Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6927
Peer-review started: December 5, 2021
First decision: January 25, 2022
Revised: January 26, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: July 16, 2022
Processing time: 211 Days and 23.1 Hours
The prognostic role of the skeletal muscle mass index (SMI) derived from computed tomography (CT) imaging been well verified in several types of cancers. However, whether the SMI could serve as a reliable and valuable pre
To identify the prognostic value of the CT-derived SMI in lung cancer patients.
The PubMed, Web of Science, and Embase electronic databases were searched up to November 5, 2021 for relevant studies. The Reference Citation Analysis data
A total of 12 studies involving 3002 patients were included. The pooled results demonstrated that a lower SMI was significantly related to poorer OS (HR = 1.23, 95%CI: 1.11-1.37, P < 0.001). In addition, the subgroup analyses stratified by treatment (nonsurgery vs surgery), tumor stage (advanced stage vs early stage), and tumor type (non-small cell lung cancer vs lung cancer) showed similar results.
The CT-derived SMI is a novel and valuable prognostic indicator in lung cancer and might contribute to the clinical management and treatment of lung cancer patients.
Core Tip: We searched the PubMed, Web of Science, and Embase electronic databases up to November 5, 2021, and a total of 12 studies involving 3002 patients were included. The pooled results demonstrated that a lower skeletal muscle mass index (SMI) was significantly related to poorer overall survival (P < 0.001). In addition, the subgroup analyses stratified by treatment (nonsurgery vs surgery), tumor stage (advanced stage vs early stage), and tumor type (non-small cell lung cancer vs lung cancer) showed similar results. The computed tomography-derived SMI is a novel and valuable prognostic indicator in lung cancer and might contribute to the clinical management and treatment of lung cancer patients.
- Citation: Pan XL, Li HJ, Li Z, Li ZL. Prognostic value of computed tomography derived skeletal muscle mass index in lung cancer: A meta-analysis. World J Clin Cases 2022; 10(20): 6927-6935
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6927.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6927
Lung cancer is the leading cause of tumor-related deaths worldwide and can be categorized into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC)[1,2]. Despite considerable advances in the clinical diagnosis, treatment, and management of lung cancer, the overall prognosis of lung cancer patients remains poor[3,4]. The tumor-node-metastasis (TNM) staging system is still the most authoritative tool to assess the disease severity and prognosis of lung cancer patients. However, in addition to disease stage, the prognosis of lung cancer patients can be affected or predicted by many factors.
In recent years, an increasing number of common clinical indicators have been identified to play a role in the evaluation of long-term survival in lung cancer, such as the D-dimer level, albumin-to-globulin ratio (AGR), lymphocyte-to-monocyte ratio, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio[5-8]. However, these blood indicators are unstable and may be changed by a number of factors or diseases. There are also some other stable prognostic indicators, such as ctDNA and circulating tumor cells[9-11], but they are relatively expensive and cannot be widely applied in clinics.
The skeletal muscle mass index (SMI) is calculated according to computed tomography (CT) images and can reflect the nutritional status of the body to a large extent. In addition, the two indicators, the area of skeletal muscle and height, involved in the calculation of SMI are both stable and reliable. The prognostic value of SMI in several cancers has been identified, such as gastric cancer, colorectal cancer, pancreatic adenocarcinoma, and renal cell carcinoma[12-16]. However, whether SMI could serve as a reliable and valuable prognostic index in lung cancer remains unclear.
Thus, the aim of this meta-analysis was to assess the prognostic role of CT-derived SMI in lung cancer, which might contribute to the evaluation of long-term survival and the formulation of therapy strategies for lung cancer patients.
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020) checklist and has been registered in PROSPERO.
The PubMed, Web of Science, and Embase electronic databases were searched from inception to November 5, 2021. The search strategy consisted of Medical Subject Heading terms and free-text terms with logical operators. The following terms were used during the literature search: Skeletal muscle mass index, SMI, lung, pulmonary, tumor, cancer, carcinoma, neoplasm, prognostic, survival, and prognosis. In detail, the specific search strategy was as follows: (Skeletal muscle mass index OR SMI) AND (lung OR pulmonary) AND (tumor OR cancer OR carcinoma OR neoplasm) AND (prognostic OR survival OR prognosis). In addition, the reference lists of the included studies were searched to identify additional eligible studies.
The inclusion criteria were as follows: (1) Patients were pathologically diagnosed with lung cancer; (2) The SMI was calculated through CT images before antitumor treatment; and (3) The association between the SMI and overall survival (OS) was explored and assessed by hazard ratios (HRs) with 95% confidence intervals (CIs).
