mg/dL; normal < 0.30 mg/dL). It also revealed low albumin level (2.1 g/dL) and coagulopathy, including low platelet count (8.7 × 104/μL; normal range, 15-35 × 104/μL) and high international normalized ratio of prothrombin time (1.29; normal range, 0.80-1.20) due to liver cirrhosis.
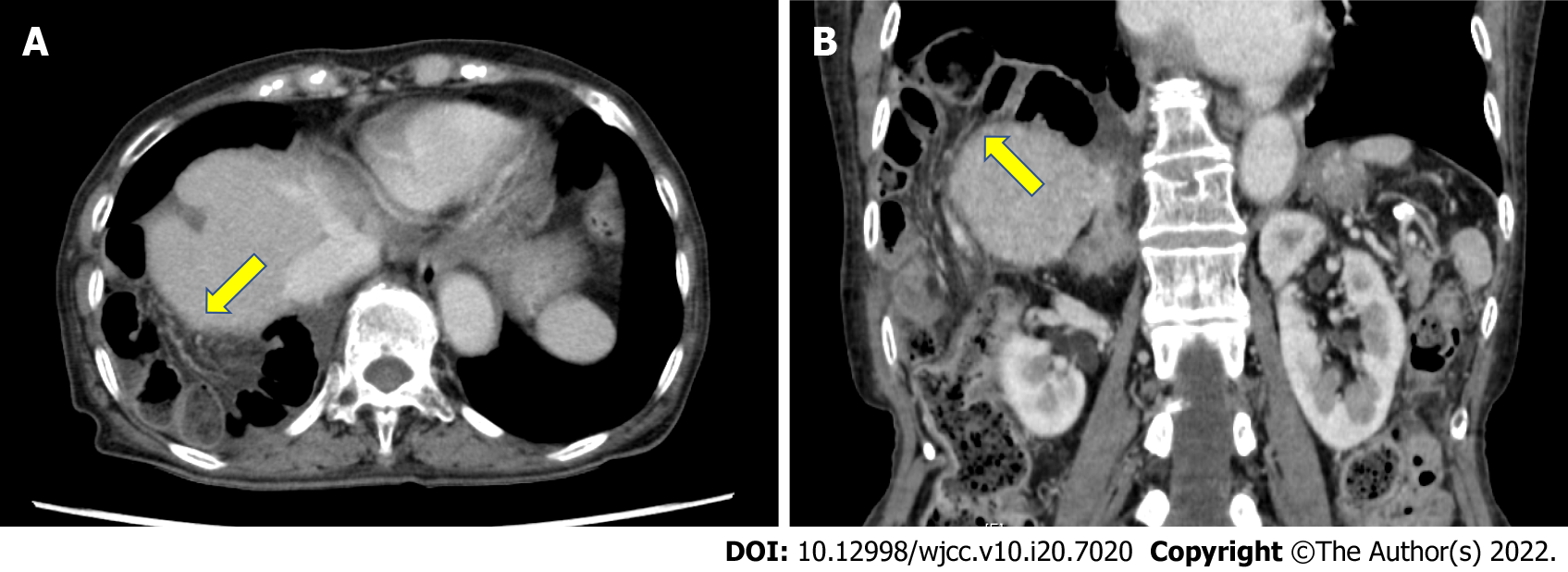
A contrast-enhanced computed tomography (CT) scan revealed small intestine incarcerated in the right thorax (Figure 2). No findings suggested intestinal ischemia.
The final diagnosis of the presented case is diaphragmatic hernia due to RFA for HCC.
