Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6890
Peer-review started: November 23, 2021
First decision: January 22, 2022
Revised: February 3, 2022
Accepted: May 16, 2022
Article in press: May 16, 2022
Published online: July 16, 2022
Processing time: 223 Days and 21.9 Hours
Repeat cesarean deliverys involve a longer surgery and more severe visceral traction than primary cesarean deliverys. The dural puncture epidural (DPE) technique provides faster and more effective analgesia for labor, but there is no sufficient evidence to indicate whether it is suitable for parturients undergoing repeat cesarean delivery.
To determine the efficacy and safety of the DPE anesthesia technique in patients undergoing repeat cesarean delivery.
Patients undergoing repeat cesarean delivery were randomly divided into the DPE and epidural anesthesia (EA) groups. A 25-G spinal needle was used for dural puncture via a 19-G epidural needle. The patients in the two groups were injected with 5 mL of 2% lidocaine followed by 15 mL of a mixture of 1% lidocaine + 0.5% ropivacaine as the epidural dosage. The primary outcome was the onset time of sensory block to the T6 dermatome level and the sensory and motor block degree.
A total of 115 women were included (EA: 57, DPE: 58). The mean time to sensory block to the T6 Level was significantly shorter in the DPE group than in the EA group (14.7 min vs 16.6 min; 95% confidence interval, 13.9 to 15.4 vs 15.8 to 17.4; P = 0.001). The cranial sensory block level was significantly higher at 5, 10, and 15 min after the initial dose in the DPE group than in the EA group (P < 0.05). The sacral sensory block level was significantly higher and the modified bromage score was significantly lower in the DPE group at each time point (P < 0.05). Adverse effects and neonatal outcomes were comparable between the two groups (P > 0.05).
The DPE technique provided higher-quality anesthesia than the EA technique, with a rapid onset of surgical anesthesia, better cranial and sacral sensory block spread and a higher motor block degree, without increasing the incidence of maternal or fetal side effects in patients undergoing repeat cesarean delivery.
Core Tip: This study aimed to explore a comparatively superior anesthesia technique for repeat cesarean delivery. The dural puncture epidural (DPE) anesthesia technique with 1% lidocaine combined with 0.5% ropivacaine provided higher-quality anesthesia than the epidural anesthesia (EA) technique, with a faster onset of surgical anesthesia, better cranial and sacral sensory block spread and a higher motor block degree, without increasing the incidence of maternal or fetal side effects, in patients undergoing repeat cesarean delivery. The DPE anesthesia technique might be a preferable anesthesia scheme over the EA technique.
- Citation: Wang SY, He Y, Zhu HJ, Han B. Dural puncture epidural technique provides better anesthesia quality in repeat cesarean delivery than epidural technique: Randomized controlled study. World J Clin Cases 2022; 10(20): 6890-6899
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6890.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6890
As one of the most common surgeries worldwide, the rate of cesarean delivery (CD) increases year by year[1]. According to the data, the rate of CD is 32%-33% in the United States and 55% to 85% in other highly populated countries[2]. The vast majority of women with a history of CD prefer repeat CD (RCD) when they give birth again[3]. RCD is known to be associated with greater operative difficulties, a longer surgery and more severe visceral traction due to severe intra-abdominal adhesion[4,5] or invasive placentation[6]. The incidence of intra-abdominal adhesion development after primary CD ranges from 46%-65% and increases to 43% to 75% at the third CD and to 83% at the fourth CD[7,8]. The rate of placenta accreta spectrum disorders have been reported to reach up to 2.1% along with the increase in elective RCD[6].
Spinal anesthesia is the most commonly used anesthesia technique for cesarean delivery due to its rapid onset and excellent sensory and motor blockade[9]. However, in some cases of severe adhesions and placental implantation, the action time of spinal anesthesia is not sufficient to meet the requi-rements of longer surgeries, and combined spinal-epidural (CSE) or epidural anesthesia (EA) should be performed[2]. Although CSE technology can meet the needs of prolonged surgery, it is associated with a high incidence of hypotension, similar to spinal anesthesia, which might lead to adverse maternal and fetal outcomes[9-11]. The EA technique has fewer adverse effects but is associated with a slow onset and a certain degree of failure, such as inadequate sacral spread or unilateral or patchy sensory blockade[12].
The dural puncture epidural (DPE) technique is a modification of the CSE technique that was first described for use in nonobstetric surgical patients in 1996 by Suzuki et al[13] and was subsequently applied by other investigators in obstetric patients[14]. It is performed by creating a perforation in the dura mater using a spinal needle (25-27 G) through the shaft of an epidural needle[15]. After the dural perforation is created and the free flow of cerebrospinal fluid (CSF) is confirmed, the spinal needle is withdrawn without local anesthetic injection into the subarachnoid space. The epidural catheter is normally placed in the epidural space, and local anesthetic is injected into the epidural space via the epidural catheter. The dural puncture creates a conduit for translocation of the local anesthetic from the epidural to the subarachnoid space, which is a unique characteristic of the DPE technique[13]. Studies have shown that compared with standard EA, DPE can provide higher-quality analgesia and lower pain scores in the first 10 min, with a lower rate of unilateral block for labor analgesia, with lower dosages of analgesic drugs, and without adverse maternal or fetal effects[14,16-18]. DPE has also been shown to result in improved anesthetic spread and better sacral blockade than EA in lower abdominal surgery[13] and in morbidly obese parturients undergoing CD[19]. However, limited evidence is available regarding the efficacy and safety of the DPE technique in RCD.
Therefore, we designed this double-blind, prospective, randomized trial to compare the DPE and EA techniques in elective RCD and aimed to explore whether DPE is suitable for anesthesia in RCD. The primary outcome was the onset time of anesthesia and the sensory and motor block levels.
This study was approved by the Ethics Committee of Hefei Maternal and Child Health Care Hospital of Anhui Province (No. YYLL2020-15-01) in accordance with the Declaration of Helsinki (1964), and all the patients signed the informed consent form. The study was registered prior to subject enrollment with the Chinese Clinical Trial Registry (Available from: www.chictr.org.cn; identification number is ChiCTR2100050266) with minor revision of the title and collected indicators. This manuscript adheres to the applicable CONSORT 2010 guidelines.
From January 2020 to September 2021, a total of 120 parturients at Hefei Maternal and Child Health Care Hospital of Anhui Province who were scheduled to undergo RCD with a lower uterine segment transverse incision were recruited for this study. Healthy pregnant women with an American Society of Anesthesiologists (ASA) physical status of I or II, age of 23-34 years, weight of 57-83 kg, height of 155-168 cm and a singleton fetus with cephalic presentation at 38-42 wk of gestational age were eligible. We excluded subjects with complications during pregnancy (e.g., gestational hypertension, preeclampsia, or diabetes), neurological diseases, contraindications to neuraxial anesthesia, or known fetal anomalies.
The study was designed as a double-blind, prospective, randomized controlled trial. Patients were allocated to one of two groups using a computer-generated random-number sheet (EA and DPE, n = 60 in each group). The random-number sheet was placed into a sealed envelope and was opened after the initiation of patient enrollment. Two anesthesiologists were involved in the anesthesia procedure to maintain blinding. One anesthesiologist performed the neuraxial anesthesia procedure, while the other anesthesiologist was responsible for anesthesia management and data collection; the latter anesthesiologist remained outside the operating room until the neuraxial anesthesia procedure was completed. The parturients were also blinded to the type of neuraxial procedure.
After the participant entered the room, peripheral venous access was secured using an 18-G intravenous (IV) cannula, standard monitoring including electrocardiography, noninvasive arterial blood pressure, oxygen saturation (SPO2) and heart rate (HR) monitoring was applied, and 500 mL of lactated Ringer’s solution was infused within 30 min before surgery. The obstetricians monitored the fetal HR before and after the neuraxial procedure to ensure that the fetal HR was in the normal range.
All neuraxial procedures were performed by a senior anesthesiologist at the L2-3 interspace with the patient in the left lateral decubitus position. The epidural space was accessed with a 19-G epidural needle and confirmed by the loss of resistance to saline. After confirming the epidural space, a stainless steel multiorifice epidural catheter was inserted 4 cm into the epidural space toward the cranial side in the EA group. In the DPE group, a 25-G Whitacre needle was used for dural puncture through the epidural needle with confirmation of free CSF flow. Then, the spinal needle was removed, and a stainless steel multiorifice epidural catheter was placed 4 cm into the epidural space toward the cranial side, as in the EA group. Then, patients in both groups were placed in the supine position and received oxygen at 5 L/min. After negative aspiration of CSF and blood, 5 mL of 2% lidocaine hydrochloride (excluding adrenaline) was injected as the test dose. If abnormal signs were not observed five min later, 15 mL of the mixture of 1% lidocaine + 0.5% ropivacaine (the mixture consisted of 10 mL of 2% lidocaine and 10 mL of 1% ropivacaine) was injected at a rate of 0.5 mL/s. The sensory block level was measured by acupuncture every 5 min. Surgery was allowed after the sensory block level reached the T6 dermatome. To prevent hypotension due to the supine position, the right side of the lower back was elevated with a lumbar cushion to incline 20 degrees to the left. If systolic blood pressure < 90 mmHg or mean arterial pressure < 60 mmHg, 8 µg of phenylephrine was given intravenously. If HR < 60 beats/min, 0.25 mg of atropine was given intravenously. In the case of a chill reaction, 10 mg of nalbuphine was given after the fetus was delivered. In the case of dyspnea, the assessment plan was applied, followed by mask oxygen inhalation or tracheal intubation as appropriate to improve respiratory function. If the patient complained of discomfort during the procedure, supplemental analgesics were administered by the anesthesiologist.
The primary outcome was the onset time of surgical anesthesia, which was defined as the time from the end of the initial dose to when the sensory block level reached the T6 dermatome[20]. Additional outcomes included the cranial and sacral sensory block levels, as well as the motor block degree. The sensory block levels were determined by acupuncture at 5 min, 10 min, 15 min, and 20 min after drug injection and 5 min after surgery. The motor block degree was assessed at the same timepoints using the modified bromage classification (grade 0: No motor nerve block; 1: Cannot lift leg; 2: Cannot bend knee; 3: Cannot bend the ankle)[20].
The secondary outcomes were the number of patients with cranial sensory block to the T6 Level, the number of patients with a modified bromage score reaching 3 at 15 min, intraoperative IV analgesic supplementation, the local anesthetic volume, the incidence of vasopressor administration and general intraoperative data. We also recorded the incidence of side effects, such as chills, hypotension, and postoperative headache, as well as neonatal outcomes, including the appearance, pulse, grimace, activity, and respiration (Apgar) score.
Sample size calculation: Based on the preliminary data, the mean onset time was 17.5 min in the EA group and 15.42 min in the DPE group, with standard deviations (SD) of 2.61 and 3.34, respectively. For a power of 90% and two-sided statistical significance set at 0.05, the minimum sample size calculated by G*Power (Version 3.1) was 47 patients in each group[21]. To compensate for a dropout rate of 20%, 60 patients were recruited for each group.
Data validation and analysis were carried out by SPSS for version 23.0 (IBM Corp., Armonk, NY, Unite States). The primary outcome, i.e., onset time of T6 sensory block, is presented as the 95% confidence interval (CI) around the difference in group means and was compared via the Mann–Whitney U test. Measurement data with a normal distribution are expressed as the mean ± SD and were analyzed by the Student’s t test. Nonnormally distributed data are expressed as the median (interquartile range) and were compared using the Mann–Whitney U test. The Chi-square test or Fisher’s exact test was used to compare categorical data. Repeated measurement data, including sensory block levels and motor block scores, were assessed longitudinally between groups with linear mixed modeling using the restricted maximum likelihood method and accounting for patient-level clustering (random intercept) under an unstructured model. Adjusted Bonferroni correction was used for multiple comparisons between groups at each timepoint. P < 0.05 was considered to indicate a statistically significant difference.
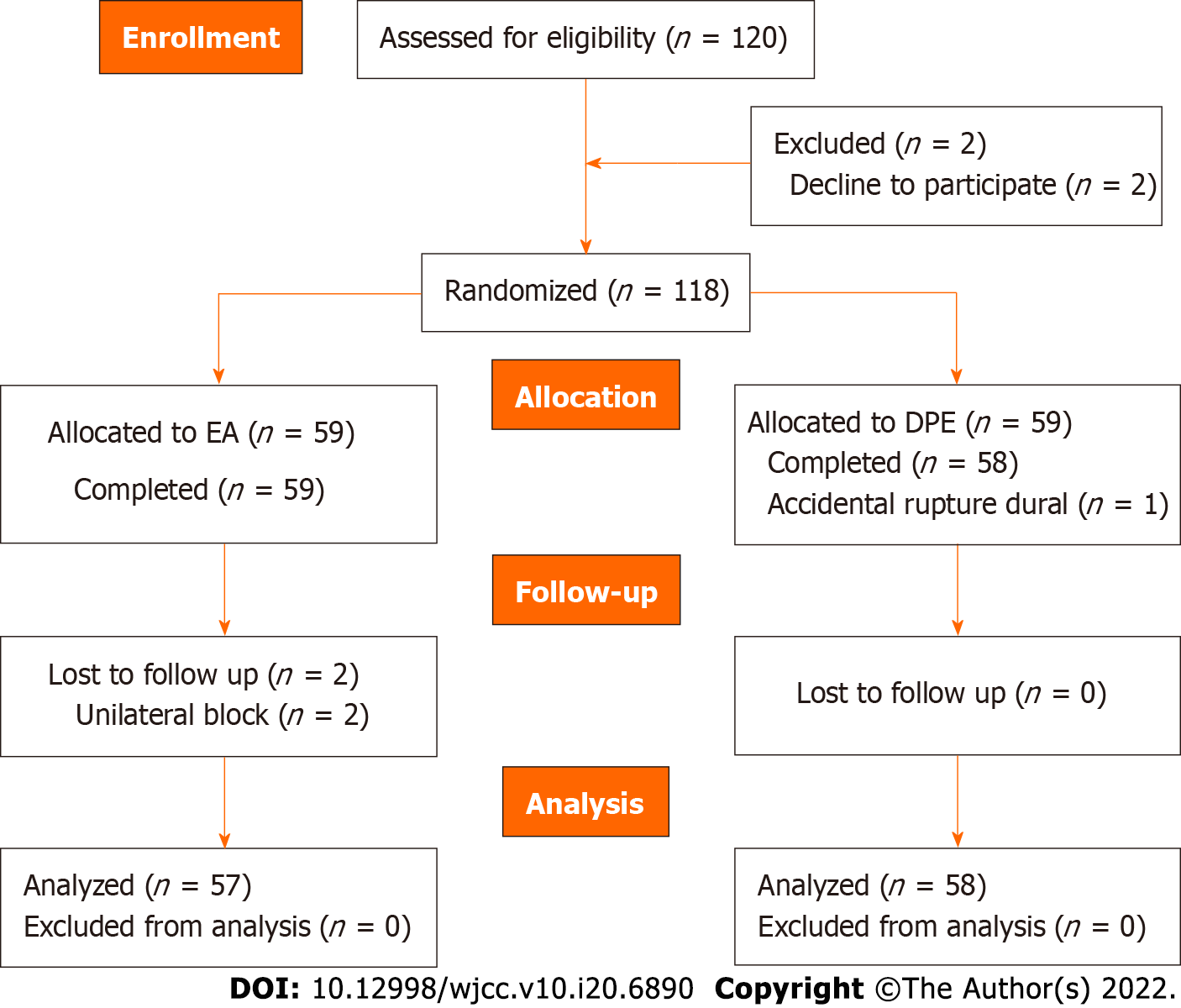
From January 2020 to September 2021, 120 subjects were recruited and randomized into the groups. Five participants were excluded due to unilateral block or accidental dural rupture with an epidural needle and were converted to general anesthesia or spinal anesthesia. Thus, data were collected from 115 subjects (Figure 1). The demographic data, including age, height, weight, body mass index, gestational weeks, hematocrit, operation time, and ASA class, were comparable between the two groups (P > 0.05, Table 1).
| EA (n = 57) | DPE (n = 58) | Z/t/χ2 | 95%CI of difference | P value | |
| Age (yr) | 28.0 (26.0-30.0) | 28.0 (25.0-30.0) | 0.766 | -1.0, 1.0 | 0.4432 |
| Height (cm) | 159.7 ± 3.8 | 160.6 ± 4.2 | 1.159 | -2.4, 0.6 | 0.2491 |
| Weight (kg) | 73.0 (69.5-75.0) | 73.5 (71.8-76.0) | 1.622 | -3.3, 0.2 | 0.1052 |
| BMI (kg/m2) | 28.2 ± 1.9 | 28.5 ± 1.7 | 0.981 | -1.0, 0.3 | 0.3292 |
| Gestational age (w) | 39.8 ± 1.1 | 39.5 ± 1.1 | 1.450 | -0.1, 0.7 | 0.1501 |
| Hb (g/l) | 116.7 ± 8.5 | 117.0 ± 8.3 | 0.223 | -3.5, 2.8 | 0.8241 |
| HCT (%) | 35.4 ± 2.3 | 35.0 ± 2.2 | 0.782 | -0.5, 1.2 | 0.4361 |
| ASA physical status | |||||
| Class 1 | 6 (10.5) | 10 (17.2) | 1.082 | 0.1, 1.7 | 0.2983 |
| Class 2 | 51 (89.5) | 48 (82.8) |
The mean time to achieve a bilateral sensory block to the T6 dermatome level was 14.7 min in the DPE group (95%CI, 13.9-15.4) and 16.6 min (95%CI, 15.8-17.4) in the EA group (Table 2). The mean difference in the onset time of sensory blockade between the two groups was 1.9 min (95%CI for the difference: -5.0 to 0.0, P = 0.001, Table 2).
| EA (n = 57) | DPE (n = 58) | Z/t/χ2 | 95%CI of difference | P value | |
| The onset time to T6 level (min) | 16.6 (15.8-17.4) | 14.7 (13.9-15.4) | 4.039 | -5.0, 0.0 | 0.0012 |
| Maximum sensory level (T) | 5 (4-6) | 5 (4-5) | 1.120 | 0.0, 1.0 | 0.2632 |
| Modified bromage score to 3, n (%) | 21 (36.8) | 45 (77.6) | 19.516 | 2.6, 13.5 | < 0.001 |
| Cranial sensory block to T6, n (%) | 30 (52.6) | 54 (93.1) | 23.915 | 3.8, 38.0 | < 0.001 |
| Vasopressor administration, n (%) | 6 (10.5) | 9 (15.5) | 0.631 | 0.5, 4.7 | 0.4273 |
| Intraoperative IV analgesic supplementation, n (%) | 16 (28.1) | 7 (12.1) | 4.600 | 0.1, 0.9 | 0.0323 |
| Local anesthetic volume (ml) | 20.0 (17.5-20.0) | 20.0 (15.0-20.0) | 0.429 | 0.0, 0.0 | 0.6682 |
| Fluid administration (ml) | 800.0 (750.0-800.0) | 800.0 (700.0-800.0) | 0.806 | 0.0, 0.0 | 0.4242 |
| Estimated blood loss (ml) | 400.0 (400.0-400.0) | 400.0 (300.0-400.0) | 1.548 | 0.0, 0.0 | 0.1222 |
| Duration of surgery (min) | 37.4 ± 8.3 | 36.3 ± 6.3 | 0.746 | -3.7, 1.7 | 0.4571 |
| Urine output (ml) | 100.0 (100.0-150.0) | 100.0 (100.0-150.0) | 0.248 | 0.0, 0.0 | 0.8042 |
| Adverse effects, n (%) | |||||
| Hypotension | 5 (8.8) | 2 (3.5) | 1.425 | 0.1, 2.0 | 0.4223 |
| Respiratory depression | 0 (0.0) | 0 (0.0) | 0.000 | 0.0, 0.0 | 1.0003 |
| Postdural headache | 0 (0.0) | 0 (0.0) | 0.000 | 0.0, 0.0 | 1.0003 |
| Nausea and vomiting | 7 (12.3) | 5 (8.6) | 0.412 | 0.2, 2.3 | 0.5213 |
| Chest distress | 5 (8.8) | 7 (12.1) | 0.334 | 0.4, 4.8 | 0.5633 |
| Dizzy | 6 (10.5) | 4 (6.9) | 0.477 | 0.2, 2.4 | 0.7193 |
| Nasal obstruction | 5 (8.8) | 6 (10.3) | 0.082 | 0.3, 4.2 | 0.7743 |
| Chills | 19 (33.3) | 14 (24.1) | 1.188 | 0.3, 1.4 | 0.2763 |
| Neonatal outcomes | |||||
| Apgar score < 8 at 1 min, n (%) | 3 (5.3) | 2 (3.4) | 0.000 | 0.1, 4.0 | 0.9843 |
| Apgar score < 8 at 5 min, n (%) | 0 (0.0) | 0 (0.0) | 0.000 | 0.0, 0.0 | 1.000 |
Table 3 presents the sensory block levels and modified bromage motor block scores at different time points after the initial dosage. The sensory block level on the cranial side was significantly higher at 5 min, 10 min and 15 min after the initial dose in the DPE group than in the EA group (P < 0.05). The sensory block level on the sacral side was significantly lower in the DPE group than in the EA group at each time point (P < 0.05). The modified bromage motor block score was significantly higher in the DPE group than in the EA group at each time point (P < 0.05).
| Group | 5 min | 10 min | 15 min | 20 min | 5 min after operation | |
| Sensory block levels of cranial side | EA (n = 57) | T11 (T10-11) | T9 (T7-T9) | T6 (T5-T7) | T5 (T4-T6) | T5 (T5-T6) |
| DPE (n = 58) | T10 (T10-11) | T8 (T7-T8) | T6 (T5-T6) | T5 (T4-T6) | T5 (T4-T6) | |
| 95%CI | 0.349-1.020 | 0.064-0.905 | 0.381-1.141 | 0.262-0.436 | 0.078-0.705 | |
| P value | < 0.001 | 0.024 | < 0.001 | 0.623 | 0.115 | |
| Ptime < 0.001, Pgroup = 0.008, Pinteraction < 0.001 | ||||||
| Sensory block levels of sacral side | EA (n = 57) | L1 (L1-L3) | L3 (L2.5-L4) | L5 (L4-S1) | S1 (S1-S1) | S1 (S1-S1) |
| DPE (n = 58) | L2 (L2-L2.3) | L4 (L3-L4) | S1 (L5-S1) | S1 (S1-S2) | S1 (S1-S2) | |
| 95%CI | 0.471-0.966 | 0.245-0.992 | 0.319-0.925 | 0.078-0.710 | 0.269-0.734 | |
| P value | < 0.001 | 0.001 | < 0.001 | 0.015 | < 0.001 | |
| Ptime < 0.001, Pgroup < 0.001, Pinteraction = 0.425 | ||||||
| Motor block score | EA (n = 57) | 0.0 (0.0-0.0) | 1.0 (0.0-1.0) | 1.0 (1.0-2.0) | 2.0 (1.0-3.0) | 3.0 (2.0-3.0) |
| DPE (n = 58) | 1.0 (1.0-1.0) | 1.0 (1.0-2.0) | 2.5 (2.0-3.0) | 3.0 (3.0-3.0) | 3.0 (3.0-3.0) | |
| 95%CI | 0.443-0.757 | 0.631-1.153 | 0.773-1.354 | 0.507-1.008 | 0.210-0.636 | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Ptime < 0.001, Pgroup < 0.001, Pinteraction < 0.0011 | ||||||
The maximum sensory block level in both groups was T5 (P = 0.303). The number of patients with cranial sensory block to the T6 Level at 15 min was 30 in the EA group and 54 in the DPE group (52.6% vs 93.1%, P < 0.001). The number of patients with a modified bromage score reaching 3 at 15 min was 21 in the EA group and 45 in the DPE group (36.8% vs 77.6%, P < 0.001).
Sixteen subjects in the EA group and seven in the DPE group complained of pain during surgery and required intraoperative IV analgesic supplementation (28.1% vs 12.1%, P = 0.032, Table 2). There were no significant differences in terms of vasopressor administration, local anesthetic volume, fluid administration, estimated blood loss, duration of surgery, urine output, neonatal outcomes or adverse effects (P > 0.05, Table 2).
The main finding of this study was that the onset of surgical anesthesia was faster with the DPE technique than the standard EA technique. The DPE technique provided higher-quality anesthesia than the EA technique, with superior cranial and sacral coverage and a higher motor block degree, without increasing the incidence of side effects.
EA is a popular and safe technique and has a sufficient duration of action for RCD. However, it has the drawback of a long onset time and limited anesthesia plane spread, and IV rescue analgesia is often needed[22]. The DPE technique is a modified anesthesia method based on CSE; the dura is punctured using a spinal needle, and local anesthetics are introduced into the epidural space via an epidural catheter instead of directly into the subarachnoid space[14]. The theoretical basis is that perforation of the spinal dura facilitates the infiltration of local anesthetics into the subarachnoid space[15]. As early as 1998, Leach et al[23] observed the translocation of epidural dye into the subarachnoid space through an accidental dural puncture via a tuohy needle (gauge not documented), which verified the theory. Previous studies have shown that the DPE technique using a 25-G or 26-G Whitacre needle resulted in earlier and greater sacral spread than the EA technique[12,13,24]. In addition, an in vitro study demonstrated notable lidocaine flux into the subarachnoid space via an 18-G or 24-G needle puncture but no flux via a 27-G needle puncture[25]. Thomas et al[26] also found that the DPE technique with a 27-G needle did not provide improved labor analgesia quality compared with the EA technique. Therefore, a larger spinal needle aperture may play a critical role in transmeningeal flux; however, the aperture of the spinal needle should be limited to no larger than 25 G to control the risk of postdural puncture headache and abnormally extensive blockade.
Aside from the spinal needle, as mentioned above, Layera et al[27] found that the transmeningeal flux of anesthetic may depend on many other variables, including the pressure gradient between the epidural space and the subarachnoid space, the distance between the puncture location and site of epidural drug administration, the pressure of epidural bolus injection, and the patient’s posture and epidural compliance, which may vary by age and height[18,23]. The local anesthetic volume might affect the transmeningeal flux of anesthetics by altering the pressure gradient between the epidural space and the subarachnoid space. Chau et al[28] used an identical DPE technique via a 25-G Whitacre needle with different epidural dosing regimens (20 mL of 0.125% bupivacaine and 12 mL of 0.25% bupivacaine) in different studies and suggested that a more dilute, higher-volume initial bolus was associated with a more rapid onset of thoracic sensory blockade and greater median cranial spread[15]. The concentration of the local anesthetic solution and transferability of local anesthetics may also affect the translocation of the medications from the epidural space to the subarachnoid space[18]. A low concentration of ropivacaine was used by Wang et al[24] and Song et al[18] in the DPE technique for labor analgesia and resulted in a faster onset and better sacral block than the EA technique. In our study, a 15-ml mixture of 1% lidocaine and 0.5% ropivacaine as the epidural loading dose was used for the first time to induce anesthesia and resulted in a faster onset of surgical anesthesia and better spread than the EA technique. Clement et al[29] found that bupivacaine exhibited a slower transmeningeal flux than lidocaine in rabbit models. Further studies are warranted to determine the difference in transmeningeal flow between bupivacaine and ropivacaine.
Although the motor block degree reported by previous studies was comparable between the DPE and EA techniques for labor analgesia [15,18,24], the DPE technique resulted in significantly higher motor block scores at each time point than the EA technique in our study. One possible reason is that the concentration of local anesthetics was significantly higher than that in other studies applied for labor analgesia, in which a low concentration of ropivacaine produced motor-sensory separation. We did not record the hemodynamic data since a previous study reported that the hemodynamic stability produced by the DPE technique was better than that of the CSE technique and comparable to that of the EA technique[15]. The rate of vasopressor administration in our study was observed to be comparable between the two groups, which indirectly supports this result.
Neither postdural puncture headache nor respiratory depression was observed in either group. The incidence of other side effects, including chest distress, nausea and vomiting, nasal obstruction, maternal hypotension and chills, was comparable between the two groups[14]. While the symptom of nasal obstruction was rarely reported in other studies, we observed 5 cases (8.8%) in the EA group and 6 cases (10.3%) in the DPE group, which was related to the high thoracic sensory blockade and was not followed by decreasing SPO2 or chest distress. Neonatal outcomes also did not differ between the DPE and EA groups, indicating that the DPE technique used in anesthesia for repeat cesarean delivery was as safe for the mother and the fetus as the EA technique.
This study has several limitations. First, although our results suggest that DPE yielded a superior bilateral block, we did not record the rate of asymmetrical neuraxial block but excluded such patients from the study in the follow-up stage because patients with asymmetrical neuraxial block had to undergo conversion to general anesthesia to complete the surgery. Second, in patients undergoing RCD, adhesions in the epidural space may also affect the spread of the local anesthetic solution in the epidural space as well as its translocation into the subarachnoid space, which might increase the bias of the data.
In summary, the DPE technique provided rapid-onset surgical anesthesia and higher-quality anesthesia compared with the EA technique, with superior cranial and sacral sensory block spread and a higher motor blockage degree, without increasing the incidence of maternal or fetal side effects in patients undergoing RCD.
Repeat cesarean delivery (RCD) involves a longer surgery and more severe visceral traction than primary cesarean deliverys, and the rate of RCD is increasing year by year. As an improvement upon the combined spinal-epidural (CSE) technique, the dural puncture epidural (DPE) technique has been reported to provide faster and more effective labor analgesia; however, insufficient data from among parturients undergoing RCD were available. This study aimed to determine whether the DPE technique is superior to the epidural anesthesia (EA) technique in parturients undergoing repeat cesarean Delivery.
The aim of this study was to overcome the drawbacks of the slow onset and limited blockade spread of the EA technique. The DPE technique might provide a faster onset and better spread than the EA technique while providing more stable hemodynamics than the CSE technique; hence, this technique might be superior to the EA and CSE techniques.
The objective of this study was to find a better anesthesia method for repeat cesarean delivery.
This was a double-blind, prospective, randomized controlled trial.
The DPE technique provided a faster onset of surgical anesthesia, better cranial and sacral sensory spread and higher motor block degree without increasing the incidence of maternal or fetal side effects when compared with the EA technique in patients undergoing RCD.
The DPE technique provided higher-quality anesthesia than the EA technique when used in patients undergoing repeat cesarean delivery.
Future research will explore the short-term and long-term potential complications of the DPE technique.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Sabouri AS, United States; Simionescu AA, Romania S-Editor: Guo XR L-Editor: A P-Editor: Chen YX
| 1. | Abbas AM, Khalaf M, Abdel-Reheem F, El-Nashar I. Prediction of pelvic adhesions at repeat cesarean delivery through assessment of striae gravidarum score: A cross-sectional study. J Gynecol Obstet Hum Reprod. 2020;49:101619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Ioscovich A, Gozal Y, Shatalin D. Anesthetic considerations for repeat cesarean section. Curr Opin Anaesthesiol. 2020;33:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Ming Y, Li M, Dai F, Huang R, Zhang J, Zhang L, Qin M, Zhu L, Yu H. Dissecting the current caesarean section rate in Shanghai, China. Sci Rep. 2019;9:2080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Mooij R, Mwampagatwa IH, van Dillen J, Stekelenburg J. Association between surgical technique, adhesions and morbidity in women with repeat caesarean section: a retrospective study in a rural hospital in Western Tanzania. BMC Pregnancy Childbirth. 2020;20:582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Cai Q, Gong H, Fan M, Chen W, Cai L. The analgesic effect of tramadol combined with butorphanol on uterine cramping pain after repeat caesarean section: a randomized, controlled, double-blind study. J Anesth. 2020;34:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Zeng C, Yang M, Ding Y, Duan S, Zhou Y. Placenta accreta spectrum disorder trends in the context of the universal two-child policy in China and the risk of hysterectomy. Int J Gynaecol Obstet. 2018;140:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Lyell DJ. Adhesions and perioperative complications of repeat cesarean delivery. Am J Obstet Gynecol. 2011;205:S11-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Awonuga AO, Fletcher NM, Saed GM, Diamond MP. Postoperative adhesion development following cesarean and open intra-abdominal gynecological operations: a review. Reprod Sci. 2011;18:1166-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Zhou Y, Yu Y, Chu M, Zhang Y, Yu X, Chen G. Comparison of Metaraminol, Phenylephrine, and Norepinephrine Infusion for Prevention of Hypotension During Combined Spinal-Epidural Anaesthesia for Elective Caesarean Section: A Three-Arm, Randomized, Double-Blind, Non-Inferiority Trial. Drug Des Devel Ther. 2022;16:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Xu W, Drzymalski DM, Ai L, Yao H, Liu L, Xiao F. The ED50 and ED95 of Prophylactic Norepinephrine for Preventing Post-Spinal Hypotension During Cesarean Delivery Under Combined Spinal-Epidural Anesthesia: A Prospective Dose-Finding Study. Front Pharmacol. 2021;12:691809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Van de Velde M. Low-dose spinal anesthesia for cesarean section to prevent spinal-induced hypotension. Curr Opin Anaesthesiol. 2019;32:268-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Bakhet WZ. A randomized comparison of epidural, dural puncture epidural, and combined spinal-epidural without intrathecal opioids for labor analgesia. J Anaesthesiol Clin Pharmacol. 2021;37:231-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Suzuki N, Koganemaru M, Onizuka S, Takasaki M. Dural puncture with a 26-gauge spinal needle affects spread of epidural anesthesia. Anesth Analg. 1996;82:1040-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Gunaydin B, Erel S. How neuraxial labor analgesia differs by approach: dural puncture epidural as a novel option. J Anesth. 2019;33:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Contreras F, Morales J, Bravo D, Layera S, Jara Á, Riaño C, Pizarro R, De La Fuente N, Aliste J, Finlayson RJ, Tran DQ. Dural puncture epidural analgesia for labor: a randomized comparison between 25-gauge and 27-gauge pencil point spinal needles. Reg Anesth Pain Med. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Lu YY, Cai JJ, Jin SW, Wang CH, Zhou YF, Hu MP, Li J. [Application of dural puncture epidural technique for labor analgesia]. Zhonghua Yi Xue Za Zhi. 2020;100:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Heesen M, Rijs K, Rossaint R, Klimek M. Dural puncture epidural versus conventional epidural block for labor analgesia: a systematic review of randomized controlled trials. Int J Obstet Anesth. 2019;40:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Song Y, Du W, Zhou S, Zhou Y, Yu Y, Xu Z, Liu Z. Effect of Dural Puncture Epidural Technique Combined With Programmed Intermittent Epidural Bolus on Labor Analgesia Onset and Maintenance: A Randomized Controlled Trial. Anesth Analg. 2021;132:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Mychaskiw G 2nd, Panigrahi T, Ray T, Shah S. Intentional puncture of the dural space as an aid to epidural placement in a morbidly obese parturient. J Miss State Med Assoc. 2001;42:303-305. [PubMed] |
| 20. | Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiol Scand Suppl. 1965;16:55-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 379] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26400] [Cited by in RCA: 34629] [Article Influence: 1923.8] [Reference Citation Analysis (0)] |
| 22. | Yan W, Xiong Y, Yao Y, Zhang FJ, Yu LN, Yan M. Continuous intravenous infusion of remifentanil improves the experience of parturient undergoing repeated cesarean section under epidural anesthesia, a prospective, randomized study. BMC Anesthesiol. 2019;19:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Leach A, Smith GB. Subarachnoid spread of epidural local anaesthetic following dural puncture. Anaesthesia. 1988;43:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Wang J, Zhang L, Zheng L, Xiao P, Wang Y, Zhou M. A randomized trial of the dural puncture epidural technique combined with programmed intermittent epidural boluses for labor analgesia. Ann Palliat Med. 2021;10:404-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Bernards CM, Kopacz DJ, Michel MZ. Effect of needle puncture on morphine and lidocaine flux through the spinal meninges of the monkey in vitro. Implications for combined spinal-epidural anesthesia. Anesthesiology. 1994;80:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Thomas JA, Pan PH, Harris LC, Owen MD, D'Angelo R. Dural puncture with a 27-gauge Whitacre needle as part of a combined spinal-epidural technique does not improve labor epidural catheter function. Anesthesiology. 2005;103:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Layera S, Bravo D, Aliste J, Tran DQ. A systematic review of DURAL puncture epidural analgesia for labor. J Clin Anesth. 2019;53:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Chau A, Bibbo C, Huang CC, Elterman KG, Cappiello EC, Robinson JN, Tsen LC. Dural Puncture Epidural Technique Improves Labor Analgesia Quality With Fewer Side Effects Compared With Epidural and Combined Spinal Epidural Techniques: A Randomized Clinical Trial. Anesth Analg 2017, 124: 560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 29. | Clement R, Malinovsky JM, Le Corre P, Dollo G, Chevanne F, Le Verge R. Cerebrospinal fluid bioavailability and pharmacokinetics of bupivacaine and lidocaine after intrathecal and epidural administrations in rabbits using microdialysis. J Pharmacol Exp Ther. 1999;289:1015-1021. [PubMed] |