The exclusion criteria were as follows: (1) The HRs with 95%CIs were not directly reported in articles; (2) Reviews, meeting abstracts, letters, editorials, or case reports; and (3) Overlapping or duplicated data.
The Reference Citation Analysis databases were used during the literature searching and selection.
The following information was collected from the included studies: The first author, publication year, country, sample size, treatment (nonsurgery vs surgery), TNM stage, cutoff value of the SMI, tumor type, and HR with corresponding 95%CI.
The quality of the included studies was evaluated according to the Newcastle Ottawa Scale (NOS), and studies with an NOS score of 6 or higher were defined as high-quality studies[17].
The literature retrieval, selection, data extraction, and quality assessment were all conducted by two investigators independently. Any disagreement was resolved by team discussion.
All statistical analyses were conducted with STATA 12.0 software (College Station, TX, United States). The HRs with 95%CIs were calculated to assess the association between the SMI and OS. Heterogeneity was evaluated by Cochran’s Q test and Higgins I2 statistic; P < 0.10 and/or I2 > 50% were defined as significant heterogeneity among studies, and the random effects model was applied for the pooled effect estimates; otherwise, the fixed effects model was used[18]. Subgroup analyses stratified by the treatment, tumor stage, and tumor type were further conducted. Sensitivity analysis for OS was performed by removing individual studies from the meta-analysis each time. Begg’s funnel plot and Egger’s test were conducted to evaluate publication bias. Significant publication bias was defined as a P-value less than 0.05, and the trim-and-fill method was applied to assess the influence of potentially unpublished papers on the stability of the pooled results[19].
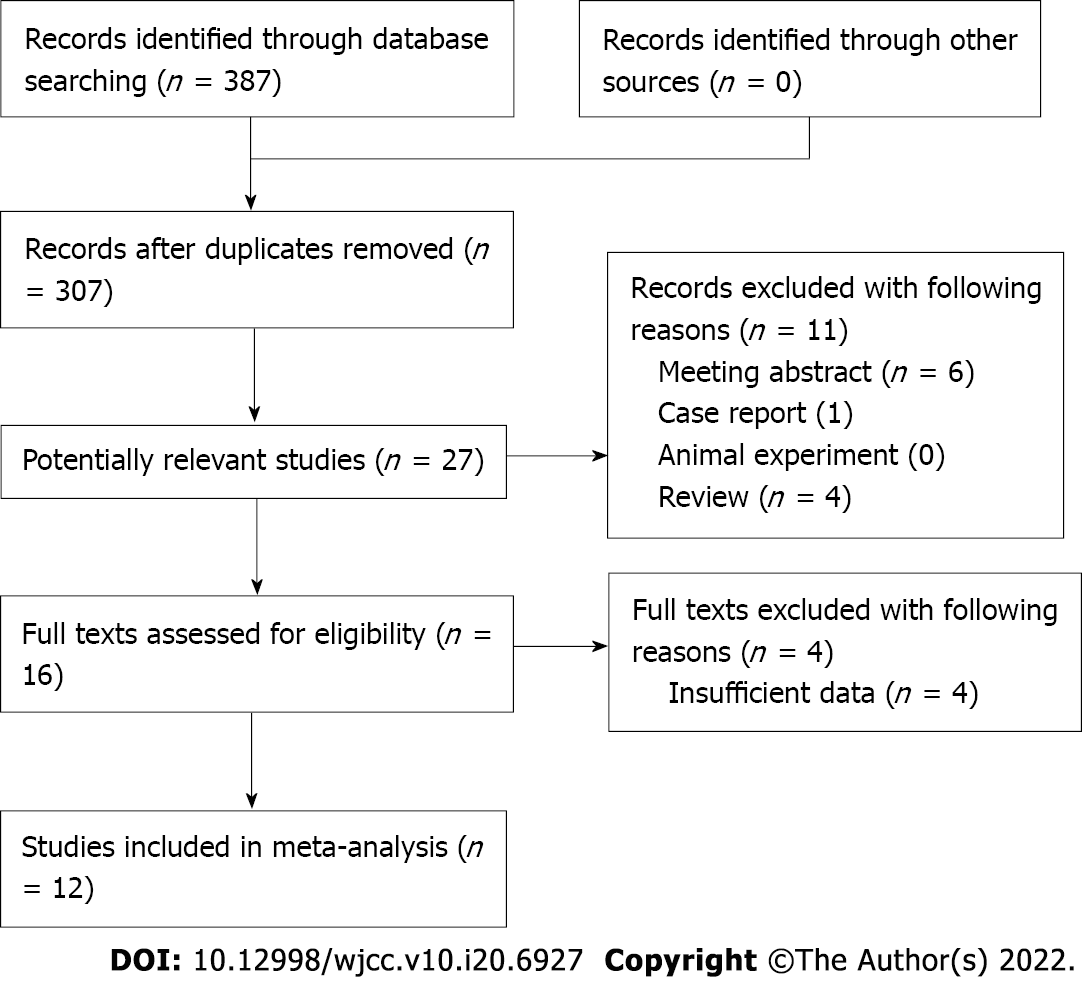
The detailed literature retrieval and selection process is presented in Figure 1. Ultimately, a total of 12 relevant retrospective studies were included in this meta-analysis[20-31].
A total of 3002 lung cancer patients were enrolled among the 12 studies, with sample sizes ranging from 46 to 734. In most included studies, the patients were diagnosed at an advanced stage and received nonsurgical treatment. In addition, most studies only included NSCLC patients, and all studies were of high quality, with an NOS score of 6 or higher (Table 1).
| Ref. | Year | Country | Sample size | Treatment | TNM stage | Threshold of SMI (cm2/m2) | Tumor type | NOS |
| Jafri et al[20] | 2015 | United States | 112 | Non-surgery | IV | 40 | NSCLC | 7 |
| Suzuki et al[22] | 2016 | Japan | 90 | Surgery | I | Male: 43.75; female: 41.10 | NSCLC | 7 |
| Sjøblom et al[21] | 2016 | Norway | 734 | Non-surgery | III-IV | NR | NSCLC | 7 |
| Shoji et al[24] | 2017 | Japan | 147 | Surgery | I | Male: 43.75; female: 41.10 | NSCLC | 7 |
| Nattenmüller et al[23] | 2017 | Germany | 200 | Non-surgery | I-IV | NR | LC | 7 |
| Roch et al[28] | 2020 | France | 142 | Non-surgery | NR | Male: 52.4; female: 38.5 | NSCLC | 6 |
| Abbass et al[25] | 2020 | United Kingdom | 643 | Non-surgery | III-IV | Male: 43; female: 41 | LC | 6 |
| Dolan et al[26] | 2020 | United Kingdom | 119 | Non-surgery | I-III | Male: 53; female: 41 | NSCLC | 6 |
| Magri et al[27] | 2019 | Israel | 46 | Non-surgery | IV | NR | LC | 6 |
| Katsui et al[29] | 2021 | Japan | 60 | Non-surgery | III | Male: 43; female: 24 | NSCLC | 7 |
| Lee et al[30] | 2021 | Republic of Korea | 70 | Non-surgery | IIIB-IV | Male: 46; female: 29 | SCC | 6 |
| Yang et al[31] | 2021 | China | 639 | Non-surgery | IIIB-IV | Male: 32.48; female: 27.82 | NSCLC | 7 |
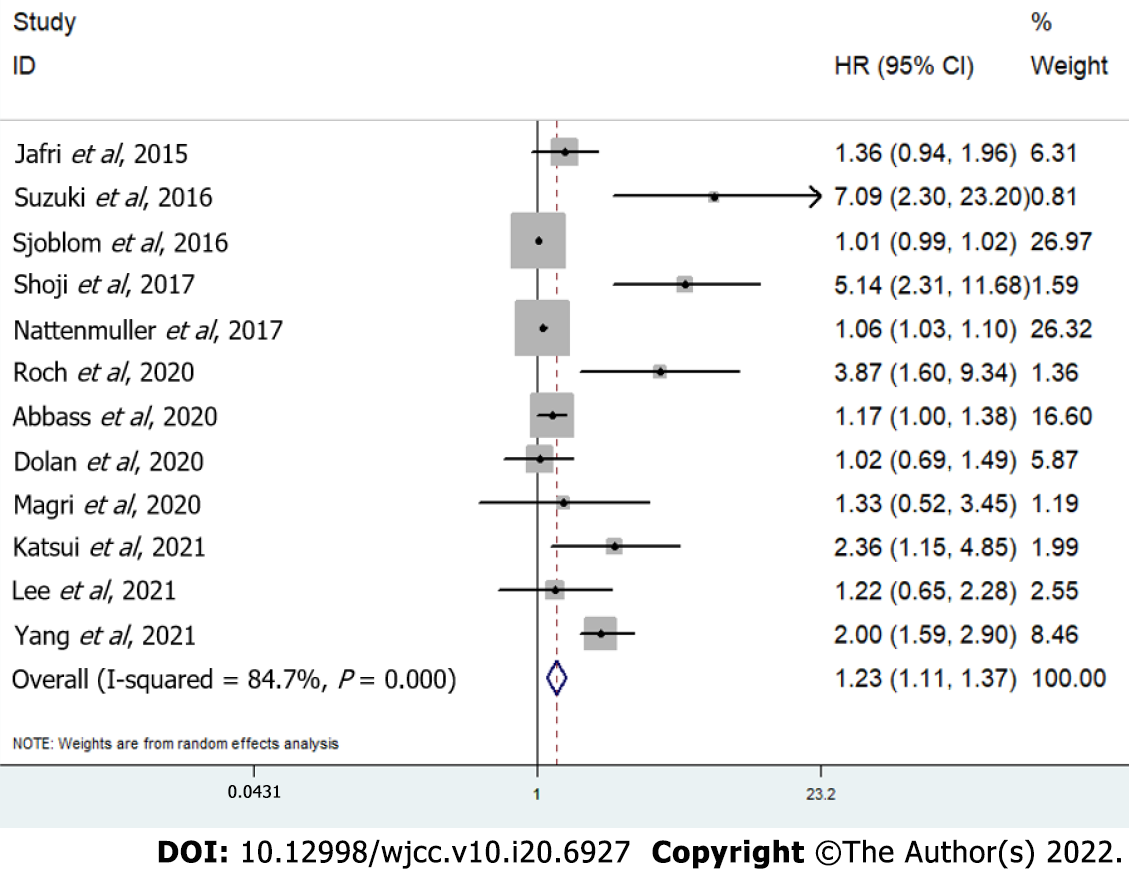
The pooled results demonstrated that a lower SMI was significantly related to poorer OS in lung cancer patients (HR = 1.23, 95%CI: 1.11-1.37, P < 0.001; I2 = 84.7%, P < 0.001) (Figure 2). Then, subgroup analyses based on the treatment [nonsurgery (HR = 1.15, 95%CI: 1.06-1.26, P = 0.002) vs surgery (HR = 5.71, 95%CI: 2.94-11.10, P < 0.001)], tumor stage [advanced stage (HR = 1.34, 95%CI: 1.07-1.68, P = 0.011) vs early stage (HR = 5.71, 95%CI: 2.94-11.10, P < 0.001)], and tumor type [NSCLC (HR = 1.97, 95%CI: 1.33-2.93, P = 0.001) vs lung cancer (HR = 1.07, 95%CI: 1.03-1.11, P < 0.001)] were performed, which showed similar results (Table 2). In addition, according to the subgroup analysis, the treatment strategy and tumor stage might be potential sources of heterogeneity (Table 2).
| No. of studies | HR | 95%CI | P value | I2 (%) | P value | |
| Overall survival | 12 | 1.23 | 1.11-1.37 | < 0.001 | 84.7 | < 0.001 |
| Treatment | ||||||
| Non-surgery | 10 | 1.15 | 1.06-1.26 | 0.002 | 80.4 | < 0.001 |
| Surgery | 2 | 5.71 | 2.94-11.10 | < 0.001 | 0.0 | 0.655 |
| Tumor stage | ||||||
| Advanced stage | 7 | 1.34 | 1.07-1.68 | 0.011 | 80.8 | < 0.001 |
| Early stage | 2 | 5.71 | 2.94-11.10 | < 0.001 | 0.0 | 0.655 |
| Tumor type | ||||||
| Non-small cell lung cancer | 9 | 1.97 | 1.33-2.93 | 0.001 | 87.3 | < 0.001 |
| Lung cancer | 3 | 1.07 | 1.03-1.11 | < 0.001 | 0.0 | 0.471 |
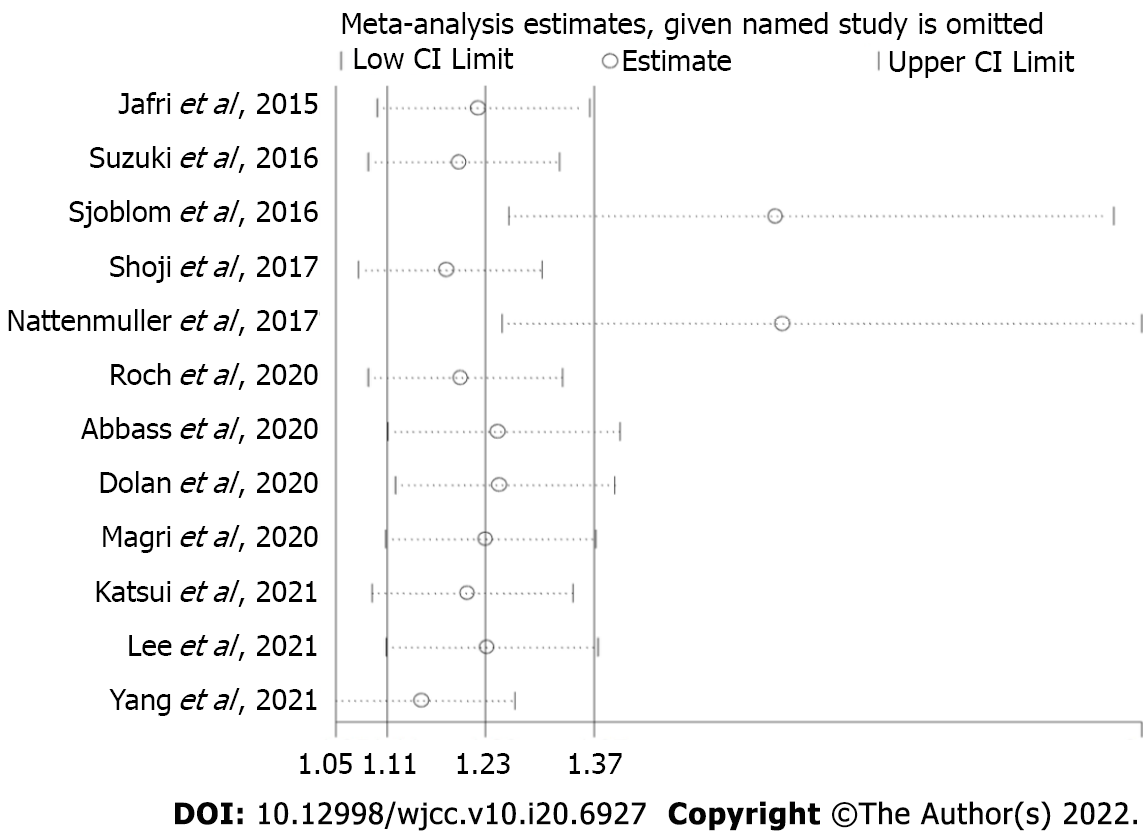
The sensitivity analysis indicated that the results of this meta-analysis were stable and that none of the included studies had a significant impact on the overall results (Figure 3).
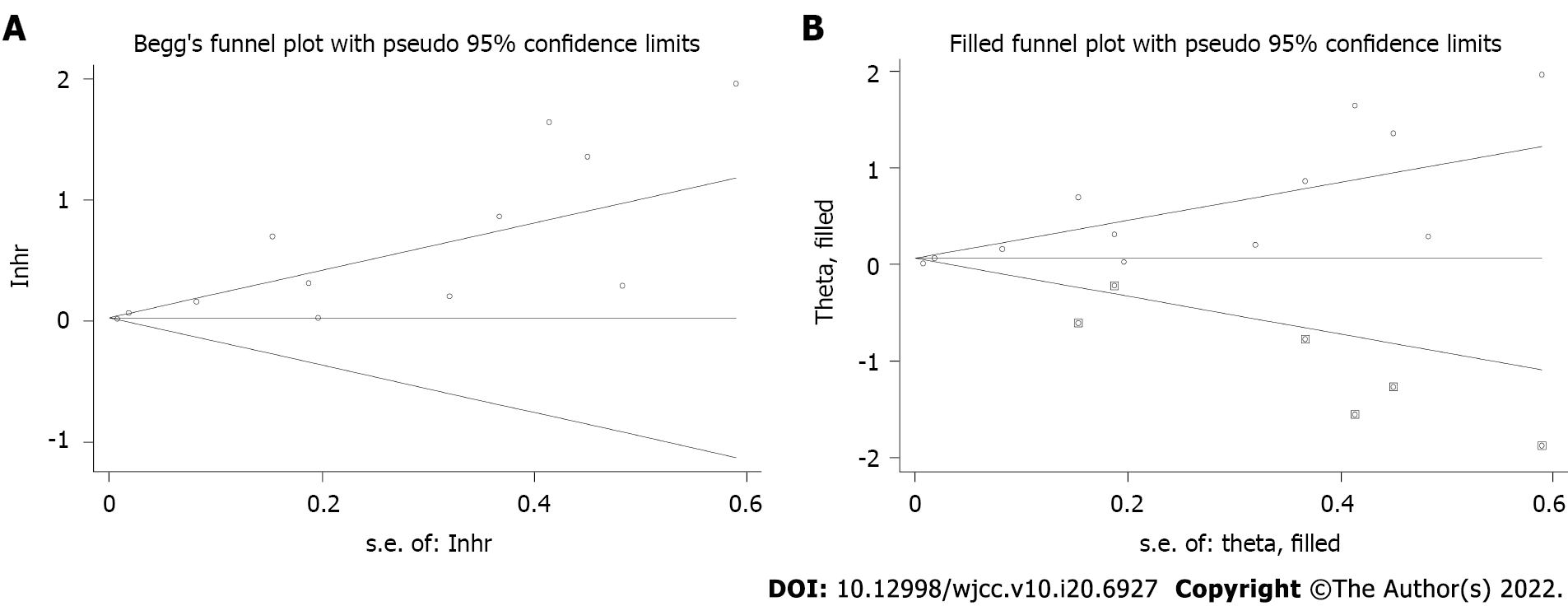
Begg’s funnel plot was asymmetric (Figure 4A), and Egger’s test was significant (P < 0.001); therefore, significant publication bias was observed. The trim-and-fill method was used to detect potentially unpublished articles and their impact on the overall results. Six potentially unpublished papers were identified (Figure 4B), and the pooled HR was 1.019 (95%CI: 1.005-1.033, P = 0.006) and 1.063 (95%CI: 0.949-1.192, P = 0.293) after combining these six studies, respectively. Thus, the six potentially unpublished studies might impact the overall results, and more high-quality studies are still needed to verify the above findings.
The current meta-analysis demonstrated that a lower pretreatment CT-derived SMI was significantly associated with poorer OS in lung cancer patients and might serve as a reliable and valuable prognostic indicator in lung cancer. The results of subgroup analyses based on the treatment, tumor stage, and tumor type all further verified the above findings.
The SMI is a novel indicator reflecting nutritional status, and it is well known that the nutritional condition of the body is essential for the prognosis of lung cancer patients. The clinical role of a number of nutritional indicators has been widely explored in lung cancer. Li et al[6] included eight studies involving 3496 patients and demonstrated that a low pretreatment AGR was a predictor of poor OS (HR = 1.88, 95%CI: 1.49-2.38, P < 0.001) and disease-free survival (DFS) (HR = 2.09, 95%CI: 1.56-2.81, P < 0.001) in lung cancer[6]. In addition, Li et al[32] included ten relevant studies involving 5085 patients and showed that a low prognostic nutritional index calculated based on the peripheral serum albumin level and total lymphocyte count was significantly related to unfavorable OS (HR = 1.72, 95%CI: 1.43-2.06, P = 0.000) in lung cancer, especially in NSCLC (HR = 1.93, 95%CI: 1.56-2.37, P = 0.000)[32]. Furthermore, a high pretreatment controlling nutritional status score calculated based on the peripheral serum albumin level, total blood cholesterol level, and total lymphocyte count was identified to be positively correlated with poor OS (HR = 1.63, 95%CI: 1.40-1.88, P < 0.001), DFS/recurrence-free survival (HR = 1.65, 95%CI: 1.35-2.01, P < 0.001), and postoperative complications (odds ratio = 1.58, 95%CI: 1.21-2.06, P = 0.001) in NSCLC patients[33]. However, the clinical application of these indices is severely limited because they are unstable and could be affected by many factors.
In most of the included studies, the patients were divided into high or low SMI groups according to the values of SMI. However, the thresholds of SMI in the included studies were different, which means that the optimal cutoff values of SMI in different groups of lung cancer should be inconsistent. Although most relevant studies differentiated cutoff values based on sex, we deem that age should also be considered because age is a very important factor affecting the basic nutritional status. Thus, more rigorously differentiated thresholds should be applied in future relevant studies. In addition, SCLC is a pathological type with a high degree of malignancy and rapid progression, and most SCLC patients are diagnosed at an advanced stage. SCLC patients are prone to recurrence and metastasis, and the application of the current staging system for SCLC is extremely limited clinically. Unfortunately, none of the included studies focused on this type of lung cancer and explored the prognostic value of the SMI in SCLC. However, we believe that the SMI might be a novel and valuable predictor of survival and therapeutic effects in SCLC patients. Thus, we hope that more scholars could pay attention to the clinical role of the SMI in SCLC in the future.
There are several limitations in this meta-analysis. First, all included studies were retrospective, and the sample sizes were relatively small. Second, more specific subgroup analyses could not be conducted due to the lack of detailed data. Third, significant heterogeneity was observed in our meta-analysis, but the sources of heterogeneity were not identified.
The CT-derived SMI is a novel and valuable prognostic indicator in lung cancer and might contribute to the clinical management and treatment of lung cancer patients. However, more prospective high-quality studies are still needed to verify the above findings.
The prognostic role of the skeletal muscle mass index (SMI) calculated through computed tomography (CT) images in several types of cancers has been demonstrated.
Whether the SMI could serve as a reliable and valuable predictor for long-term survival in lung cancer remains unclear.
To verify the prognostic value of the CT-derived SMI in lung cancer patients.
Several electronic databases were searched up to November 5, 2021 for relevant studies. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to assess the association of the SMI with the overall survival (OS) of lung cancer patients. All statistical analyses were performed with STATA 12.0 software.
The pooled results demonstrated that a lower SMI was significantly related to poorer OS (HR = 1.23, 95%CI: 1.11-1.37, P < 0.001). In addition, the subgroup analyses stratified by treatment (nonsurgery vs surgery), tumor stage (advanced stage vs early stage), and tumor type (non-small cell lung cancer vs lung cancer) showed similar results.
The CT-derived SMI is a novel and valuable prognostic indicator in lung cancer.
The SMI might contribute to the clinical management and treatment of lung cancer patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Geng J, China; Ozdemir HI, Turkey A-Editor: Liu X, China S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 1775] [Article Influence: 443.8] [Reference Citation Analysis (1)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2930] [Article Influence: 732.5] [Reference Citation Analysis (7)] |
| 3. | American Cancer Society Cancer Statistics 2021 Report. J Nucl Med. 2021;62:12N. [PubMed] |
| 4. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11902] [Article Influence: 2975.5] [Reference Citation Analysis (4)] |
| 5. | Li J, Wang Y, Li J, Che G. Prognostic Value of Pretreatment D-Dimer Level in Small-Cell Lung Cancer: A Meta-Analysis. Technol Cancer Res Treat. 2021;20:1533033821989822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Li J, Wang Y, Wu Y, Li J, Che G. Prognostic Value of Pretreatment Albumin to Globulin Ratio in Lung Cancer: A Meta-Analysis. Nutr Cancer. 2021;73:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Hu X, Xu W, Wang H, Huang Y, Che G. Prognostic value of a novel scoring system using inflammatory response biomarkers in non-small cell lung cancer: A retrospective study. Thorac Cancer. 2019;10:1402-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Huang D, Xu WY, Wang YW, Che GW. Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Non-Small Cell Lung Cancer: A Meta-Analysis. Oncol Res Treat. 2019;42:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Xia L, Mei J, Kang R, Deng S, Chen Y, Yang Y, Feng G, Deng Y, Gan F, Lin Y, Pu Q, Ma L, Lin F, Yuan Y, Hu Y, Guo C, Liao H, Liu C, Zhu Y, Wang W, Liu Z, Xu Y, Li K, Li C, Li Q, He J, Chen W, Zhang X, Kou Y, Wang Y, Wu Z, Che G, Chen L, Liu L. Perioperative ctDNA-Based Molecular Residual Disease Detection for Non-Small Cell Lung Cancer: A Prospective Multicenter Cohort Study (LUNGCA-1). Clin Cancer Res. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 10. | Li H, Li B, Pan Y, Zhang Y, Xiang J, Sun Y, Yu X, He W, Hu H. Preoperative Folate Receptor-Positive Circulating Tumor Cell Level Is a Prognostic Factor of Long Term Outcome in Non-Small Cell Lung Cancer Patients. Front Oncol. 2020;10:621435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Yang L, Yan X, Chen J, Zhan Q, Hua Y, Xu S, Li Z, Wang Z, Dong Y, Zuo D, Xue M, Tang Y, Herschman HR, Lu S, Shi Q, Wei W. Hexokinase 2 discerns a novel circulating tumor cell population associated with poor prognosis in lung cancer patients. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Kim EY, Jun KH, Kim SY, Chin HM. Body mass index and skeletal muscle index are useful prognostic factors for overall survival after gastrectomy for gastric cancer: Retrospective cohort study. Medicine (Baltimore). 2020;99:e23363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Kang Z, Cheng L, Li K, Shuai Y, Xue K, Zhong Y, Chen L. Correlation between L3 skeletal muscle index and prognosis of patients with stage IV gastric cancer. J Gastrointest Oncol. 2021;12:2073-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Lee J, Suh J, Song C, You D, Jeong IG, Hong B, Hong JH, Kim CS, Ahn H. Association Between Sarcopenia and Survival of Patients with Organ-Confined Renal Cell Carcinoma after Radical Nephrectomy. Ann Surg Oncol. 2022;29:2473-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Malik M, Michalak M, Radecka B, Gełej M, Jackowska A, Filipczyk-Cisarż E, Hetman K, Foszczyńska-Kłoda M, Kania-Zembaczyńska B, Mańka D, Orlikowska M, Rogowska-Droś H, Bodnar L. Prognostic Value of Sarcopenia in Metastatic Colorectal Cancer Patients Treated with Trifluridine/Tipiracil. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Rom H, Tamir S, Van Vugt JLA, Berger Y, Perl G, Morgenstern S, Tovar A, Brenner B, Benchimol D, Kashtan H, Sadot E. Sarcopenia as a Predictor of Survival in Patients with Pancreatic Adenocarcinoma After Pancreatectomy. Ann Surg Oncol. 2022;29:1553-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 2017;5:80-84. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 554] [Article Influence: 69.3] [Reference Citation Analysis (82)] |
| 18. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46343] [Article Influence: 2106.5] [Reference Citation Analysis (3)] |
| 19. | Wang Y, Li J, Chang S, Dong Y, Che G. Risk and Influencing Factors for Subsequent Primary Lung Cancer After Treatment of Breast Cancer: A Systematic Review and Two Meta-Analyses Based on Four Million Cases. J Thorac Oncol. 2021;16:1893-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 20. | Jafri SH, Previgliano C, Khandelwal K, Shi R. Cachexia Index in Advanced Non-Small-Cell Lung Cancer Patients. Clin Med Insights Oncol. 2015;9:87-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Sjøblom B, Grønberg BH, Wentzel-Larsen T, Baracos VE, Hjermstad MJ, Aass N, Bremnes RM, Fløtten Ø, Bye A, Jordhøy M. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr. 2016;35:1386-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Suzuki Y, Okamoto T, Fujishita T, Katsura M, Akamine T, Takamori S, Morodomi Y, Tagawa T, Shoji F, Maehara Y. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer. 2016;101:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Nattenmüller J, Wochner R, Muley T, Steins M, Hummler S, Teucher B, Wiskemann J, Kauczor HU, Wielpütz MO, Heussel CP. Prognostic Impact of CT-Quantified Muscle and Fat Distribution before and after First-Line-Chemotherapy in Lung Cancer Patients. PLoS One. 2017;12:e0169136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Shoji F, Matsubara T, Kozuma Y, Haratake N, Akamine T, Takamori S, Katsura M, Toyokawa G, Okamoto T, Maehara Y. Relationship Between Preoperative Sarcopenia Status and Immuno-nutritional Parameters in Patients with Early-stage Non-small Cell Lung Cancer. Anticancer Res. 2017;37:6997-7003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Abbass T, Dolan RD, MacLeod N, Horgan PG, Laird BJ, McMillan DC. Comparison of the prognostic value of MUST, ECOG-PS, mGPS and CT derived body composition analysis in patients with advanced lung cancer. Clin Nutr ESPEN. 2020;40:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Dolan RD, Maclay JD, Abbass T, Colville D, Buali F, MacLeod N, McSorley ST, Horgan PG, McMillan DC. The relationship between 18F-FDG-PETCT-derived tumour metabolic activity, nutritional risk, body composition, systemic inflammation and survival in patients with lung cancer. Sci Rep. 2020;10:20819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Magri V, Gottfried T, Di Segni M, Urban D, Peled M, Daher S, Stoff R, Bar J, Onn A. Correlation of body composition by computerized tomography and metabolic parameters with survival of nivolumab-treated lung cancer patients. Cancer Manag Res. 2019;11:8201-8207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Roch B, Coffy A, Jean-Baptiste S, Palaysi E, Daures JP, Pujol JL, Bommart S. Cachexia - sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer. 2020;143:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 29. | Katsui K, Ogata T, Sugiyama S, Yoshio K, Kuroda M, Hiraki T, Kiura K, Maeda Y, Toyooka S, Kanazawa S. Sarcopenia is associated with poor prognosis after chemoradiotherapy in patients with stage III non-small-cell lung cancer: a retrospective analysis. Sci Rep. 2021;11:11882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Lee J, Kim EY, Kim E, Kim KG, Kim YJ, Kim YS, Ahn HK, Lee SW. Longitudinal changes in skeletal muscle mass in patients with advanced squamous cell lung cancer. Thorac Cancer. 2021;12:1662-1667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Yang M, Tan L, Xie L, Hu S, Liu D, Wang J, Li W. Factors That Improve Chest Computed Tomography-Defined Sarcopenia Prognosis in Advanced Non-Small Cell Lung Cancer. Front Oncol. 2021;11:754975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Li D, Yuan X, Liu J, Li C, Li W. Prognostic value of prognostic nutritional index in lung cancer: a meta-analysis. J Thorac Dis. 2018;10:5298-5307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Peng J, Hao Y, Rao B, Cao Y. Prognostic impact of the pre-treatment controlling nutritional status score in patients with non-small cell lung cancer: A meta-analysis. Medicine (Baltimore). 2021;100:e26488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |