Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6876
Peer-review started: March 10, 2022
First decision: April 13, 2022
Revised: April 22, 2022
Accepted: May 21, 2022
Article in press: May 21, 2022
Published online: July 16, 2022
Processing time: 116 Days and 15.8 Hours
Bruxism is a rhythmic masticatory muscle activity that occurs involuntarily in a non-physiologically functional state. There is a lack of research classifying the functional status of masticatory muscles in patients with different mandibular movement types (centric clenching or eccentric grinding) of bruxism.
To assess the differences of the masticatory muscle activity in patients with different types of bruxism.
A total of 21 subjects with centric bruxism (CB) and 21 subjects with eccentric bruxism (ECB) were screened from college students according to a questionnaire and their tooth wear features. Sixteen subjects with no bruxism were also recruited. The surface electromyography (EMG) signals of the temporalis anterior (TA) and superficial masseter muscle (MM) were measured in different mandibular positions and during the chewing task. The EMG amplitude and chewing cycle duration parameters were then analyzed.
The CB group showed fewer muscle maximal motor units, with the MM being more pronounced, a higher proportion of muscle contractions to be recruited for the same load of chewing activity, and a longer chewing cycle. The ECB group showed more TA maximal motor units and higher MM activity on the non-working side in unilateral chewing.
CB mainly affects the MM, and patients with CB show reduced masticatory muscle contraction efficiency and chewing cycle efficiency. ECB mainly affects the TA, and patients with ECB show enhanced contraction of non-functional lateral muscle bundles.
Core Tip: Bruxism can be divided into types by the nature of the mandibular movement: Centric bruxism is dominated by centric clenching movements and eccentric bruxism by eccentric grinding movements. This study found that eccentric bruxism mainly involved the temporalis anterior on the working side and the masseter muscle on the non-working side, with the temporalis anterior predominating, and the patients showed enhanced contraction of non-functional lateral muscles; centric bruxism mainly involved the temporalis anterior and the masseter muscle bilaterally, of which the masseter muscle was predominant, and the patients showed reduced masticatory muscle contraction efficiency and chewing cycle efficiency.
- Citation: Lan KW, Jiang LL, Yan Y. Comparative study of surface electromyography of masticatory muscles in patients with different types of bruxism. World J Clin Cases 2022; 10(20): 6876-6889
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6876.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6876
Bruxism is a rhythmic masticatory muscle activity that occurs involuntarily in a non-physiologically functional state, causing rhythmic, intermittent teeth grinding or clenching[1]. According to the type of mandibular movement, bruxism can be divided into centric bruxism (CB), which is dominated by centric clenching movements, and eccentric bruxism (ECB), which is dominated by eccentric grinding movements[2]. Patients with CB can experience it both while they are awake and while they are asleep, but those with ECB experience one or the other but never both.
Tooth wear is an important sign of bruxism. Patients with different types of bruxism have different tooth contact patterns. The resulting different tooth wear characteristics can be used as a basis for the diagnostic classification of bruxism[3]. CB shows tooth wear in the corresponding tooth contact areas during intercuspal position and ECB reflects tooth wear in the corresponding tooth contact areas during eccentric movement of the mandible. Okura et al[4] found that tooth grinding in bruxism patients may lead to severe wear of the canine, and that there is a close relationship between mandibular movements, masticatory electromyographic activity patterns, and tooth wear. Tooth clenching may also lead to crown deformation and minor movement with small localized wear surfaces in the cusp-fossa contact region[5].
Previous works did not distinguish between types of mandibular movement in bruxism, which has made it impossible to develop suitable treatments for different types of bruxism[1]. The pattern of mandibular movement is directly related to the temporalis anterior (TA) and masseter muscle (MM). TA muscle fibers start from the temporal fossa and end vertically downward at the coronal eminence, and their main role is to lift the mandible upward, maintain the stability of the mandibular postural position, and control mandibular movements in the lateral plane[6,7]. MM superficial muscle fibers start from the maxillary eminence and zygomatic arch and end posteriorly downward at the mandibular angle, and their main role is to make the mandible move forward and upward. The lateral grinding movement is dominated by the contraction of the working side TA and the non-working side MM, but the TA is more active[7]. Bilateral TA and MM play a role in lifting the jaw upward during clenching, but the MM is the primary muscle that generates the occlusal force[8]. We hypothesized that ECB may affect non-axial muscle fibers, such as the temporalis, while CB may affect axial muscle fibers (masseter muscle).
Masticatory muscle contraction is accompanied by myocyte bioelectric activity. Electromyography is commonly used to evaluate muscle fiber action potential activity. It helps researchers analyze neuromuscular activation patterns, evaluate the functional state of the muscle, and monitor changes in the muscle patterns of the stomatognathic system[9,10]. The electromyographic amplitude is positively correlated with the number of motor units recruited by muscle contraction and with the motor unit firing rate[10]. The contractile activity of the TA and MM is closely related to masticatory function. Surface electromyography (sEMG) of the TA and MM can be used to assess the masticatory muscle function status[11].
The aim of this study was to investigate the effect of early bruxism on masticatory muscle function by analyzing the characteristics of TA and MM contraction electromyographic signals during different mandibular positions and chewing activities in young adult patients with different types of bruxism, and to provide a physiological basis for the diagnostic classification and the selection of appropriate treatment options for early bruxism patients with different types of mandibular movements.