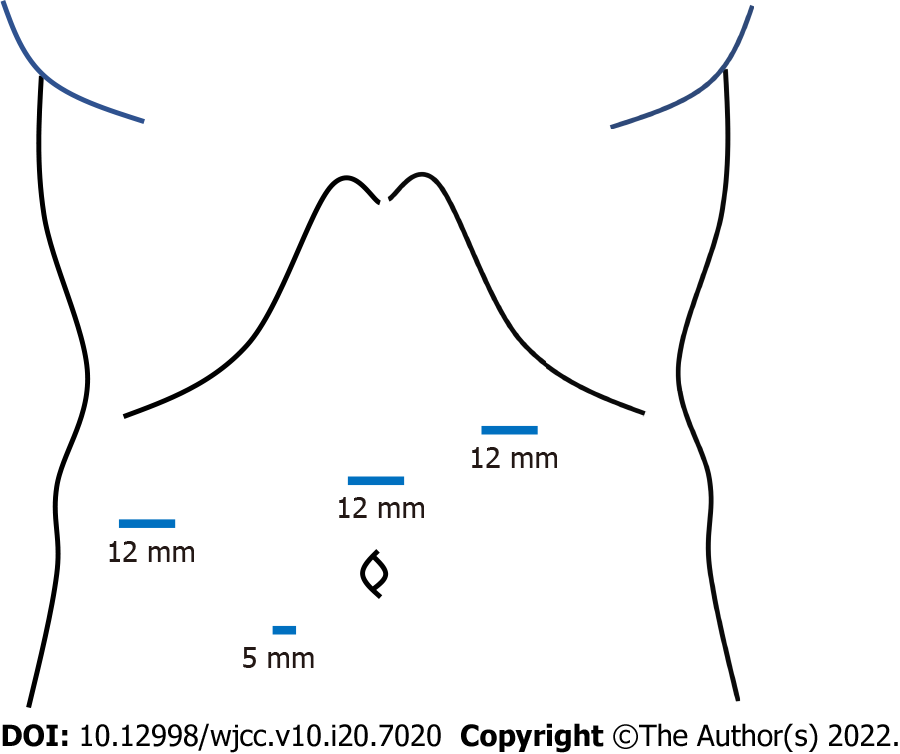
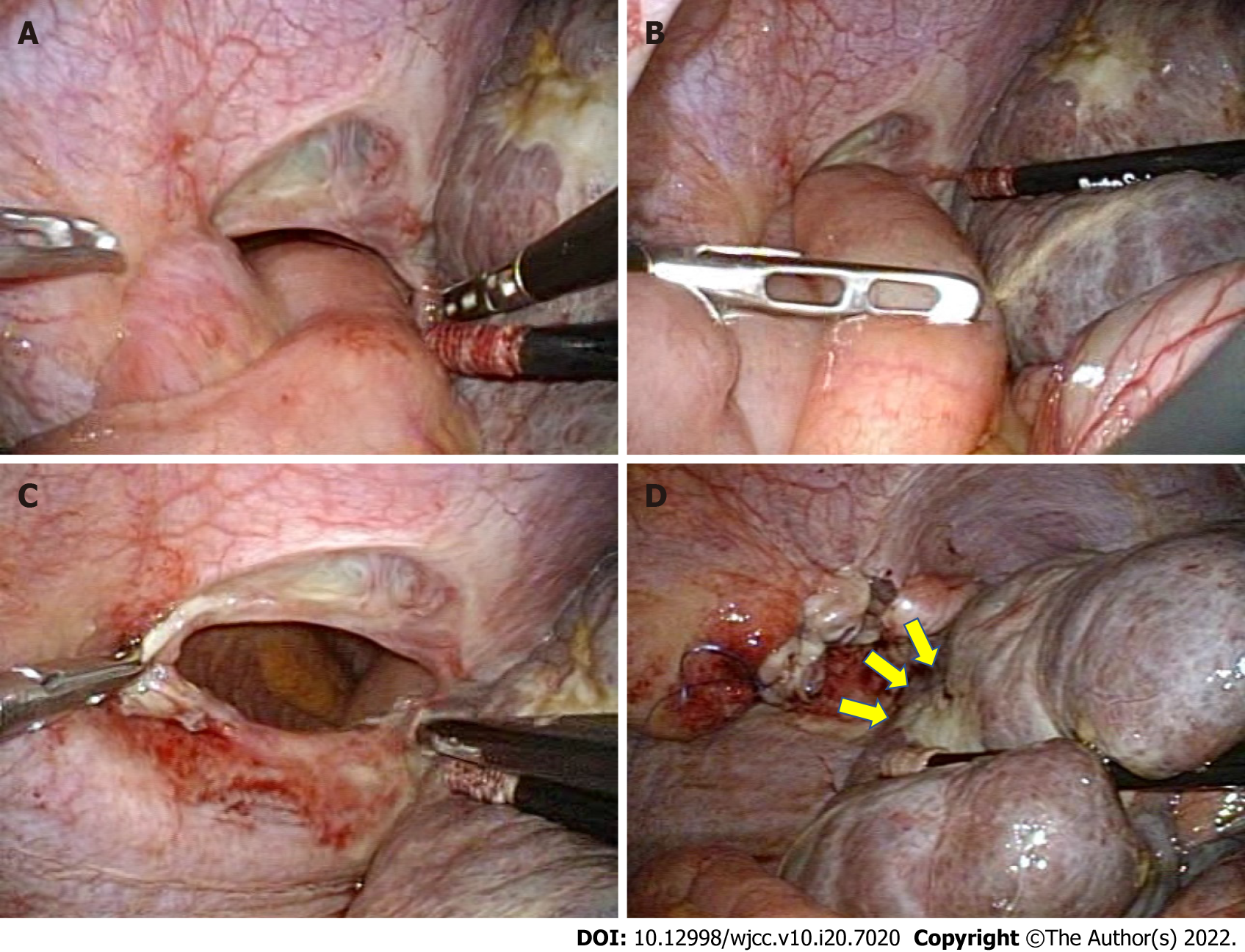
The patient immediately underwent emergency surgery. The patient underwent laparoscopic hernia repair in the dorsosacral position under general anesthesia. Four trocars were inserted into the abdomen (Figure 3). The first 12-mm trocar was introduced in the left-upper abdomen using the open-entry technique so as to avoid adhesions between the abdominal wall and visceral organs due to the previous surgery. After pneumoperitoneum by carbon dioxide insufflation, three more trocars were inserted at the right lateral abdomen, the mid-upper abdomen (12-mm trocars for operator) and near the umbilicus (a 5-mm trocar for scopist). Small intestine had slipped through the diaphragmatic defect and was observed to be incarcerated in the right thorax (Figure 4A). The small intestine was gently pulled back into the abdominal cavity using laparoscopic bowel-grasping forceps (Figure 4B). Bowel resection was not required. The hernia defect was estimated to be approximately 5 cm in diameter (Figure 4C). Intra-abdominal air pressure was reduced from 8 mmHg to 6 mmHg because the intrathoracic air pressure was increased through the defect and the pulmonary ventilation volume was decreased.
The defect was repaired using synthetic non-absorbable monofilament polypropylene sutures (3-0 PROLENE; Ethicon Inc., Somerville, NJ, United States) in the running fashion (Figure 4D). No drainage tube was placed. The operative duration was 76 min, and the estimated blood loss was < 5 mL.
The patient’s postoperative course was favorable, and the patient was discharged on postoperative day 12. The diaphragmatic hernia has not recurred 24 mo after the surgery.
Diaphragmatic hernia associated with RFA is an uncommon complication. However, diaphragmatic hernia is fatal for patients of liver cirrhosis. Therefore, it is important to recognize the risks of diaphragmatic hernia and provide prompt treatment. Twenty cases of diaphragmatic hernia due to RFA have been reported in English including our case. The background of the patients and the details of RFA are given in Table 1[8-21]. The details of diaphragmatic hernia and the treatment are presented in Table 2. The median age of the cases under study was 71 years [Interquartile range (IQR) 61-79]. There were 11 (55%) males and 9 females (45%) in the current study. The most common (13 patients, 65%) cause underlying liver diseases in patients was Hepatitis C. In the present study, 16 patients (80%) had the tumor located in S8. Diaphragmatic hernia tends to occur frequently after RFA for S8 HCC, as the location of the tumor is adjacent to the diaphragm. Physical and thermal damage to the diaphragm can result in a defect in diaphragm because of poor wound healing in patients with liver cirrhosis[22].
| Case | Ref. | Age | Sex | Underlying liver disease | Child-Pugh classification | Tumor location (size) | Guiding modality | Artificial ascites/pleural effusion | Type of needle | The number of RFA |
| 1 | Koda et al[8], 2003 | 61 | F | HB | B | S6, S8 (15 mm, 10 mm, 25 mm) | Sonography | None | Expandable | 2 |
| 2 | Shibuya et al[9], 2006 | 72 | M | AH | NA | S4/S8 (28 mm) | Sonography | None | Expandable | 2 |
| 3 | di Francesco et al[10], 2008 | 49 | M | AH and HC | NA | S8 (54 mm) | NA | None | Cool-tip | 1 |
| 4 | Yamagami et al[12], 2011 | 71 | F | HC | B | S7 (24 mm) | CT | None | Cool-tip | 1 |
| 5 | Singh et al[11], 2011 | 46 | F | AH and HB | A | S2/S3, S5/S8 (17 mm, 18 mm) | Sonography | None | Cool-tip | 1 |
| 6 | Kim et al[13], 2013 | 61 | M | AH | A | S5, S8 (13 mm, 11 mm) | Sonography | None | Cool-tip | 2 |
| 7 | Zhou et al[14], 2013 | 61 | F | HB | NA | S8 (15 mm) | NA | NA | NA | 1 |
| 8 | Nakamura et al[15], 2014 | 81 | M | HC | NA | S4, S8 (19 mm, 24 mm) | Sonography | None | Cool-tip | 1 |
| 9 | Nomura et al[16], 2014 | 62 | M | HC | C | S8 (21 mm) | Sonography | None | Cool-tip | 1 |
| 10 | Saito et al[17], 2015 | 81 | M | HC | C | S3, S5, S5/S8, S8 (NA) | NA | NA | NA | 3 |
| 11 | Abe et al[18], 2016 | 72 | F | HC | B | S5 (NA) | NA | NA | NA | Several times |
| 12 | Nagasu et al[19], 2017 | 49 | M | AH | A | S4 (17 mm) | Sonography | None | Cool-tip | Several times |
| 13 | Nagasu et al[19], 2017 | 79 | F | HC | B | S8 (19 mm) | Sonography | None | Cool-tip | Several times |
| 14 | Nagasu et al[19], 2017 | 68 | M | HC | C | S8 (26 mm) | CT | None | Expandable | 1 |
| 15 | Nagasu et al[19], 2017 | 70 | F | HC | C | S6 (23 mm) | Sonography | None | Cool-tip | 1 |
| 16 | Nagasu et al[19], 2017 | 65 | M | HC | B | S8 (21 mm) | Sonography | None | Cool-tip | 1 |
| 17 | Nagasu et al[19], 2017 | 76 | F | HC | A | S8 (20 mm) | Sonography | None | Cool-tip | Several times |
| 18 | Morito et al[20], 2021 | 78 | M | HC | NA | S6/S7, S8 (NA) | Thoracoscopic | Artificial pleural effusion | NA | 2 |
| 19 | Ushijima et al[21], 2021 | 82 | M | HC | B | S6, S4/S5, S8 (NA) | NA | NA | NA | 3 |
| 20 | Current case | 83 | F | AIH | B | S8 (20 mm) | Sonography | Artificial pleural effusion | Expandable | 2 |
| Case | Ref. | Times from last RFA (mo) | Symptoms | Herniated viscera | Size of hernia orifice (cm) | Necrosis of intestines | Surgical approach | Suture/mesh | Postoperative complication | Prognosis |
| 1 | Koda et al[8], 2003 | 32 | Dyspnea | Colon | 5 | No | OS | Suture | Hemorrhage from rupture of the HCC | Died of HCC rupture |
| 2 | Shibuya et al[9], 2006 | 18 | Right upper abdominal pain and dyspnea | Small intestine | NA | NA | Surgery | Suture | None | Alive |
| 3 | di Francesco et al[10], 2008 | 15 | Nausea and vomiting | Small intestine | 3 | No | OS | Suture | None | Alive |
| 4 | Yamagami et al[12], 2011 | 36 | Dyspnea | Colon | NA | No | CM | - | - | Alive |
| 5 | Singh et al[11], 2011 | 19 | Right upper abdominal pain and dyspnea | Colon | 5 | No | LS | Non-absorbable interrupted suture | None | Alive |
| 6 | Kim et al[13], 2013 | 9 | None | Mesenteric fat | 2 | No | CM | - | - | Alive |
| 7 | Zhou et al[14], 2013 | 12 | Lower abdominal pain, nausea and vomiting | Transverse colon | 4 | Yes | OS | Suture | None | Alive |
| 8 | Nakamura et al[15], 2014 | 18 | Right upper abdominal pain and dyspnea | Small intestine | 5 | Yes | OS | Non-absorbable interrupted suture | None | Alive |
| 9 | Nomura et al[16], 2014 | 96 | Nausea | Right colon | 4 | No | LS | Non-absorbable interrupted suture | Recurrence of diaphragmatic hernia | Alive |
| 10 | Saito et al[17], 2015 | 28 | Right upper abdominal pain | Small intestine | 4 | No | OS | Suture | Liver failure | Died of liver failure |
| 11 | Abe et al[18], 2016 | 15 | Right upper abdominal pain and dyspnea | Transverse colon | 10 | No | OS | Non-absorbable suture | None | Alive |
