Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6981
Peer-review started: October 30, 2021
First decision: March 23, 2022
Revised: April 5, 2022
Accepted: June 4, 2022
Article in press: June 4, 2022
Published online: July 16, 2022
Processing time: 247 Days and 8.5 Hours
Schwannomatosis is a rare disease characterized by multiple schwannomas of the whole body. Although benign, schwannomatosis that occurs in important areas of the body, such as the brain and spinal canal, can cause considerable disability and mortality. The disease is rare, frequent and relapsing, and this poses a diagnostic and therapeutic challenge.
A 40-year-old male had multiple masses all over his body, starting at the age of 19. Four years prior, he started to experience a progressive decrease in muscle strength in both lower limbs and developed urinary and defecation dysfunctions, and gradual paralysis. One month prior, the patient developed pain and numbness in his left forearm. The patient had undergone five surgical procedures for this disease in our department. Based on the family history, imaging examinations, pathological biopsy and molecular biological examinations, the diagnosis of schwannomatosis was confirmed. This time, the patient was admitted to our hospital again for a 6th operation because of the pain and numbness in his left forearm. After the operation, the patient's symptoms improved significantly; the patient recovered and was discharged from the hospital. At the last telephone follow-up, the patient reported a poor general condition but was alive.
Here, we report a rare case of schwannomatosis. We conducted 15 years of patient follow-up and treatment, and analyzed the timing of surgery and patient psychology. This case will further extend our overall understanding of the diagnosis and treatment of this rare tumor.
Core Tip: Schwannomatosis is a rare disease characterized by multiple schwannomas throughout the body. There are only a few reports of clinical cases of schwannomatosis. Here, we describe a patient who has been followed up for 15 years and has been treated with surgery 6 times. According to the family history, magnetic resonance imaging scan results, immunohistochemistry results and larger sequencing results, the patient was confirmed to have schwannomatosis. We summarized and discussed this case and reviewed the literature on the pathogenesis, clinical manifestations, clinical diagnosis and treatment of schwannomatosis.
- Citation: Li K, Liu SJ, Wang HB, Yin CY, Huang YS, Guo WT. Schwannomatosis patient who was followed up for fifteen years: A case report. World J Clin Cases 2022; 10(20): 6981-6990
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6981.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6981
Schwannomatosis, also known as neurofibromatosis type 3, can be familial or sporadic and is a tumor-prone syndrome characterized by multiple schwannomas in the central and peripheral nervous systems. It has an incidence of approximately 1/70000 and a prevalence of approximately 1/126000[1]. Schwannomatosis differs from type 1 and type 2 neurofibromatosis because it has few skin manifestations, may exhibit malignant transformation, and shows no bilateral vestibular nerve involvement[2,3].
At present, the pathogenesis of schwannomatosis is still unclear, and existing studies have shown that abnormal expression of the SMARCB1, LZTR1 and NF2 genes is involved in the occurrence and development of the disease[4]. Germline mutations in SMARCB1 or LZTR1 were found in 86% of familial and 40% of sporadic schwannomatosis patients, while NF2 was mostly somatic[4,5]. The main clinical manifestations of schwannomatosis are chronic pain, the occurrence of masses, and neurological symptoms in the corresponding area of innervation when the tumor compresses the nerve[6]. Treatment mainly involves surgery and is for symptomatic patients, however there is no specific treatment available. Because of its rarity and predisposition to multiple occurrences, neurofibromas that occur in important tissues, such as in the craniocerebral or spinal canal, have fairly high rates of disability, and late complications can also lead to high fatality rates. Surgical treatment cannot cure this disease completely, and the patient often needs multiple operations, which are associated with additional risks. These operations and risks seriously reduce patients’ quality of life, threaten their life and health, and impose heavy burdens on the patients' families and society.
Here, we report a patient who was followed up for 15 years to obtain a more comprehensive understanding of this rare disease, which can further improve the diagnosis and treatment of these patients.
A 40-year-old male was admitted to our hospital on July 10, 2020, with a chief complaint of “Multiple tumors occurring throughout the body for 21 years, with pain and numbness of the left forearm for 1 mo.”
Twenty-one years prior to this report, the patient began to develop multiple tumors throughout his body without any obvious causative factors. The tumors progressively increased in size and were accompanied by neurological dysfunction in the areas in which the tumors were growing. The patient developed a progressive decrease in the muscle strength of both lower limbs with urinary and fecal dysfunction, and gradual paralysis began 4 years before this report. The patient developed pain and numbness in the left forearm 1 mo before this report, and his symptoms progressively worsened without any known aggravating or relieving factors. There were no café au lait spots on his skin, and he had no recent hearing or visual impairments, dizziness, headache, cough, hemoptysis, nausea, vomiting, or any significant changes in his weight.