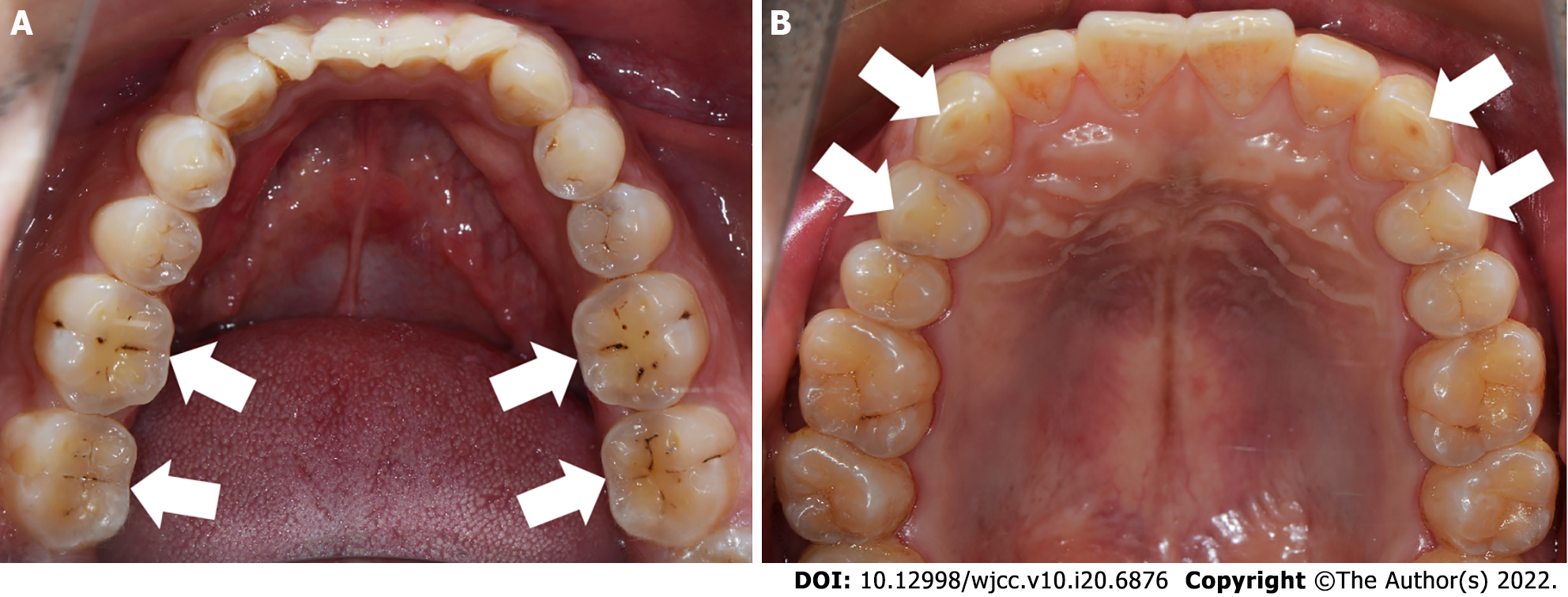
Subjects: The inclusion criteria for bruxism in this study were as follows: (1) Being informed by others of the presence of bruxism (teeth grinding or clenching) within the last 6 mo; and (2) One or more of the following symptoms or signs: Abnormal tooth wear, masticatory muscle fatigue, or pain on rising in the morning. A total of 158 students from Sun Yat-sen University who self-reported the presence of bruxism were screened and divided into CB and ECB groups based on tooth wear characteristics. In addition, non-bruxism subjects were recruited as a non-bruxism group (NB). The grouping criteria were as follows: Subjects who showed teeth wear associated with the contact area between the lower anterior teeth and the lingual surface of the upper anterior teeth, and/or the wear of the support cusp of the posterior teeth and the "cup"-shaped wear surface at the central fossa were included in the CB group (Figure 1A); those who had horizontal tooth wear associated with the canine and the guide cusp of the posterior teeth were included in the ECB group (Figure 1B); and subjects who did not have self-reported or clinical signs/symptoms associated with bruxism were included in the NB group.
The exclusion criteria for all of the subjects were as follows: (1) History of systemic disease; (2) Anxiety or long-term use of psychotropic medication; (3) Obstructive sleep apnea hypoventilation syndrome; (4) Abnormal maxillofacial skeletal morphology, severe malocclusion, and history of orthodontics; (5) Temporomandibular joint related disorders; (6) Active caries, periodontal disease, and oral mucosal disease; (7) History of occlusal splint treatment, maxillofacial trauma or surgery, or prosthetic treatment within the last 6 mo; (8) More than two missing teeth (except the third molar); (9) The presence of other non-bruxism factors that may lead to tooth defects; (10) Unilateral chewing habit; and (11) Some chewing habits that may affect the masticatory muscles (e.g., habitual gum chewing).
A total of 42 subjects (21 females) with bruxism were finally included. These included 21 subjects (15 females) in the CB group with a mean age of 22.86 ± 1.98 years and 21 subjects (6 females) in the ECB group with a mean age of 21.29 ± 1.90 years. A total of 16 subjects (5 females) were recruited in the NB group with a mean age of 21.94 ± 2.41 years. None of the subjects had teeth that exceeded grade 2 with respect to tooth wear as classified in the Tooth Wear Evaluation System 2.0[12].
Food samples: Food samples were selected from commercial products of gelatinous candy and almonds. Samples of similar shape, volume, and weight were selected. The candies were cylindrical in shape, each with a volume of 1.5 cm3 and weight of 2 g. The almonds had a volume of 1 cm3 and weight of 1 g each.
sEMG system operation protocol: The BioEMG III system (Bioresearch Associates, Inc., Milwaukee, WI, United States) was used for the sEMG testing. When the subject clenches, the most obvious muscle contraction on the skin will be found by palpation as the TA and MM electrode localization points. Electrodes were applied to the point parallel to the direction of the muscle fiber alignment[13], while the reference electrode was applied to the skin surface of the spinous process of the seventh cervical vertebra. Bilateral TA and MM sEMG signals were recorded at a sampling rate of 1000 Hz as the subject completed the assigned movements.
Subject movement requirements: All tests were completed during the same period during the day. Subjects were tested in the same quiet room. The subject was asked to remain in a seated position with the head upright and the Frankfurt plane parallel to the ground. Subjects were instructed to practice until they mastered the specified movements, and then completed the following procedures:
Mandibular postural position (MPP): The subject relaxed the jaw without any mandibular movement or occlusion.
Intercuspal position maximal voluntary clenching (ICP-MVC): The subject rapidly clenched from MPP to ICP, maintained the maximal clenching for 5 s, and then relaxed to MPP. The subject was allowed to rest for 3 min before repeating the clenching again, for a total of three repetitions.
Free chewing preparation: Subjects were given candy/almonds to chew freely to familiarize them with the chewing process and food texture, and they were asked to chew freely for at least 20 s (> 21 cycles) at the frequency that they were habituated to without recording EMG data.
Free chewing: Two trials, one with candy and one with almond, were conducted. Subjects were given one candy or almond to chew freely and were asked not to swallow it until the experimenter told them to stop chewing. This chewing task was repeated five times (one candy/almond for each chewing task), and EMG data were recorded with 3-min interval between each chewing task and 5-min interval between each trial (i.e., each type of food).
Unilateral chewing preparation: Subjects were given candy/almonds to chew unilaterally on the left and right side for at least 20 s (> 21 chews) at the frequency they were habituated to without recording EMG data.
Unilateral chewing: Subjects were given one candy/almond to repeat the unilateral chewing preparation process three times on each side with 3 min intervals, and the EMG data was recorded with 5 min intervals for each chewing side and type of food. Subjects were asked not to swallow food, like in the free chewing task.
The analysis of the experimental data was done by one experimenter. To avoid bias, the experimenter analyzed the data without knowing the inclusion group of the subjects.
EMG data: The mean EMG values (4 s) of the bilateral TA and MM of the MPP and ICP-MVC were analyzed. The normalized muscle activity ratio (NMA ratio) of the MPP for each muscle was calculated as (EMG recorded during MPP/EMG recorded during ICP-MVC)%. The mean EMG value of the ICP-MVC was used as a reference value to calculate the percentage of the MPP EMG amplitude against the reference value[6].