| 12 | Nagasu et al[19], 2017 | 17 | None | None | NA | No | OS | Interrupted suture | None | Alive |
| 13 | Nagasu et al[19], 2017 | 9 | Abdominal pain | Small intestine | NA | No | OS | Interrupted suture | None | Alive |
| 14 | Nagasu et al[19], 2017 | 21 | Abdominal pain | Mesenteric fat | NA | No | OS | Interrupted suture | None | Died of liver failure |
| 15 | Nagasu et al[19], 2017 | 8 | Dyspnea | Colon | NA | Yes | OS | Interrupted suture | None | Died of liver failure |
| 16 | Nagasu et al[19], 2017 | 16 | Abdominal pain | Colon | NA | No | OS | Interrupted suture | None | Died of liver failure |
| 17 | Nagasu et al[19], 2017 | 6 | None | None | NA | No | OS | Interrupted suture | None | Alive |
| 18 | Morito et al[20], 2021 | 12 | Nausea and abdominal pain | Small intestine | 8 | Yes | OS | Non-absorbable interrupted suture | None | Alive |
| 19 | Ushijima et al[21], 2021 | 16 | Dyspnea | Transverse colon | 2 | No | LS | Non-absorbable suture and mesh | None | Alive |
| 20 | Current case | 28 | Right upper abdominal pain | Small intestine | 5 | No | LS | Non-absorbable running suture | None | Alive |
In most cases including ours, RFA was performed under sonographic guidance. Yamagami et al[12] reported that the tip of the RFA electrode is relatively difficult to detect by sonography as compared to CT while performing RFA for HCC located close to the diaphragm. According to the surgical findings, the scar on the liver caused by the first RFA was close to the hernia orifice (Figure 4D), suggesting that the first RFA had caused the diaphragmatic hernia. In only 2 out of 20 cases, RFA was performed using artificial pleural effusion, while in 18 cases (90%) RFA was performed without using artificial pleural effusion or ascites. Wang and Kao[23] have reported that the use of artificial ascites protected the abdominal wall and adjacent organs from burn injuries during RFA for HCC. Clinicians and radiologists should therefore consider the use of artificial ascites during RFA to prevent diaphragmatic heat injury. Furthermore, some studies have reported that laparoscopic RFA is also useful for preventing physical injury to the diaphragm[24-26].
The median duration of time between occurrence of hernia and the previous RFA was 17 mo (IQR 12-25) in the current study. Diaphragmatic hernia is a late-onset complication of RFA. In the present case, diaphragmatic hernia occurred 28 mo after the final RFA. With the progression of liver atrophy, the space between the diaphragm and the liver enlarges, and intestines can move onto the liver, a phenomenon called Chilaiditi syndrome[27]. Clinicians should be aware of the possibility of the occurrence of delayed-onset diaphragmatic hernia after RFA.
Diaphragmatic hernia is a fatal disease that generally requires emergency surgery. However, 2 cases took conservative management because there were no symptoms of a strangulated hernia and they considered the risks of surgery[12,13]. The best surgical approach to treat diaphragmatic hernia has not been established. Liver cirrhosis is an important risk factor in surgery due to the factors, such as coagulopathy, poor nutritional status, adaptive immune dysfunction, cirrhotic cardiomyopathy, and renal and pulmonary dysfunction[28]. In 4 cases out of 20 cases, the laparoscopic approach was adopted. The laparoscopic approach is safer and more feasible than open surgery, considering the possibility of postoperative complications followed by reduced collateral circulation in the abdominal wall[16,29]. Furthermore, the laparoscopic approach is useful for securing a field of view over the surgical site, as the location of the hernia defect is deep. However, insufficient respiratory function may preclude the laparoscopic approach because of the risks of pneumoperitoneum and pneumothorax. In our case, we reduced abdominal air pressure from 8 mmHg to 6 mmHg because thoracic air pressure increased through the hernia orifice and pulmonary ventilation volume decreased.