The patient had undergone 5 surgical treatments in our department for schwannomatosis (Table 1), and he had no other significant medical history.
| Time | Cardinal symptom | Therapeutic method | Postoperative follow-up |
| September 12, 2006 | Pain in the back, neck and chest for 5 yr with walking dysfunction for 1 mo | Posterior cervical and thoracic vertebral canal tumor resection was performed | After the operation, the patient's pain and lower limb muscle strength improved, recovered, and the patient was discharged from hospital, and lived normally |
| January 5, 2011 | Lumbago with numbness of both lower limbs for 1 mo | Posterior cervical and thoracic vertebral canal tumor resection was performed | After the operation, the patient's pain and lower limb muscle strength improved, recovered, and the patient was discharged from hospital and lived normally |
| March 9, 2013 | Low back pain with numbness and fatigue of both lower limbs for 3 mo | Thoracolumbar intraspinal tumor resection was performed | After operation, the symptoms did not improve, and the muscle strength of both lower limbs gradually decreased, accompanied by persistent pain in chest, waist and both lower limbs |
| August 2, 2013 | Persistent pain in chest, waist and lower limbs for 5 mo | Thoracic and lumbar posterior tumor resection + pelvic anterior tumor resection + thoracoscopic thoracic tumor resection wereperformed | The pain and muscle strength were improved after the operation, which could meet the needs of his daily life |
| October 17, 2018 | Repeated neck and upper limb pain for 2 mo with paraplegia of both lower limbs | Posterior cervical spinal cord tumor resection | After the operation, his neck and upper limb pain was relieved, but the muscle strength of lower limbs was not significantly improved. After the neck incision had healed, the patient was discharged from hospital |
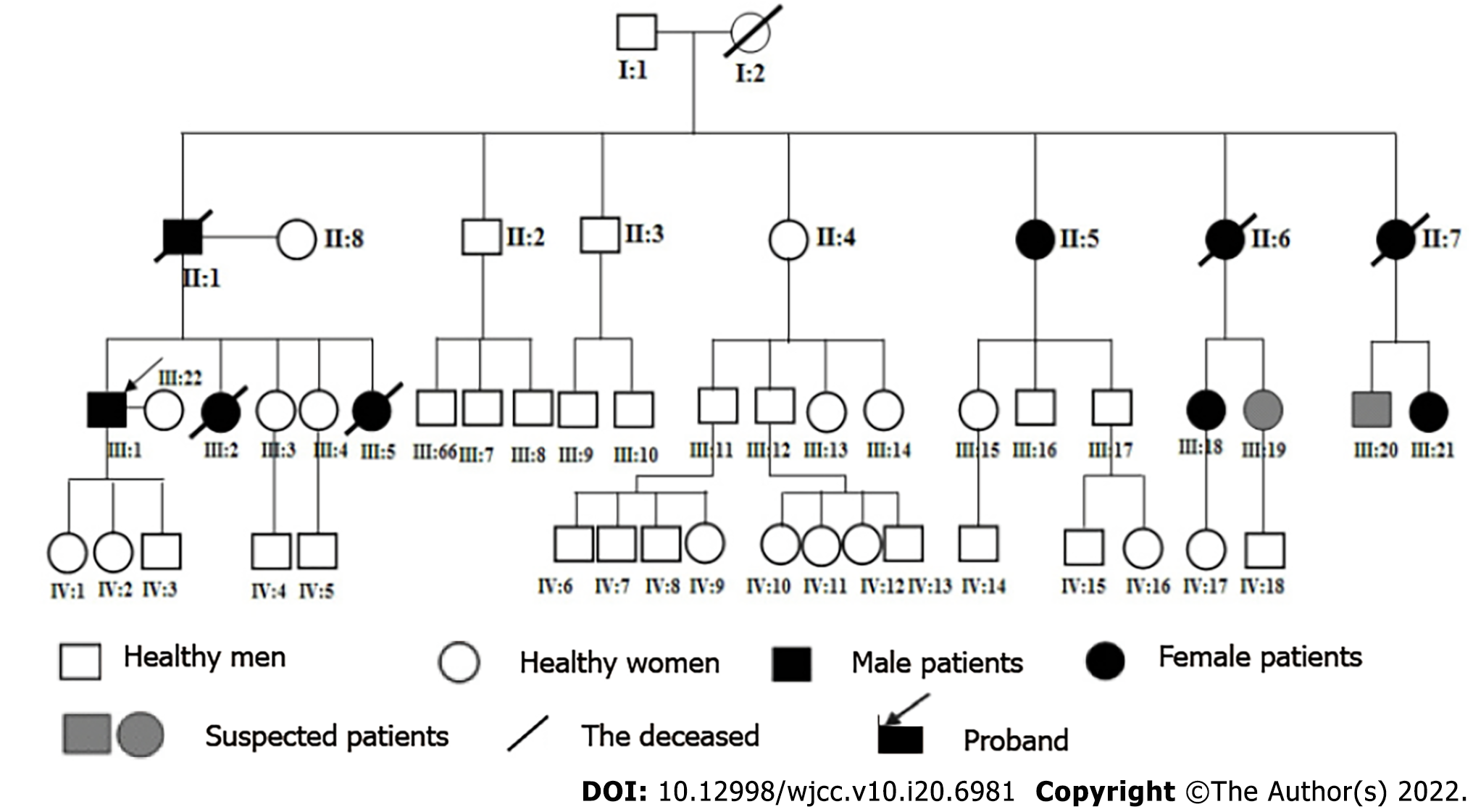
The patient had a familial schwannomatosis that was also present in 48 of his family members, 9 of whom were currently ill and 5 of whom died of the disease (Figure 1). The patient began to show symptoms at the age of 19 and has suffered from this disease for 21 years. We contacted the patient for the first time 15 years ago, and he had already undergone several operations in our department during the follow-up period.