Referring to the analysis of masticatory EMG by Benjamin et al[14], a chewing cycle was manually marked between the onset of the chewing potential pulse and the next pulse. The first chewing cycle duration was analyzed individually, and then every five chewing cycles in the following 20 chews were defined as one STEP. A total of five chewing stages (the first chewing cycle and four STEPs) are included in a chewing task. The average duration of each stage, as well as the total duration of four STEPs, was analyzed. This analysis method was applied to all the chewing tasks in this study.
The studies of Martinez-Gomis et al[15] and Fuentes et al[6] were referred to for the judgment of the chewing preferred side (PS). Subjects were asked to recall their chewing PS and then required to eat five candies/almonds (one piece/one chewing for a total of five chewing tasks) freely. During each chewing, after excluding the first chewing cycle, the side on which the subject chewed more than 10 times out of the following 20 chews was selected as the chewing PS. In the five chewing tasks, the side that was selected (left/right) more than two times was the chewing PS of this food (candy/almond) in the subject. If the PS that the subject recalled was different from the one observed in free chewing, the latter result prevailed.
For the EMG recordings (16 s) of the unilateral chewing task, the root-mean-square (RMS) values of the EMG amplitudes of the bilateral TA and MM were evaluated with a mean value of 50 ms[16,17]. In unilateral chewing, the side of chewing is the working side (WS) and the side without chewing is the non-working side (NWS). The RMS values of EMG amplitudes for each muscle in each unilateral chewing task were normalized according to the following formula[17]:
Normal muscle activity (NMA) ratio = (RMS values of EMG during each chewing task/EMG recorded during ICP-MVC)%.
The ICP activity index (ICP Ac) was calculated using the bilateral EMG values in the ICP MVC[18]. The relevant formula is as follows:
ICP Ac = (left MM + right MM - left TA - right TA)/(left TA + right TA + left MM + right MM) × 100%.
The EMG amplitude of ICP-MVC was used as the main indicator. The sample size was determined using the sample size estimation formula of the multiple-sample one-way ANOVA design with a statistical power of 80%.
Statistical analyses were performed using the statistical software SPSS (version 25.0, IBM, United States), and data are described the mean ± standard deviation. The significance level (α) was set at 0.05. The Shapiro-Wilk test for normality and the Levene test for homogeneity of variance were applied.
ANOVA with a factorial design (three groups and two genders) was used for indicators that were normally distributed, and if the interaction effects were not significant, the primary effects of the different groups were tested and further compared using the Fisher's least significant difference (for homogeneity of variance) or the Bonferroni test (for heterogeneity of variance). If the interaction effects were significant, the individual effects of each group were compared under the same gender (unless otherwise specified in the results section, and the interaction effects were insignificant by default). The Kruskal-Wallis test was used to test for differences between groups for indicators that did not have normal distributions.
A paired-samples t-test was used to compare the difference between the mean duration of the first chewing cycle and the subsequent total 20 cycles within the same group.
The mean chewing cycle duration at each chewing stage was statistically tested using three-way repeated measures ANOVA (three groups, two genders, and five stages). If the Mauchly's sphericity test was significant, the Greenhouse-Geisser correction was applied.
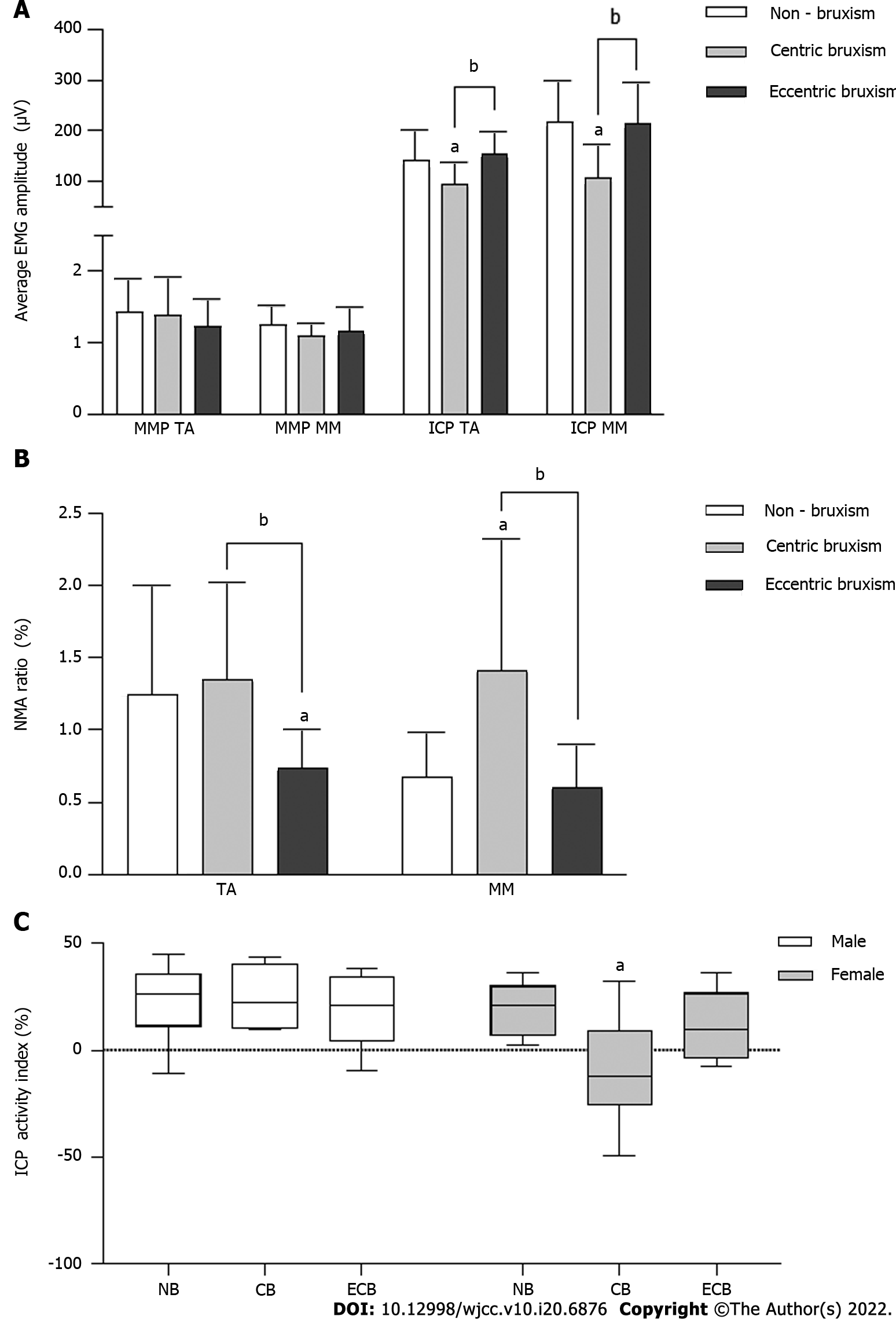
In the MPP, there was no significant difference in the average EMG amplitude between the groups (P > 0.05). In the ICP MVC, the average EMG amplitudes in the CB group (TA: 94.15 ± 44.13 μV; MM: 107.27 ± 64.83 μV) were significantly lower than those in the NB group (TA: 141.98 ± 58.48 μV; MM: 218.32 ± 81.41 μV) and ECB group (TA: 153.65 ± 44.19 μV; MM: 215.21 ± 80.05 μV) (P < 0.05) (Figure 2A).
The TA NMA ratio of MPP in the ECB group (0.74 ± 0.26%) was significantly lower than that in the NB group (1.25 ± 0.75%) and CB group (1.35 ± 0.68%) (P < 0.05), while the MM NMA ratio of MPP in the CB group (1.42 ± 0.91%) was significantly higher than that in the NB group (0.67 ± 0.31%) and ECB group (0.61 ± 0.29%) (P < 0.05) (Figure 2B).
The interaction effect of group and gender on the ICP Ac was statistically significant (Figure 2C). The Ac of the CB group was significantly lower than that of the NB group in females (P < 0.05), while there was no significant difference between all groups in males (P > 0.05).
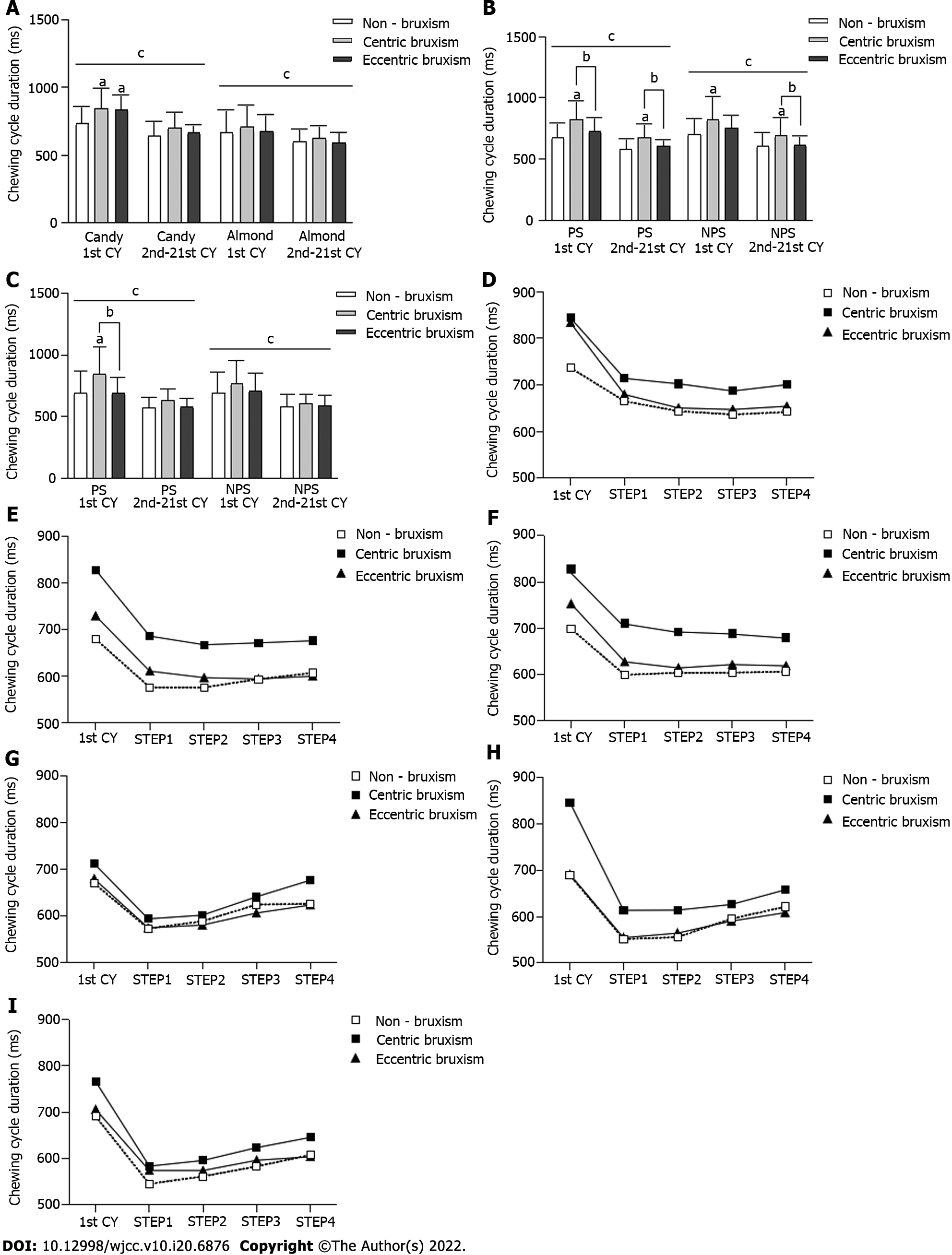
In all of the chewing tests (Figure 3A-C), the duration of the first chewing cycle was significantly longer than that of the subsequent total 20 chewing cycles (P < 0.05). In the free-chewing candies test, the duration of the first chewing cycle was significantly longer in the CB and ECB groups than in the NB group (P < 0.05) (Figure 3A). In the unilateral chewing candies test, the duration of the chewing cycle was significantly longer in the CB group than in the ECB and NB groups (P < 0.05) (Figure 3B). In the PS unilateral chewing almond test, the duration of the first chewing cycle was significantly longer in the CB group than in the ECB and NB groups (P < 0.05) (Figure 3C).
Statistical tests of the duration of the chewing cycle at each stage of the chewing process showed that the three-factor interaction effect and the two-factor interaction effect were not statistically significant. There was a significant difference in the duration of the chewing cycle at different stages of chewing (P < 0.05), but there was no significant difference between the different groups (P > 0.05) (Figure 3D-I).