We repaired the diaphragmatic hernia by non-absorbable running sutures. In most cases, the hernia repair was performed by non-absorbable interrupted sutures. Regardless of the suture techniques, absorbable sutures should not be used to prevent the recurrence of hernia[30]. On the other hand, we did not use a mesh owing to the possibility of HCC recurrence, as the use of a mesh patch could preclude another RFA. However, if the diaphragmatic hernia recurs without the need for bowel resection, the use of mesh should be considered.
RFA for HCC located close to the diaphragm should be performed using artificial ascites under CT guidance to prevent an injury to the diaphragm. Clinicians should also monitor patients who have undergone RFA, staying alert to the possibility of delayed-onset diaphragmatic hernia. Laparoscopic treatment of iatrogenic diaphragmatic hernia is effective and minimally invasive.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu SZ, China A-Editor: Yao QG, China S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4070] [Article Influence: 581.4] [Reference Citation Analysis (6)] |
| 2. | Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 530] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 3. | Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 496] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 4. | Kong WT, Zhang WW, Qiu YD, Zhou T, Qiu JL, Zhang W, Ding YT. Major complications after radiofrequency ablation for liver tumors: analysis of 255 patients. World J Gastroenterol. 2009;15:2651-2656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 353] [Article Influence: 58.8] [Reference Citation Analysis (1)] |
| 7. | Maeda M, Saeki I, Sakaida I, Aikata H, Araki Y, Ogawa C, Kariyama K, Nouso K, Kitamoto M, Kobashi H, Sato S, Shibata H, Joko K, Takaki S, Takabatake H, Tsutsui A, Takaguchi K, Tomonari T, Nakamura S, Nagahara T, Hiraoka A, Matono T, Koda M, Mandai M, Mannami T, Mitsuda A, Moriya T, Yabushita K, Tani J, Yagi T, Yamasaki T. Complications after Radiofrequency Ablation for Hepatocellular Carcinoma: A Multicenter Study Involving 9,411 Japanese Patients. Liver Cancer. 2020;9:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Koda M, Ueki M, Maeda N, Murawaki Y. Diaphragmatic perforation and hernia after hepatic radiofrequency ablation. AJR Am J Roentgenol. 2003;180:1561-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Shibuya A, Nakazawa T, Saigenji K, Furuta K, Matsunaga K. Diaphragmatic hernia after radiofrequency ablation therapy for hepatocellular carcinoma. AJR Am J Roentgenol. 2006;186:S241-S243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | di Francesco F, di Sandro S, Doria C, Ramirez C, Iaria M, Navarro V, Silvestry S, Needleman L, Frank A. Diaphragmatic hernia occurring 15 mo after percutaneous radiofrequency ablation of a hepatocellular cancer. Am Surg. 2008;74:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Singh M, Singh G, Pandey A, Cha CH, Kulkarni S. Laparoscopic repair of iatrogenic diaphragmatic hernia following radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2011;41:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Yamagami T, Yoshimatsu R, Matsushima S, Tanaka O, Miura H, Nishimura T. Diaphragmatic hernia after radiofrequency ablation for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2011;34 Suppl 2:S175-S177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Kim JS, Kim HS, Myung DS, Lee GH, Park KJ, Cho SB, Joo YE, Choi SK. A case of diaphragmatic hernia induced by radiofrequency ablation for hepatocellular carcinoma. Korean J Gastroenterol. 2013;62:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Zhou M, He H, Cai H, Chen H, Hu Y, Shu Z, Deng Y. Diaphragmatic perforation with colonic herniation due to hepatic radiofrequency ablation: A case report and review of the literature. Oncol Lett. 2013;6:1719-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Nakamura T, Masuda K, Thethi RS, Sako H, Yoh T, Nakao T, Yoshimura N. Successful surgical rescue of delayed onset diaphragmatic hernia following radiofrequency ablation for hepatocellular carcinoma. Ulus Travma Acil Cerrahi Derg. 2014;20:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Nomura R, Tokumura H, Furihata M. Laparoscopic repair of a diaphragmatic hernia associated with radiofrequency ablation for hepatocellular carcinoma: lessons from a case and the review of the literature. Int Surg. 2014;99:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Saito T, Chiba T, Ogasawara S, Inoue M, Wakamatsu T, Motoyama T, Kanogawa N, Suzuki E, Ooka Y, Tawada A, Matsubara H, Yokosuka O. Fatal Diaphragmatic Hernia following Radiofrequency Ablation for Hepatocellular Carcinoma: A Case Report and Literature Review. Case Rep Oncol. 2015;8:238-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Abe T, Amano H, Takechi H, Fujikuni N, Sasada T, Yoshida M, Yamaki M, Nakahara M, Noriyuki T. Late-onset diaphragmatic hernia after percutaneous radiofrequency ablation of hepatocellular carcinoma: a case study. Surg Case Rep. 2016;2:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Nagasu S, Okuda K, Kuromatsu R, Nomura Y, Torimura T, Akagi Y. Surgically treated diaphragmatic perforation after radiofrequency ablation for hepatocellular carcinoma. World J Gastrointest Surg. 2017;9:281-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Morito A, Nakagawa S, Imai K, Uemura N, Okabe H, Hayashi H, Yamashita YI, Chikamoto A, Baba H. Successful surgical rescue of delayed onset diaphragmatic hernia following radiofrequency ablation using a thoracoscopic approach for hepatocellular carcinoma: a case report. Surg Case Rep. 2021;7:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Ushijima H, Hida JI, Yane Y, Kato H, Ueda K, Kawamura J. Laparoscopic repair of diaphragmatic hernia after radiofrequency ablation for hepatocellular carcinoma: Case report. Int J Surg Case Rep. 2021;81:105728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kayashima H, Maeda T, Harada N, Masuda T, Guntani A, Ito S, Matsuyama A, Hamatake M, Tsutsui S, Matsuda H, Ishida T. Risk factors for incisional hernia after hepatic resection for hepatocellular carcinoma in patients with liver cirrhosis. Surgery. 2015;158:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Wang CC, Kao JH. Artificial ascites is feasible and effective for difficult-to-ablate hepatocellular carcinoma. Hepatol Int. 2015;9:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Herbold T, Wahba R, Bangard C, Demir M, Drebber U, Stippel DL. The laparoscopic approach for radiofrequency ablation of hepatocellular carcinoma--indication, technique and results. Langenbecks Arch Surg. 2013;398:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Jiang K, Zhang W, Su M, Liu Y, Zhao X, Wang J, Yao M, Ogbonna J, Dong J, Huang Z. Laparoscopic radiofrequency ablation of solitary small hepatocellular carcinoma in the caudate lobe. Eur J Surg Oncol. 2013;39:1236-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Tanaka K, Kojima T, Hiraguchi E, Hashida H, Noji T, Hirano S. Laparoscopy-Guided Transthoracic Transdiaphragmatic Radiofrequency Ablation for Hepatic Tumors Located Beneath the Diaphragm. J Laparoendosc Adv Surg Tech A. 2016;26:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Orr J, Elson CO 3rd. Abdominal Pain With an Unusual Radiographic Image. Gastroenterology. 2016;151:241-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Lopez-Delgado JC, Ballus J, Esteve F, Betancur-Zambrano NL, Corral-Velez V, Mañez R, Betbese AJ, Roncal JA, Javierre C. Outcomes of abdominal surgery in patients with liver cirrhosis. World J Gastroenterol. 2016;22:2657-2667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Belli G, D'Agostino A, Fantini C, Cioffi L, Belli A, Russolillo N, Langella S. Laparoscopic incisional and umbilical hernia repair in cirrhotic patients. Surg Laparosc Endosc Percutan Tech. 2006;16:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Muysoms FE, Antoniou SA, Bury K, Campanelli G, Conze J, Cuccurullo D, de Beaux AC, Deerenberg EB, East B, Fortelny RH, Gillion JF, Henriksen NA, Israelsson L, Jairam A, Jänes A, Jeekel J, López-Cano M, Miserez M, Morales-Conde S, Sanders DL, Simons MP, Śmietański M, Venclauskas L, Berrevoet F; European Hernia Society. European Hernia Society guidelines on the closure of abdominal wall incisions. Hernia. 2015;19:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 384] [Article Influence: 38.4] [Reference Citation Analysis (0)] |