There were no deformities in his spine or limbs, and many old surgical scars could be seen in the cervicothoracic, lumbar and dorsal areas. A mass, approximately 5 by 4 cm in size, was seen on the ulnar side of the left forearm; the mass was raised, without any necrosis or ulceration. The pedicle of the lump was firm, and the position of the mass was fixed to the underlying tissues. The surrounding skin temperature was normal, and the skin sensation in the left ulnar forearm and palm (the hand muscles, ring finger and little finger area) was decreased. Skin pigmentation was seen in both of his lower limbs, and his skin sensation was decreased bilaterally from his umbilical area to his inguinal area. There was no skin sensation below his inguinal area, and his sensation was absent in the sellar area. His lower limb muscle strength was 0 in both lower limbs, and the muscle strength of both of his upper limbs was grade IV, with a (+) Babinski sign.
All the patient’s tumor markers, biochemical examinations, liver and kidney functional markers and electrolytes were within normal ranges.
Magnetic resonance imaging (MRI) examination of the cervical spine showed that the spinous processes and laminae of C2-T1 were absent and showed postoperative changes. There were multiple abnormal signals in the spinal canal at the C2, C3 and T1 Levels; the largest signal was at the C3 Level, and the size was approximately 8 mm × 12 mm × 19 mm.
MRI examination of the thoracic spine showed that some spinous processes and laminae were diseased, and that they also had postoperative changes. The structure of the thoracic spinal canal was disordered. Multiple irregular and nodular abnormal signals were found, so multiple lesions were considered. MRI examination of the lumbar spine showed that the spinous processes and laminas of the 12th thoracic vertebrae and the 2nd lumbar vertebral body were absent, that they also showed post
MRI examination of the pelvic cavity showed multiple abnormal cystic signals of different sizes in the pelvic cavity, and multiple compartments were found in the pelvic cavity. Multiple schwannomas were considered, and the largest lesions were approximately 75 mm × 60 mm × 83 mm (Figure 2).
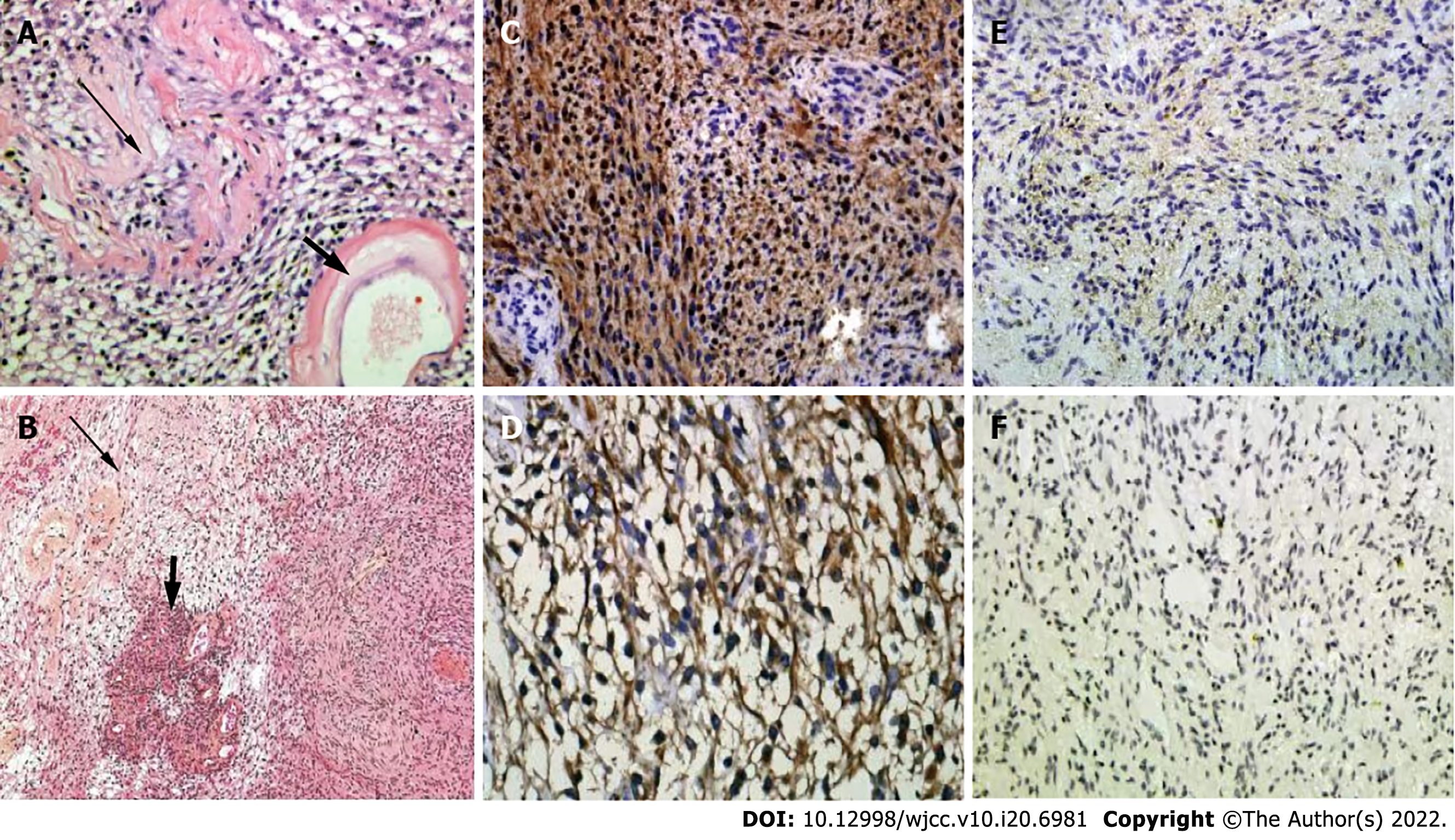
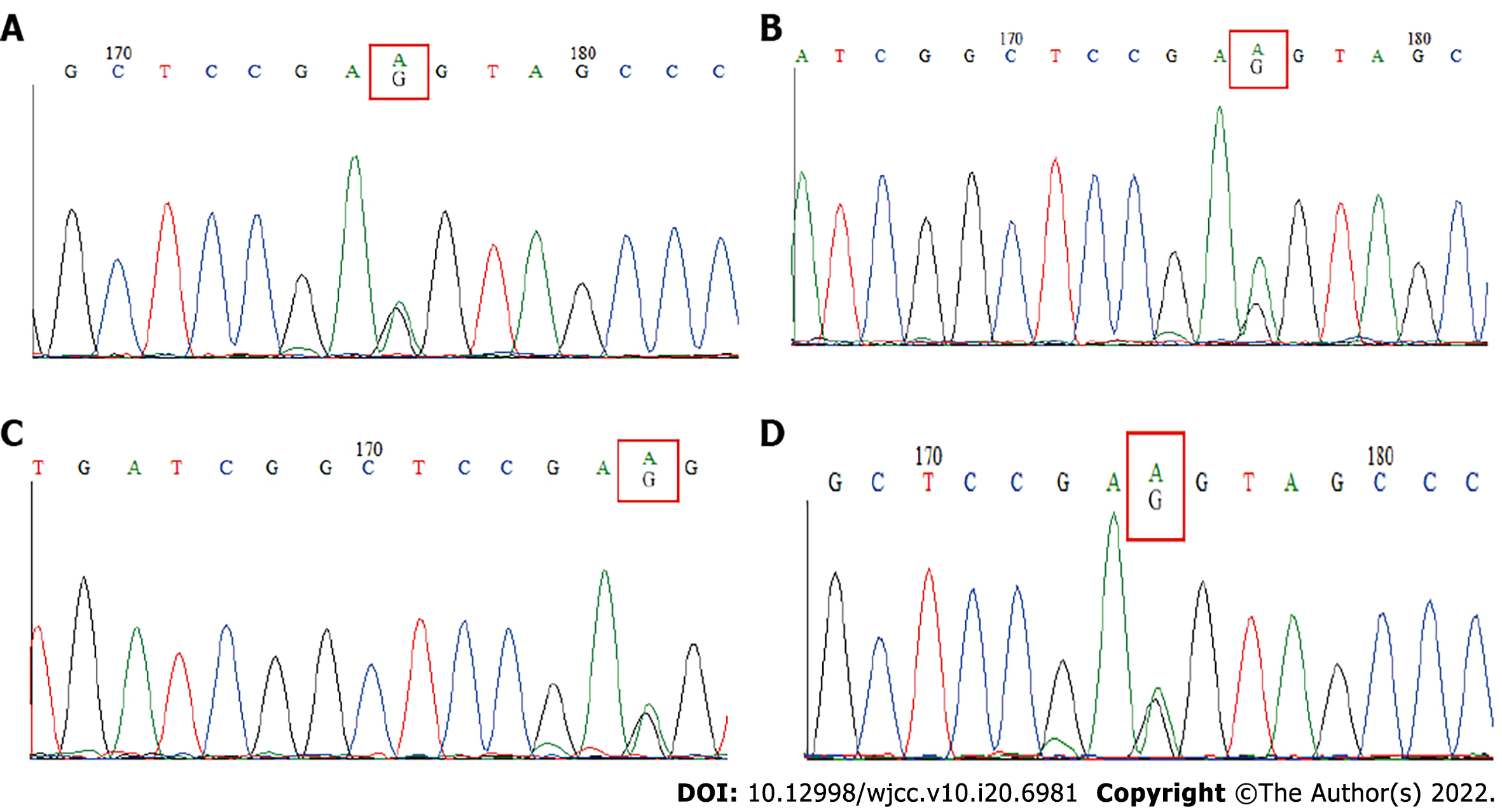
The combination of information about the patient's medical history, signs, imaging examination, previous pathological examinations, immunohistochemistry (Figure 3) and Sanger sequencing (Figure 4) led to schwannomatosis as the final diagnosis, hence neurofibromatosis type 1 (NF1) and neurofibromatosis type 2 (NF2) were excluded according to the international diagnostic criteria.