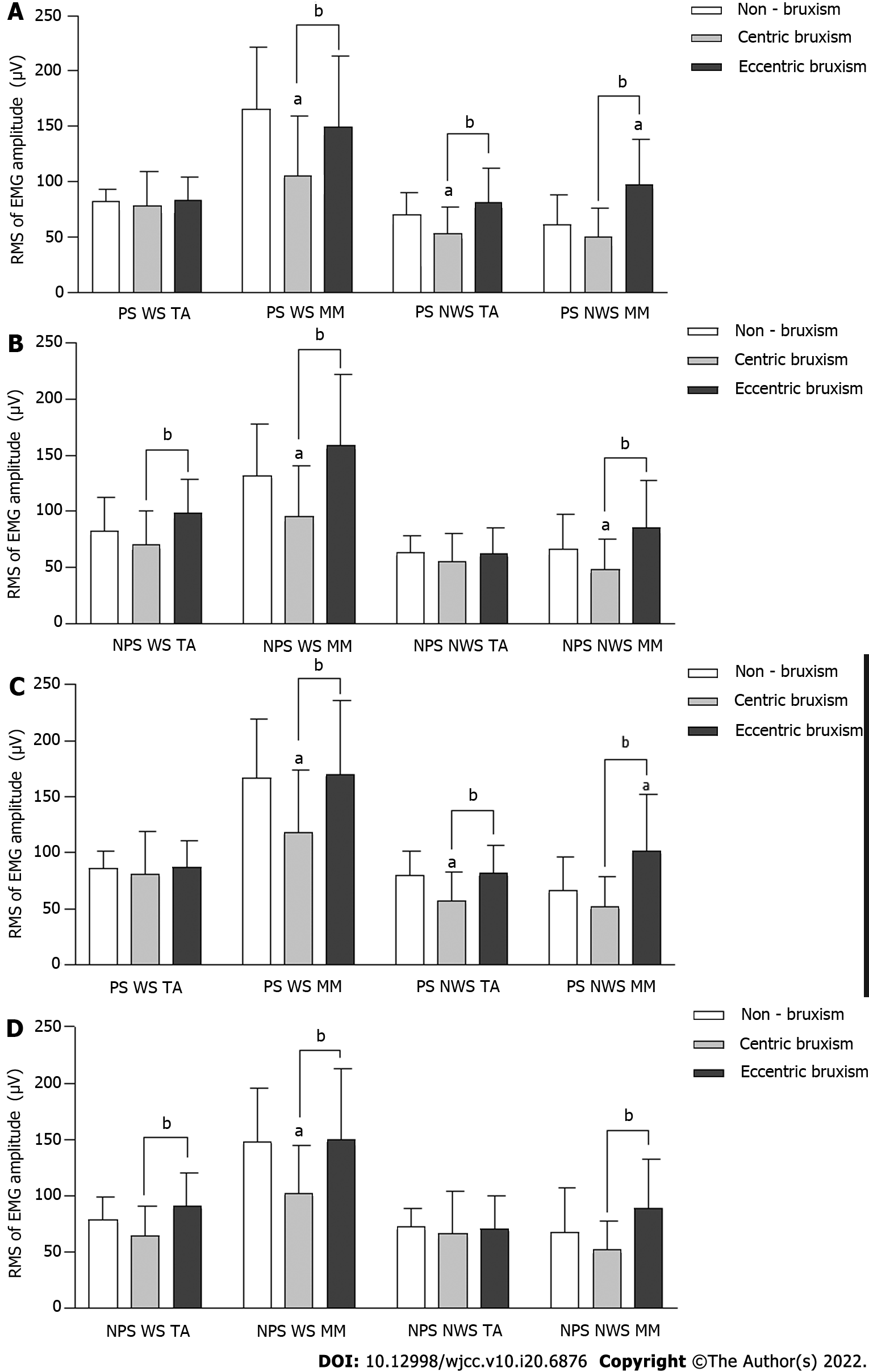
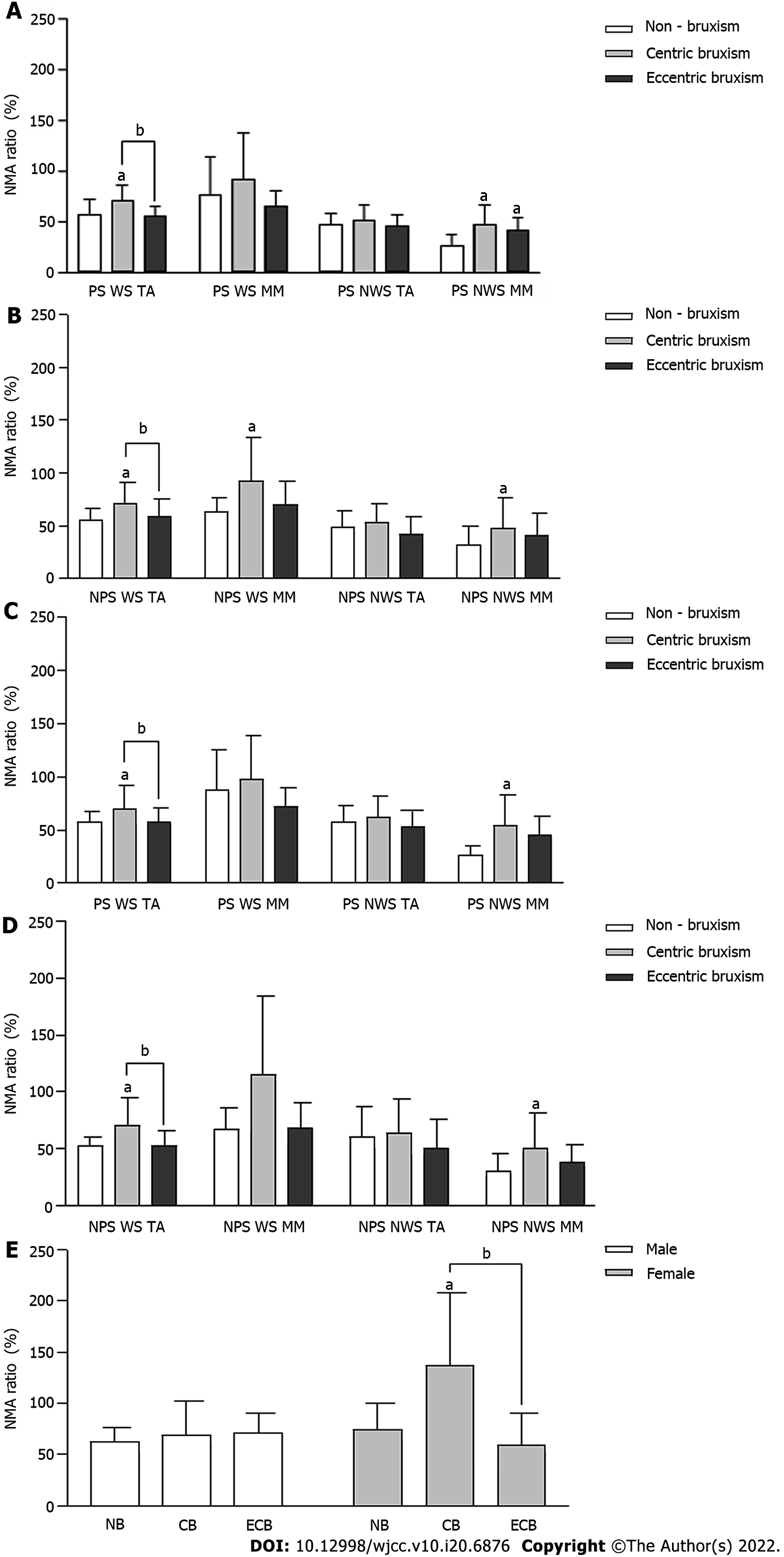
The root mean square values of MM and TA EMG amplitudes in the CB group were always significantly lower than those in the ECB and NB groups (P < 0.05). During PS chewing, the root mean square values of MM EMG amplitude on the NWS in the ECB group was always higher than that in the NB group (P < 0.05). The effect of the interaction effect between the group and gender on the root mean square values of NWS TA EMG amplitude during NPS chewing almond was statistically significant, but there was no significant difference between the groups under the same gender (P > 0.05) (Figure 4).
The NMA ratio of the WS TA in the CB group was always significantly higher than that in the ECB and NB groups (P < 0.05). The NMA ratio of the NWS MM in the CB group was significantly higher than that in the NB group (P < 0.05) (Figure 5A-D). The effect of the interaction between the group and gender on the NMA ratio of the WS MM in NPS chewing almond was statistically significant, and the NMA ratio in the CB group was significantly higher than that in the NB and ECB groups in females (P < 0.05), while there was no significant difference between all groups in males (P > 0.05) (Figure 5E).
Diagnosis of bruxism is based on self-reports, clinical examination, and instrumentation[1,3,19]. Although polysomnography (PSG) testing is believed to provide critical evidence for the diagnosis of bruxism, PSG testing only focuses on the frequency of electromyographic episodes or bursts and does not take into account the amplitude of electromyographic activity[1]. The electromyographic signal from PSG only indicates that the subject is experiencing mandibular movements and does not demonstrate that teeth contact is occurring, which may lead to mandibular movements being mistaken for bruxism. This study used the most commonly used clinical diagnostic methods for bruxism (self-reporting and clinical examination)[19], in which subjects with bruxism included in the study must have teeth grinding/clenching informed by others, so the diagnostic method used in this study is more objective and accurate than the PSG test.
Tooth wear characteristics were an important basis for the grouping of bruxism in this study. However, tooth wear may be the result of the cumulative effect of multiple factors (mechanical/ chemical)[20]. Tooth wear may also be the result of previous bruxism and may not be a valid predictor of current bruxism activity, so a comprehensive analysis of the relationship between tooth wear and bruxism in conjunction with self-reports, other clinical examinations, and instrumental findings is warranted[1,4]. The subjects included in this study were informed by others of the presence of teeth grinding/clenching within the last 6 mo, which ensured the inclusion of subjects with active bruxism. A combination of self-reports and clinical examination also excluded the subjects who had tooth defects caused by issues other than bruxism.
The EMG results of our study supported the hypothesis. The main muscles involved in ECB are the TA on the working side and MM on the non-working side, with the TA predominating; the muscles involved in CB patients are bilateral TA and MM, with the MM predominating. Compared with the NB group, the ECB group exhibited an increased number of TA maximal motor units during ICP MVC, prolonged neuromuscular activity, reduced firing frequency of TA motor neurons, and significantly reducing TA NMA ratio in the MPP. While in the CB group, the number of muscle maximal motor units was reduced, but the MM was reduced to a greater extent, resulting in a significant increased MM NMA ratio in the MPP. In previous studies, there were no significant differences in the TA and MM EMG amplitudes between bruxism and non-bruxism subjects in the MPP and ICP MVC[16,21]. These studies did not differentiate the mandibular movement types of bruxism subjects, resulting in findings that did not reflect the true situation.
In our study, the degree of MM contribution to clenching was weakest in the females of the CB group. ICP Ac can be used to compare the relative contribution of the MM and TA to the bite force[18]. Previous studies showed that men have higher tolerance to muscle fatigue than women[22]. There are differences in muscle anatomy between the sexes. It has been reported that, in young adults, TA and MM are thinner in women than in men[23]. CB resulted in MM fatigue, which further reduced the extent of the MM contribution to clenching in women. We found a significantly reduced MM contribution to clenching in only the females of the CB group, suggesting pronounced involvement of MM in these individuals.
This study suggests that masticatory muscle contraction efficiency was significantly less pronounced in the CB group. There were fewer muscle maximal motor units in both the TA and MM during chewing, and the proportion of myofiber motor unit activation (NMA ratio) was greater than in the other two groups, while the ECB and NB groups had similar EMG activity. This suggests that the masticatory muscles were more significantly involved in the CB patients. Palinkas et al[21] suggested that the reason for the low EMG signal during mastication in a bruxism patient is that the masticatory muscles are in a fatigued state with fewer motor units recruited by muscle contraction. Compared to mild bruxism patients, patients with severe bruxism have lower masticatory muscle contraction efficiency and show a higher NMA ratio during food chewing[24].
Compared with the NB group, the degree of EMG activity of both the WS TA and the NWS MM were enhanced in the ECB group. Under normal conditions, unilateral contraction of the MM can move the mandible toward the contracted side[8]. The presence of an abnormal increase in MM activity on the non-working side indicates that ECB can reinforce non-functional lateral muscle bundle contraction.
Compared to one previous study[24], this study was progressive in that a combination of questioning and free-chewing observations was used to differentiate and confirm the chewing PS. Although subjects who chewed unilaterally were excluded, subjects still chewed on a preferred side[8]. The comparative analysis based on the chewing preferred side/non-preferred side was superior to the comparative analysis based on the left side/right side, so the former was chosen in this study to improve the comparability of the results.
EMG activity can be used to assess the efficiency of the chewing cycle[25]. In this study, the CB showed inefficient chewing cycle. The CB group always had a longer chewing cycle duration than the other two groups, and the CB group showed significant differences during unilateral candy chewing. An analysis of each stage of the chewing process showed that the CB group always had the longest chewing cycle duration throughout mastication. One possible reason for this is that the masticatory muscles of CB patients become fatigued, and the muscle contraction capacity is diminished. Another possible reason is that clenching increases the depth of the central fossa of the posterior teeth and lengthens chewing trajectory, leading to longer chewing cycle duration.
It has been reported that the duration of the first chewing cycle is always longer than that of the subsequent chewing cycles[14]. Therefore, it was reasonable to separate out the first chewing cycle as a separate analysis in this study. Quantifying the chewing cycle duration at different chewing stages can reflect changes in the masticatory process. A similar change trend of chewing cycle duration in the different groups in this study indicated that there was no significant effect of the different types of bruxism on changes in the mastication process.
This study showed that masticatory muscle function is more significantly adversely affected in CB patients. It has been established that long-term motor loading can affect muscle capillary supply[26] or lead to muscle fiber damage[27]. The weaker muscle functional status in the CB group compared to the ECB group may be explained by the fact that bruxism behaviors may last longer in the CB group, i.e., clenching may occur both during the day and night. Compared to the tooth grinding in ECB patients, the sound of clenching in CB patients is more insidious and easily ignored by patients. Patients do not usually realize that they are clenching until painful symptoms appear in the masticatory muscles. Bruxism may lead to adverse consequences such as changes in occlusion and temporomandibular joint disorders in the long term[1,28]. However, it is not clear whether bruxism causes painful symptoms in the masticatory muscles[29]. Because previous studies did not classify bruxism based on the type of mandibular movement, in which the majority of bruxism patients included may have been ECB patients and CB was disregarded, no strong evidence of painful fatigue of the masticatory muscles due to bruxism was found. Clinicians should pay attention to the early diagnosis and intervention of CB to avoid the aggravation of its damage to the function of the stomatognathic system.
Previous studies mostly recommended occlusal splints (especially stabilization occlusal splint) as the primary treatment for bruxism. The occlusal splint is an appliance that reestablishes and maintains intermaxillary distance, which is often used to prevent the adverse effects of bruxism on the function of the stomatognathic system - to reduce excessive muscle activity, to evenly distribute occlusal forces, to relieve TMJ symptoms, and to prevent increased tooth wear[30-32]. However, due to the uncertainty of the cause of bruxism, previous treatments have not been able to eliminate bruxism[33]. The temporary occlusion provided by the occlusal splint should help to reorganize neuromuscular reflex activity, reduce the occurrence of bruxism, and restore the function of the masticatory muscles[34]. The appropriate type of occlusal splint should be selected depending on the mandibular movement characteristics of different types of bruxism.
Clenching behavior in CB patients is mainly involved in the bilateral TA and MM (predominantly MM). The axial force is increased because the direction of the muscle fiber alignment approximates the line of occlusal force, and patients show cup-shaped tooth wear and axial muscle impairment. Therefore, the muscle balance occlusal splint[35] should be used to release the tension of masticatory muscles caused by clenching, bring the mandible nearly into the postural position, reduce the contraction tension of the vertical muscle bundle, and maintain the coordination and stability of masticatory muscle function.
ECB is caused by the enhancement of non-functional lateral muscle bundles, which should be restricted. The buccal-pterygoid occlusal splint can be used to restrict the lateral mandibular movement, reduce the abnormal excitability of the muscle bundle, reorganize the neuromuscular reflex activity, and reduce the occurrence of teeth grinding to prevent the aggravation of tooth wear[36].
The subjects in this study were university students, so the study only reflects the characteristics of a single population in one age group and of a specific educational background. Although the high degree of homogeneity and compliance of the subjects made the results more objective, it also showed the limitations of the subject, such as the relatively short duration of bruxism. The study results mainly reflected the effect of early bruxism on the function of the masticatory muscles. Further studies should be extended to other types of populations in order to fully reflect the effects of bruxism on the function of the stomatognathic system.
The following conclusion can be drawn from the present experimental population: ECB mainly involves the temporalis anterior on the working side and the masseter muscle on the non-working side, with the temporalis anterior predominating, and the patients show enhanced contraction of non-functional lateral muscles; CB mainly involves the temporalis anterior and the masseter muscle bilaterally, of which the masseter muscle was predominant, and the patients show reduced masticatory muscle contraction efficiency and chewing cycle efficiency.
Bruxism is a rhythmic masticatory muscle activity that occurs involuntarily in a non-physiologically functional state. Previous studies have classified bruxism mainly according to its phase of occurrence. However, this classification does not provide targeted guidance for the treatment of bruxism. Bruxism can also be classified according to the type of mandibular movement: Centric bruxism (CB), which is dominated by centric clenching movements, and eccentric bruxism (ECB), which is dominated by eccentric grinding movements. There is a dearth of research classifying the functional status of masticatory muscles in patients with different mandibular movement types of bruxism.
This study analyzed the differences in the functional status of the masticatory muscles affected by different types of bruxism (CB and ECB), thus illustrating the need for diagnostic classification and corresponding treatment for patients with different types of bruxism.
To reflect the mainly involved types of masticatory muscle and the functional status of the masticatory muscle in patients with different types of bruxism.
A total of 21 CB subjects and 21 ECB subjects were screened from college students according to a questionnaire and their tooth wear features. Sixteen non-bruxism subjects were also recruited. The surface electromyography signals of the temporalis anterior (TA) and superficial masseter muscle (MM) were measured in different mandibular positions and during the chewing task. The electromyography amplitude and chewing cycle duration parameters were then analyzed.
The CB group showed a reduced number of muscle maximal motor units, with the MM being more pronounced, a higher proportion of muscle contractions to be recruited for the same load of chewing activity, and the longer chewing cycle duration. The ECB group showed an increased number of TA maximal motor units, and higher MM activity on the non-working side the in unilateral chewing. This study provides a myoelectrophysiological basis for the diagnostic classification of bruxism based on the type of mandibular movement. Since the subjects selected for this study were college students, the effect of bruxism on populations containing people at other age levels is not clear.
CB mainly affects the MM, and the patients show reduced masticatory muscle contraction efficiency and chewing cycle efficiency. ECB mainly affects the TA, and the patients show enhanced contraction of non-functional lateral muscle bundles.
The effects of different types of bruxism on people of other ages should be explored in further study to provide a comprehensive picture of the effects of bruxism on masticatory muscle function.
We thank prof. Zhang JX for providing guidance on the statistical analysis of this research. We thank Huang QX, Yang GH, and Chou LH for clinical assistance during the course of this research work. The participation of volunteers was greatly appreciated.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nassar G, France; Shopova D, Bulgaria A-Editor: Zhu JQ, China S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Lobbezoo F, Ahlberg J, Raphael KG, Wetselaar P, Glaros AG, Kato T, Santiago V, Winocur E, De Laat A, De Leeuw R, Koyano K, Lavigne GJ, Svensson P, Manfredini D. International consensus on the assessment of bruxism: Report of a work in progress. J Oral Rehabil. 2018;45:837-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 730] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 2. | Dawson PE. Functional occlusion from TMJ to smile design. 1st ed. St Louis: Mosby, 2007: 333-341. |
| 3. | Yan Y, Ling JQ, Wang JH, Hao YT. A study of craniomaxillofacial morphological characteristics of bruxism patients by cephalometric analysis. Zhonghua Kouqiang Yixue Yanjiu Zazhi. 2008;2:368-375. [DOI] [Full Text] |
| 4. | Okura K, Shigemoto S, Suzuki Y, Noguchi N, Omoto K, Abe S, Matsuka Y. Mandibular movement during sleep bruxism associated with current tooth attrition. J Prosthodont Res. 2017;61:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Ruiz JL. Seven signs and symptoms of occlusal disease: the key to an easy diagnosis. Dent Today. 2009;28:112-113. [PubMed] |
| 6. | Fuentes AD, Martin C, Bull R, Santander H, Gutiérrez MF, Miralles R. Natural mediotrusive contact: does it affect the masticatory and neck EMG activity during tooth grinding? Cranio. 2016;34:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Cecílio FA, Regalo SC, Palinkas M, Issa JP, Siéssere S, Hallak JE, Machado-de-Sousa JP, Semprini M. Ageing and surface EMG activity patterns of masticatory muscles. J Oral Rehabil. 2010;37:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | He SG, Yu HY. Oral anatomy and physiology. 8th ed. Beijing: People's Medical Publishing House. 2020;110-241. |
| 9. | de Rossi M, Palinkas M, de Lima-Lucas B, Santos CM, Semprini M, Oliveira LF, Hallak-Regalo I, Bersani EO, Miglioranca R, Siéssere S, Hallak-Regalo SC. Masticatory muscle activity evaluation by electromyography in subjects with zygomatic implants. Med Oral Patol Oral Cir Bucal. 2017;22:e392-e397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Clark BC, Manini TM, Thé DJ, Doldo NA, Ploutz-Snyder LL. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. J Appl Physiol (1985). 2003;94:2263-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Hugger A, Hugger S, Schindler HJ. Surface electromyography of the masticatory muscles for application in dental practice. Current evidence and future developments. Int J Comput Dent. 2008;11:81-106. [PubMed] |
| 12. | Wetselaar P, Wetselaar-Glas MJM, Katzer LD, Ahlers MO. Diagnosing tooth wear, a new taxonomy based on the revised version of the Tooth Wear Evaluation System (TWES 2.0). J Oral Rehabil. 2020;47:703-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Ferrario VF, Tartaglia GM, Luraghi FE, Sforza C. The use of surface electromyography as a tool in differentiating temporomandibular disorders from neck disorders. Man Ther. 2007;12:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Le Révérend B, Saucy F, Moser M, Loret C. Adaptation of mastication mechanics and eating behaviour to small differences in food texture. Physiol Behav. 2016;165:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Martinez-Gomis J, Lujan-Climent M, Palau S, Bizar J, Salsench J, Peraire M. Relationship between chewing side preference and handedness and lateral asymmetry of peripheral factors. Arch Oral Biol. 2009;54:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Lucas Bde L, Barbosa Tde S, Pereira LJ, Gavião MB, Castelo PM. Electromyographic evaluation of masticatory muscles at rest and maximal intercuspal positions of the mandible in children with sleep bruxism. Eur Arch Paediatr Dent. 2014;15:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Ferrario VF, Sforza C. Coordinated electromyographic activity of the human masseter and temporalis anterior muscles during mastication. Eur J Oral Sci. 1996;104:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Ferrario VF, Sforza C. Biomechanical model of the human mandible in unilateral clench: distribution of temporomandibular joint reaction forces between working and balancing sides. J Prosthet Dent. 1994;72:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. |
Wang MQ, Xie QF, Li XJ.
Occlusion. 4th ed. |
| 20. | Abe S, Yamaguchi T, Rompré PH, De Grandmont P, Chen YJ, Lavigne GJ. Tooth wear in young subjects: a discriminator between sleep bruxers and controls? Int J Prosthodont. 2009;22:342-350. [PubMed] |
| 21. | Palinkas M, Bataglion C, de Luca Canto G, Machado Camolezi N, Theodoro GT, Siéssere S, Semprini M, Regalo SC. Impact of sleep bruxism on masseter and temporalis muscles and bite force. Cranio. 2016;34:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Sun Y, Qin Z, Wan JJ, Wang PY, Yang YL, Yu JG, Hu BH, Su DF, Luo ZM, Liu X. Estrogen weakens muscle endurance via estrogen receptor-p38 MAPK-mediated orosomucoid (ORM) suppression. Exp Mol Med. 2018;50:e463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Palinkas M, Nassar MS, Cecílio FA, Siéssere S, Semprini M, Machado-de-Sousa JP, Hallak JE, Regalo SC. Age and gender influence on maximal bite force and masticatory muscles thickness. Arch Oral Biol. 2010;55:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Palinkas M, Seidel Coscarella L, Hirono Hotta T, Bataglion C, De Luca Canto G, Corrêa de Mello E, Maria Napolitano Gonçalves L, Siéssere S, Cecilio Hallak Regalo S. Influence of sleep bruxism severity on masticatory efficiency: electromyographic analysis. Arch Ital Biol. 2019;157:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Palinkas M, Cecilio FA, Siéssere S, Borges Tde F, de Carvalho CA, Semprini M, de Sousa LG, Regalo SC. Aging of masticatory efficiency in healthy subjects: electromyographic analysis--Part 2. Acta Odontol Latinoam. 2013;26:161-166. [PubMed] |
| 26. | Larsson B, Björk J, Kadi F, Lindman R, Gerdle B. Blood supply and oxidative metabolism in muscle biopsies of female cleaners with and without myalgia. Clin J Pain. 2004;20:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Larsson SE, Bengtsson A, Bodegård L, Henriksson KG, Larsson J. Muscle changes in work-related chronic myalgia. Acta Orthop Scand. 1988;59:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Ommerborn MA, Giraki M, Schneider C, Fuck LM, Handschel J, Franz M, Hans-Michael Raab W, Schäfer R. Effects of sleep bruxism on functional and occlusal parameters: a prospective controlled investigation. Int J Oral Sci. 2012;4:141-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Jiménez-Silva A, Peña-Durán C, Tobar-Reyes J, Frugone-Zambra R. Sleep and awake bruxism in adults and its relationship with temporomandibular disorders: A systematic review from 2003 to 2014. Acta Odontol Scand. 2017;75:36-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 30. | Shopova D, Bozhkova T, Yordanova S, Yordanova M. Case Report: Digital analysis of occlusion with T-Scan Novus in occlusal splint treatment for a patient with bruxism. F1000Res. 2021;10:915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Kosturkov D, Taneva I, Uzunov T. Examination of pulp innervation of teeth with abrasion. Problems of Dental Medicine. 2020;46:12-17. |
| 32. |
Bozhkova T, Shopova D.
T-Scan Novus System in the Management of Splints-Pilot Study. |
| 33. | Taneva I, Uzunov T, Milanov N. Complete digital approach for bruxism management. Problems of Dental Medicine. 2020;46:18-27. |
| 34. | Pita MS, Ribeiro AB, Garcia AR, Pedrazzi V, Zuim PR. Effect of occlusal splint thickness on electrical masticatory muscle activity during rest and clenching. Braz Oral Res. 2011;25:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Yan Y, Jiang LL, Lan KW, Feng YF, Chen JM, Wu MH, inventor; Guangzhou Zhiyuanchuangda Patent Agency Inc. assignee. The muscle balance occlusal splint. Chinese patent CN 202120549626.3. 2021, Mar 17. |
| 36. | Yan Y, Wang JH, Feng YF, Jiang LL, Chen JM, Wu MH, inventor; Guangzhou Sanhuan Patent Agency Inc. assignee. Maxillary buccal-pterygoid splint. Chinese patent CN 201620908577.7. 2017, Apr 10. |