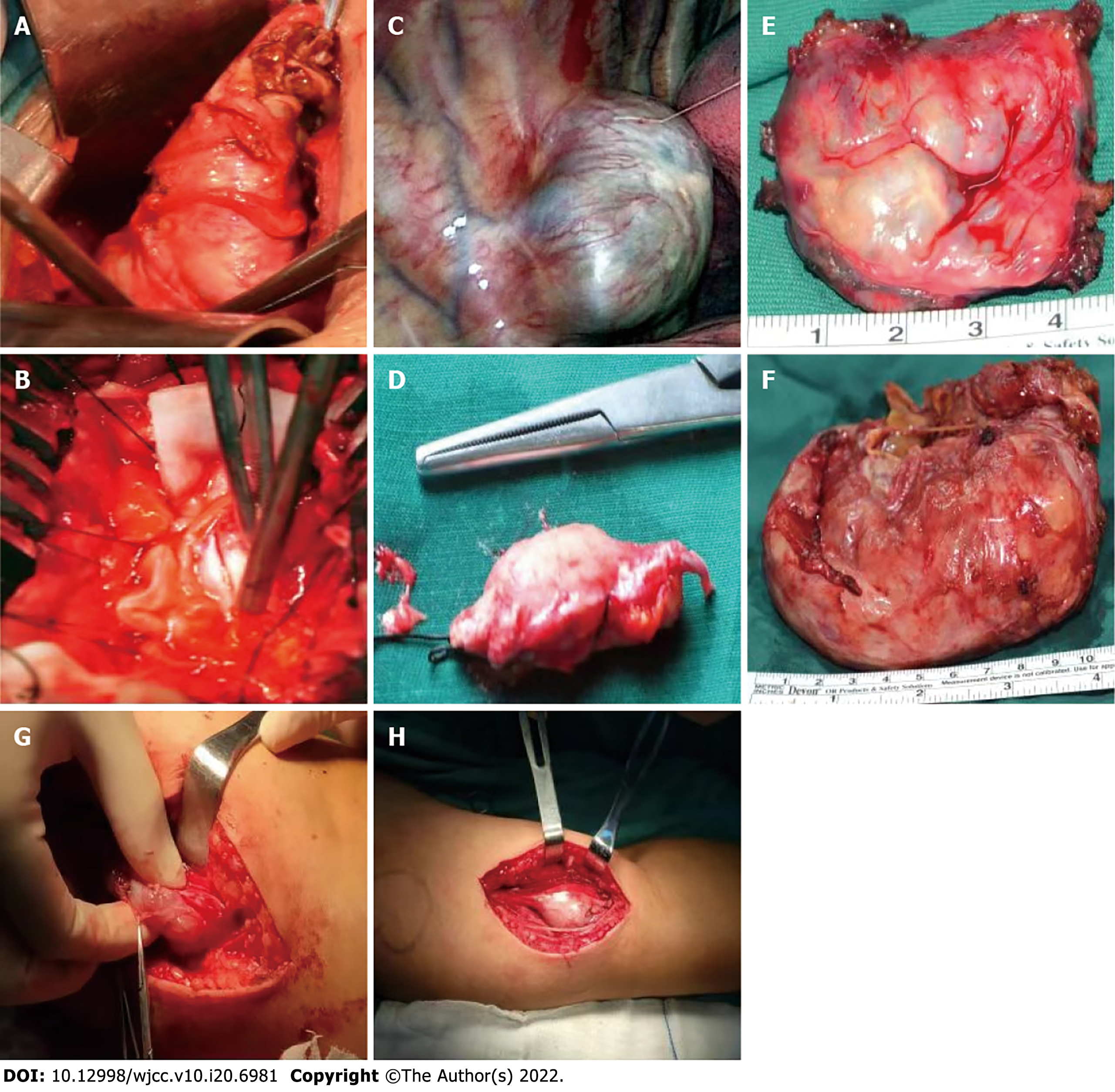
The patient had multiple tumors in his spinal cord and had bilateral lower extremity paralysis for 4 years, which resulted in a prolonged traumatic period for the patient, however no radical resection surgery was necessary. After considering the patient's needs, the patient's quality of life was improved, as much as possible, with treatment, and the remaining limb function was preserved. At present, the treatment resolved his pain and numbness that was present on the ulnar side of his left hand. After his symptoms improved in comparison with his chief complaints at the preoperative examination, we performed "tumor resection of the left upper limb + nerve exploration" on the third day after admission. During the operation, we found a tumor that was approximately 5 cm × 4 cm in size adjacent to the left ulnar nerve, and the mass was cystic and solid (Figure 5). After the tumor was completely removed, pathological examination showed schwannomatosis. The patient was given routine oral nutritional nerve drugs (mecobalamin) and nonsteroidal anti-inflammatory drugs (celecoxib). After the operation, the symptoms of pain and numbness in his left hand improved, and the patient recovered and was discharged.
On postoperative day 2, the patient's left hand pain and numbness had improved significantly in comparison with reports of his previous symptoms. On postoperative day 7, his surgical incision had healed, and he was discharged from the hospital. During the last telephone follow-up call on August 13, 2021, the patient was still alive; he had no new symptoms when compared to the previous follow-up exam, but his overall quality of life was poor.
Schwannomatosis is a tumor-prone syndrome characterized by multiple schwannomas, which often involve the spinal nerve and peripheral nervous system. Compared with NF1 and NF2, schwannomatosis is rare, and can be familial and sporadic. The absence of bilateral involvement of the vestibular nerves is an important difference between schwannomatosis and NF2. The pathogenesis of nerve sheath tumor disease is still unclear. The available studies suggest that abnormal expression of the SMARCB1, LZTR1 and NF2 genes is involved in the development of SWNTS[4]. The SMARCB1 gene is located on chromosome 22 and encodes the SMARCB1 protein. Highly conserved among eukaryotes and widely involved in epigenetic regulation, cell cycle progression, and signal pathway crosslinking, they are expressed proteins of a class of tumor suppressor genes[7]. The LZTR1 gene, also located on chromosome 22, encodes a protein that is a member of the BTB-Kelch superfamily and is active in the Golgi complex, an effector of the CULlin 3-containing E3 ubiquitin ligase complex[8,9]. The events involved include the ’4-hit/3-step’[10-12], the loss of heterozygosity of alleles related to mitotic recombination errors[13,14], stable mutation transcription after a nontruncated mutation[15], mRNA degradation or the restart of transcription mediated by a nonsense mutation[16], an abnormal N-terminal structure of SMARCB1 protein leading to incorrect action with DNA[17], etc. The most classic mutation is the "4 strikes and 3 steps" (4-hit/3-step): At first, a germline mutation occurs in the SMARCB1 or LZTR1 gene (the first strike), then a loss of heterozygosity occurs on chromosome 22, resulting in the loss of the second SMARCB1 or LZTR1 allele and the loss of one of the NF2 alleles (the second and third strikes), and finally, there is a somatic mutation of the remaining wild-type NF2 alleles (the fourth strike)[10-12].
The first diagnostic standard of schwannomatosis that reached a consensus was in 2005. With the continuous development of genetics and molecular biology, the latest diagnostic criteria and exclusion criteria are summarized (Table 2), according to the research of Plotkin et al[18], which is of great significance for clinical diagnosis. Schwannomatosis usually affects the spine (74%) and peripheral nerves (89%), and patients most often present with clinical manifestations of chronic pain (46%) or masses (27%), as well as neurological symptoms in the corresponding innervated areas after tumor compression of nerves. Pain is usually the first symptom and is the most challenging symptom for the treatment of this disease. Additionally, chronic pain and recurrence of the tumor lead to the need for multiple surgical procedures during the course of the patient's life, and these are the aspects that can lead to symptoms of depression and anxiety, which occur in approximately 17%-39% of patients[19]. This results in some patients being less willing to treat or giving up treatment.
| Clinical diagnosis | Combined molecular and clinical diagnosis | Exclusion criteria |
| ≥ 2 nonintradermal schwannomas, 1 pathologically confirmed schwannoma and absence of bilateral vestibular schwannomas Or 1 pathologically confirmed schwannoma or intracranial meningioma and 1 affected first-degree relative | ≥ 2 pathologically confirmed schwannomas or meningiomas; ≥ 2 tumors with 22q LOH and 2 different somatic NF2 mutations Or 1 pathologically confirmed schwannoma or meningioma; Germline SMARCB1 or LZTR1 pathogenic mutation | Germline pathogenic NF2 mutation; Diagnostic criteria for NF2 fulfilled; First-degree relative with NF2; Schwannomas occur exclusively in a region of previous radiation therapy |
In this case, pain symptoms began at age 19, neurological symptoms developed at age 24, and the patient underwent his first surgical treatment. Because schwannomatosis is multiple and prone to recurrence, the patient underwent three more surgeries in the following years. The tumor along the spinal nerve distribution, involving more parts, the operation difficulty is larger, and the tumor compression nerve causes repeated pain on the body torture, which makes the patient appear to have serious psychological disorders, and the enthusiasm of treatment is greatly reduced. After September 2013, due to personal reasons, the patient refused to receive further examination and treatment. Later, the patient showed a progressive decline in muscle strength of both lower limbs and failed to receive timely treatment. Long-term spinal nerve compression led to paralysis of both lower limbs and a severe reduction in his quality of life. During follow-up, the patient developed neck and bilateral upper limb symptoms. With the encouragement of his family and us, the patient was readmitted to the hospital in October 2018 for cervical spinal surgery to further preserve the patient's remaining limb functions. This time, the patient presented ulnar nerve compression symptoms on the ulnar side of the left forearm. After surgical treatment in the hospital, the patient's symptoms were significantly improved, and he recovered and was discharged, but the previous nerve injury of the lower limb could not be recovered.
Later, we summarized the treatment experience of schwannomatosis in the past 15 years, and the choice of surgical timing is crucial. Early surgery can relieve the compression of the spinal cord and nerve by tumor tissue as soon as possible to preserve limb function to the greatest extent and improve the quality of life. However, in the process of treatment, we often ignore the psychological and mental status of patients. Based on the characteristics of the disease itself, such as multiple recurrences, most patients need to undergo multiple surgical treatments and bear more surgical risks. In addition to the tumor tissue on the nerve compression, the patient's body has suffered pain for a long time, for patients with psychological and physical double blow, which often cause serious psychological disorders, depression and anxiety, leading to some patients losing confidence in treatment and even giving up treatment. This psychological disorder also causes patients to fail to see a doctor in time, thus missing the best treatment opportunity, resulting in irreversible damage to the nervous system and reducing the quality of life and survival rate. Unfortunately, during the five-year period from August 2013 to October 2018, the patient refused to further cooperate with us for treatment and therefore missed the best treatment opportunity. When the patient was admitted to the hospital again, he had already developed irreversible injury to the spinal cord and was paralyzed and bedridden, resulting in a serious decline in his quality of life.
At present, the main treatment of schwannoma is given priority with surgical cut method disease. Symptomatic treatment was observed in patients with asymptomatic schwannoma and regular follow-up, but for patients with spinal cord and peripheral nerve compression symptoms and early surgical resection, the goal is to remove the oppression in the spinal cord and peripheral nerves, and maximum retention dominates regional neural function. At the same time, we should unite with psychologists and must not ignore the psychological treatment of patients so that patients maintain a good state of mind and are more confident with our follow-up and treatment. In the field of drug treatment, most patients need to take analgesic drugs, such as amitriptyline, pregabalin and gabapentin, at a certain treatment stage to relieve the pain[19]. Although it has been reported that bevacizumab plays a certain role in the treatment of schwannomatosis, the use of bevacizumab needs to be further verified[20]. Therefore, in the future, the pathogenesis of this disease needs to be clarified, new immune drugs and new gene targets need to be found, and these will be an important direction to improve the treatment of this rare tumor.
Here, we report a rare case of schwannomatosis. We also conducted 15 years of patient follow-up and treatment, and analyzed the timing of surgery and patient psychology. This case will further increase our overall understanding of the diagnosis and treatment of this rare tumor.
We would like to thank Dr. Guo WT for his guidance and support of this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Garbuzenko DV, Russia; Gupta SK, India; Kung WM, Taiwan; Taha MM, Egypt A-Editor: Yao QG, China S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Evans DG, Bowers NL, Tobi S, Hartley C, Wallace AJ, King AT, Lloyd SKW, Rutherford SA, Hammerbeck-Ward C, Pathmanaban ON, Freeman SR, Ealing J, Kellett M, Laitt R, Thomas O, Halliday D, Ferner R, Taylor A, Duff C, Harkness EF, Smith MJ. Schwannomatosis: a genetic and epidemiological study. J Neurol Neurosurg Psychiatry. 2018;89:1215-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Smith MJ, Kulkarni A, Rustad C, Bowers NL, Wallace AJ, Holder SE, Heiberg A, Ramsden RT, Evans DG. Vestibular schwannomas occur in schwannomatosis and should not be considered an exclusion criterion for clinical diagnosis. Am J Med Genet A. 2012;158A:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Smith MJ, Bowers NL, Bulman M, Gokhale C, Wallace AJ, King AT, Lloyd SK, Rutherford SA, Hammerbeck-Ward CL, Freeman SR, Evans DG. Revisiting neurofibromatosis type 2 diagnostic criteria to exclude LZTR1-related schwannomatosis. Neurology. 2017;88:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Kehrer-Sawatzki H, Farschtschi S, Mautner VF, Cooper DN. The molecular pathogenesis of schwannomatosis, a paradigm for the co-involvement of multiple tumour suppressor genes in tumorigenesis. Hum Genet. 2017;136:129-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Wu J, Kong M, Bi Q. Identification of a novel germline SMARCB1 nonsense mutation in a family manifesting both schwannomatosis and unilateral vestibular schwannoma. J Neurooncol. 2015;125:439-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Gonzalvo A, Fowler A, Cook RJ, Little NS, Wheeler H, McDonald KL, Biggs MT. Schwannomatosis, sporadic schwannomatosis, and familial schwannomatosis: a surgical series with long-term follow-up. Clinical article. J Neurosurg. 2011;114:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Versteege I, Sévenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, Aurias A, Delattre O. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1161] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 8. | Yaniv M. Chromatin remodeling: from transcription to cancer. Cancer Genet. 2014;207:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Geller JI, Roth JJ, Biegel JA. Biology and Treatment of Rhabdoid Tumor. Crit Rev Oncog. 2015;20:199-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Boyd C, Smith MJ, Kluwe L, Balogh A, Maccollin M, Plotkin SR. Alterations in the SMARCB1 (INI1) tumor suppressor gene in familial schwannomatosis. Clin Genet. 2008;74:358-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Hadfield KD, Newman WG, Bowers NL, Wallace A, Bolger C, Colley A, McCann E, Trump D, Prescott T, Evans DG. Molecular characterisation of SMARCB1 and NF2 in familial and sporadic schwannomatosis. J Med Genet. 2008;45:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Sestini R, Bacci C, Provenzano A, Genuardi M, Papi L. Evidence of a four-hit mechanism involving SMARCB1 and NF2 in schwannomatosis-associated schwannomas. Hum Mutat. 2008;29:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Garcia-Linares C, Fernández-Rodríguez J, Terribas E, Mercadé J, Pros E, Benito L, Benavente Y, Capellà G, Ravella A, Blanco I, Kehrer-Sawatzki H, Lázaro C, Serra E. Dissecting loss of heterozygosity (LOH) in neurofibromatosis type 1-associated neurofibromas: Importance of copy neutral LOH. Hum Mutat. 2011;32:78-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Stewart DR, Pemov A, Van Loo P, Beert E, Brems H, Sciot R, Claes K, Pak E, Dutra A, Lee CC, Legius E. Mitotic recombination of chromosome arm 17q as a cause of loss of heterozygosity of NF1 in neurofibromatosis type 1-associated glomus tumors. Genes Chromosomes Cancer. 2012;51:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Smith MJ, Walker JA, Shen Y, Stemmer-Rachamimov A, Gusella JF, Plotkin SR. Expression of SMARCB1 (INI1) mutations in familial schwannomatosis. Hum Mol Genet. 2012;21:5239-5245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Hulsebos TJ, Kenter S, Verhagen WI, Baas F, Flucke U, Wesseling P. Premature termination of SMARCB1 translation may be followed by reinitiation in schwannomatosis-associated schwannomas, but results in absence of SMARCB1 expression in rhabdoid tumors. Acta Neuropathol. 2014;128:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Allen MD, Freund SM, Zinzalla G, Bycroft M. The SWI/SNF Subunit INI1 Contains an N-Terminal Winged Helix DNA Binding Domain that Is a Target for Mutations in Schwannomatosis. Structure. 2015;23:1344-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Plotkin SR, Blakeley JO, Evans DG, Hanemann CO, Hulsebos TJ, Hunter-Schaedle K, Kalpana GV, Korf B, Messiaen L, Papi L, Ratner N, Sherman LS, Smith MJ, Stemmer-Rachamimov AO, Vitte J, Giovannini M. Update from the 2011 International Schwannomatosis Workshop: From genetics to diagnostic criteria. Am J Med Genet A. 2013;161A:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Merker VL, Esparza S, Smith MJ, Stemmer-Rachamimov A, Plotkin SR. Clinical features of schwannomatosis: a retrospective analysis of 87 patients. Oncologist. 2012;17:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Blakeley J, Schreck KC, Evans DG, Korf BR, Zagzag D, Karajannis MA, Bergner AL, Belzberg AJ. Clinical response to bevacizumab in schwannomatosis. Neurology. 2014;83:1986-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |