Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6855
Peer-review started: November 8, 2021
First decision: April 19, 2022
Revised: April 23, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: July 16, 2022
Processing time: 238 Days and 21.3 Hours
Several methods, such as finger fracture, Pean crush, cavitron ultrasonic surgical aspirator (CUSA), and water jet (WJ), are used for hepatic parenchymal dissection in liver surgery. CUSA is the conventional method in Japan. WJ is a relatively novel method for parenchymal dissection. Although it has several advantages, such as lower volume of blood loss and shorter operative time, the effect of the WJ system for hepatic dissection on the remnant liver has not yet been investigated.
To investigate and compare the effect of the WJ method vs CUSA on the remnant liver cut surface.
This observational study compared the two types of parenchymal transection methods (WJ vs CUSA) in liver surgery. In total, 24 and 40 patients who underwent hepatectomy using the WJ method and CUSA, respectively, were included in the analysis. Accordingly, the clinicopathological characteristics and clinical outcomes of 24 and 40 patients were compared. Furthermore, posto
On CT scan, the median areas of denaturation in the liver dissection planes were 522 (range: 109.5-1242) mm2 in the CUSA group and 324 (range: 93.6-1529) mm2 in the WJ group. The area did not significantly differ between the two groups; however, the denaturation thickness of the WJ group was significantly lower than that of the CUSA group [5.8 (range: 0.7-11.1) mm vs 3.3 (range: 1.7-10.4) mm, P < 0.001].
The WJ group had significantly thinner contrast-enhanced areas in the post hepatectomy detached section than the CUSA group.
Core Tip: We evaluated the denaturation degree of the liver dissection plane on computed tomography (CT) scan and compared the cavitron ultrasonic surgical aspirator and water jet (WJ) method. The perioperative outcomes were almost similar. However, the WJ method had a lower loss of contrast effect on the liver dissection plane. Moreover, we found a positive correlation between the denaturation degree on CT scans and the postoperative peak of hepatic enzymes. The reduced contrast effect on CT scan reflects thermal degeneration during hepatectomy, and the WJ method may be more advantageous in preserving the remnant functioning liver volume during hepatectomy.
- Citation: Hanaki T, Tsuda A, Sunaguchi T, Goto K, Morimoto M, Murakami Y, Kihara K, Matsunaga T, Yamamoto M, Tokuyasu N, Sakamoto T, Hasegawa T, Fujiwara Y. Influence of the water jet system vs cavitron ultrasonic surgical aspirator for liver resection on the remnant liver. World J Clin Cases 2022; 10(20): 6855-6864
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6855.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6855
Hepato-parenchymal transection is an essential step in liver surgery. Since Langenbuch[1] reported the first successful liver surgery in 1888, different hepatectomy methods have been investigated and performed[2,3]. The major tools used to dissociate the liver parenchyma include the classical finger crush, which can dissociate the liver parenchyma using the finger[4], the more modern Pean-clump crushing method[5], cavitron ultrasonic surgical aspirator (CUSA), which can dissociate the liver parenchyma by applying an oscillator with a suction device[3], and water jet (WJ) scalpel, which can dissociate the liver parenchyma with a high-pressure saline flow[6]. All these methods are used for parenchymal fragmentation in the hepatic incision, and the emerging vascular structures such as the Glissonean sheath and veins must be incised after ligation via suturing, clipping, or sealing with energy devices, depending on their diameters. Thus, the hepatic incision can be divided into two steps: incision of the liver parenchyma and incision after ligation for the emerging small vascular structures.
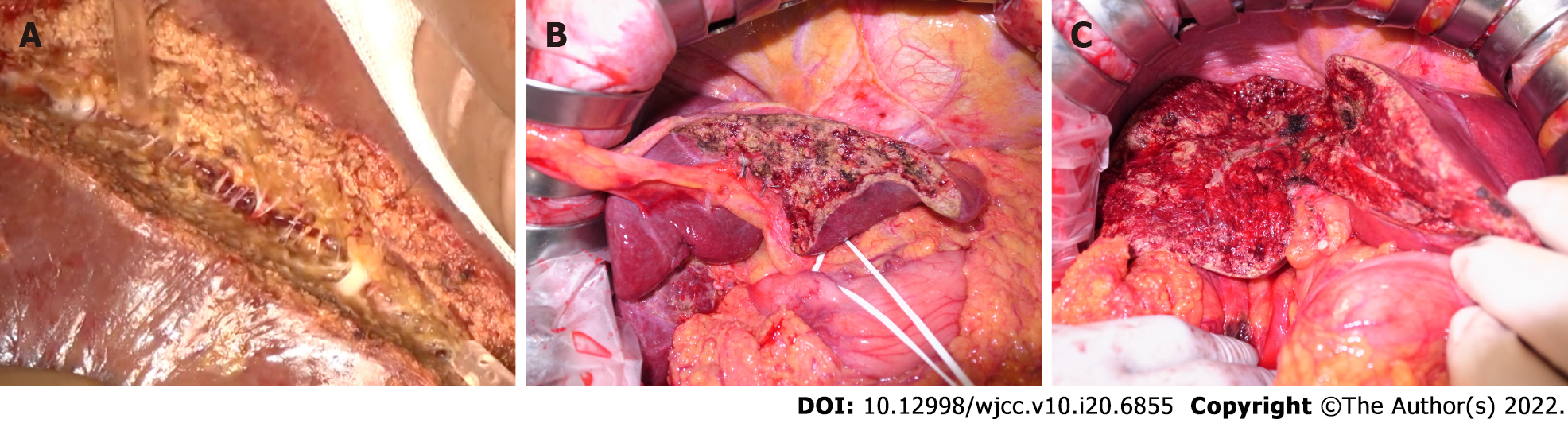
The WJ method removes hepatocytes and exposes the vascular and bile duct structures between the liver parenchyma by jetting saline[7]. Initially, high-pressure fluid cutting was a technique developed in the glass and metal industries[8]. Since the application of the WJ system in the medical field in 1982[9], it has been marketed as a commercial product (Helix Hydro-Jet®, Erbe Elektromedizin GmbH, Tübingen, Germany) since 2001. As an extension model of the WJ system, Erbejet2® (Erbe Elektromedizin GmbH, Tübingen, Germany) has been marketed and used worldwide. Hepatectomy using the WJ method is relatively novel and is associated with the absence of thermal damage caused by the dissection procedure because saline is used as a jetted effector. In our experience, among the abovementioned methods, the WJ system is the most sensitive in exposing the quite delicate vascular structures in the liver parenchyma without bleeding or bile leakage (Figure 1A-C).
Hemorrhages in the hepatic dissected surface can be treated by compression and suture hemostasis and cauterization with energy devices[10,11]. However, all these methods may cause some damage to the remnant liver. The remnant functioning liver must be sufficient to prevent postoperative liver failure in liver surgery. Therefore, damage to the hepatic resection surface must be prevented. That is, unnecessary damage leading to a lower remaining liver volume should be avoided. Several reports show the blood loss volume, operative time, and postoperative complications of different liver dissection methods[3,12,13]. However, none have examined the effect of each technique on the liver dissected surface. Considering that the WJ system may have less impact on the residual liver than CUSA, which is a conventional method, as mentioned above, the perioperative outcomes of liver dissection were retrospectively compared. Thus, the current single-center retrospective study evaluated the efficacy of the WJ system and CUSA on the liver transection cut surface during resection.
From April 2019 to April 2021, 87 patients who underwent hepatectomy at Tottori University Hospital were retrospectively assessed, and 64 consecutive patients who had contrast-enhanced computed tomography (CE-CT) scan with portal phase study on postoperative day (POD) 7 (± 2 d) were included in this study. However, 13 patients who did not undergo postoperative CE-CT scan or who had plain CT scan only because of impaired renal function or allergy to CT scan contrast agents were excluded.
All patients underwent preoperative blood tests within 2 wk before hepatectomy. The following data about preoperative factors were collected: age; sex; body mass index; a medical history of diabetes mellitus; creatinine, albumin, total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels; platelet count; prothrombin activity; Child-Pugh score and grade; and degree of liver damage. In addition, data about intra- and postoperative factors were collected according to the degree of hepatic resection (Hr 0/S, 1, 2, or more), operative time, volume of blood loss, highest postoperative AST and ALT levels, surgical complications based on the Clavien-Dindo classification, type and volume of intra- and postoperative blood transfusion, pathological diagnosis, and degree of background liver fibrosis, which was assessed microscopically according to the Inuyama Classification after Mallory-Azan or Elastica van Gieson staining[14]. Moreover, the degree of liver fibrosis was assessed, and the patients were divided into two groups: The non- and early cirrhotic (F0 + F1 + F2) group and the chronic cirrhotic (F3 + F4) group. The highest AST and ALT levels were adopted based on examining blood samples collected on PODs 1, 2, 3, 5, and 7.
All procedures carried out in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the ethics committee of Tottori University School of Medicine (approval No. 21A081). This study did not perform any animal studies. In addition, due to the study's retrospective nature, written consent from the patient was not required.
In total, 64 hepatectomies were performed at Tottori University Hospital, and two surgeons (Hanaki T and Sakamoto T) specializing in liver surgery performed all surgeries. Moreover, 40 patients underwent hepatectomies using CUSA (CUSA EXcel Plus; Integra LifeSciences Corp., Plainsboro, NJ, United States), and 24 patients had surgeries with the WJ system (ERBE JET2; Erbe Elektromedizin GmbH, Tübingen, Germany). The following settings were applied in the WJ system: saline blow for 35 bar (35 × 104 Pa), which is the default value, with continuous suction pressure of -0.8 bar (-8 × 103 Pa). Until November 2019, parenchymal dissection of the liver was performed using CUSA. Then, unless there are institutional constraints, the WJ is used as the primary technique for liver dissection. The saline-cooled monopolar soft-coagulation system (VIO300D; Erbe Elektromedizin GmbH, Tübingen, Germany) was utilized for hemostasis, particularly for bleeding in the dissected surface of the liver in both groups. The vessels exposed during parenchymal transection (1 mm or less) were dissected using coagulating shears (Harmonic 1000HDi; Ethicon, Inc., Cincinnati, OH, United States), and vessels measuring 1 mm or more were ligated with 4-0 or 3-0 silk and were dissected. In principle, during liver dissection, the intermittent Pringle's maneuver was performed with ≤ 15 min of clamping and ≥ 5 min of de-clamping. The extent of hepatic resection performed was classified as follows: Hr0 + S: hepatic resection of one subsegment or not exceeding one subsegment, Hr1: hepatic resection of one segment, and Hr2: hepatic resection of two segments according to the general rules for the clinical and pathological study of primary liver cancer[15].
All patients underwent a preoperative multidetector CT scan (64 sections) with Aquilion CX (Canon Inc., Tokyo, Japan) or Discovery CT 750HD (G.E. Healthcare, Chicago, IL, United States) on POD 7 (± 2). 580 mg of iodine/kg was administrated as the contrast agent using a mechanical injector with variable injection speeds via a plastic intravenous catheter typically placed in the median cubital vein within 30 s. A scan delay of 60 s was performed for portal phase imaging. The generated CT reconstruction images with 5-mm thickness of the upper abdomen were used for all imaging evaluations in this study.
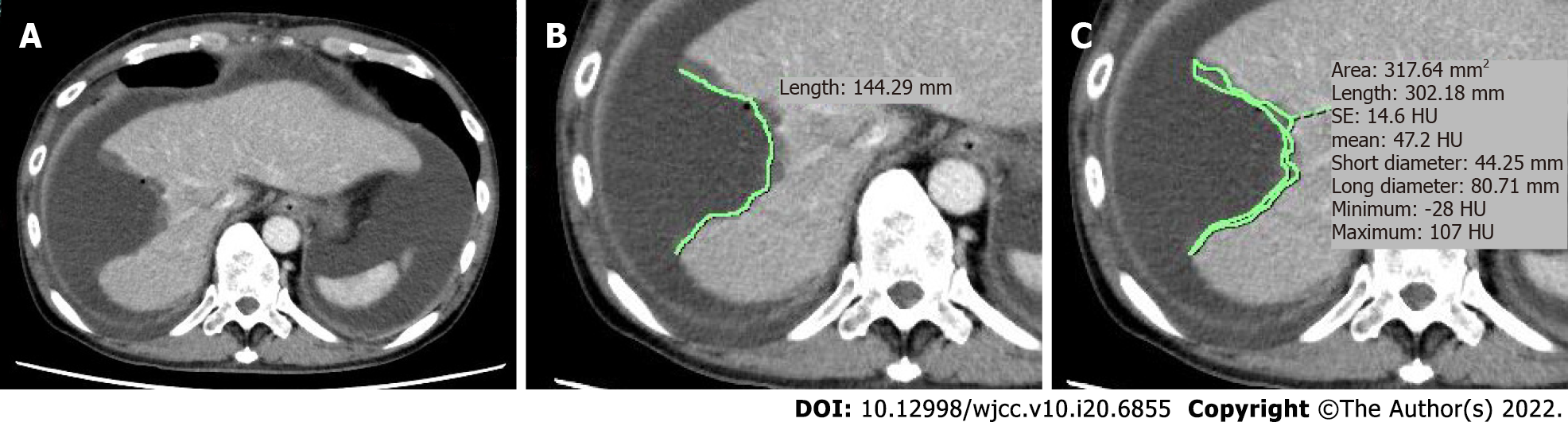
The images were saved in DICOM format and transferred to an image workstation with a dedicated image assessment software (SYNAPSE SAI viewer; FUJIFILM Corp., Tokyo, Japan). As mentioned above, the CE-CT slice of the portal phase study with the longest liver dissection length was used as the evaluation image of the dissected liver. The CT slice of the liver section with the longest dissection length (mm) was measured manually (Figure 2A). In the same slice, the area (mm2) of the liver section where the contrast effect was reduced due to thermal denaturation was also measured manually, thereby preventing fluid collection around the hepatic transection plane (Figure 2B). Next, the denatured area-to-the liver dissected length ratio was calculated as the denaturation index (DI), or denatured thickness: denatured area (mm2)/dissected length (mm) (Figure 2). When multiple hepatectomies were performed in the same liver, the abovementioned data were calculated for each slice with the longest liver dissection lengths and largest areas. The sum of the data for lengths and areas, respectively, were then used as the representative data of each patient.
All graphing and statistical analyses were performed using the Statistical Package for the Social Sciences software for Macintosh version 25.0 (IBM Corp., Armonk, NY, United States). A two-sided P value of < 0.05 was considered statistically significant. Continuous data were presented as median with range. The Mann-Whitney U test and the Fisher's exact test or the chi-square test evaluated differences in continuous and categorical variables, respectively. The correlation tests (Pearson correlation) examined relationships between numerical variables.
In total, 64 patients were included in this study. Table 1 shows the CUSA and WJ groups' pre-, intra-, and postoperative characteristics. In total, 40 (28 men and 12 women) and 24 (15 men and 9 women) patients underwent hepatectomy using CUSA and the WJ system, respectively. Preoperative factors such as age, sex, physical condition, results of the blood test including indocyanine green, and background liver status did not differ significantly between the two groups. Twenty-four (60%) patients underwent surgery using the open approach in the CUSA group. Meanwhile, in the WJ group, all patients underwent hepatectomy with the open approach, with a significantly higher laparotomy rate (P < 0.001). Most patients (82.4% in the CUSA group and 95.8% in the WJ group, P = 0.241) did not undergo extrahepatic bile duct resection. There was no significant difference in terms of Hr volume or operative time. However, the WJ group had a significantly higher volume of blood loss than the CUSA group (432 mL vs 227.5 mL, P = 0.047). By contrast, there were no significant differences in the incidence of postoperative complications, incidence and severity of biliary fistulas, and intra- and postoperative red blood cell and fresh frozen plasma transfusions. There was no in-hospital mortality in both groups. The length of postoperative hospital stay did not significantly differ between the two groups. Histological evaluation showed no significant differences in the incidence rate of chronic cirrhosis (F3 or 4) between the two groups (P = 0.778).
| Characteristics | CUSA (n = 40) | Water jet (n = 24) | P value | ||
| Pre-operative | |||||
| Age (yr) | 72 (36-87) | 73 (54-83) | 0.588 | ||
| Sex (male/female) | 28/12 | 15/9 | 0.589 | ||
| BMI (kg/m2) | 22.5 (16.0-28.0) | 22.5 (16.1-44.9) | 0.782 | ||
| ASA-PS (1/2/3/4) | 2/29/9/0 | 0/15/8/1 | 0.290 | ||
| Medical history of DM (yes/no) | 11/29 | 9/15 | 0.419 | ||
| Chemical examination of blood | |||||
| Creatine (mg/dL) | 0.72 (0.19-1.32) | 0.78 (0.48-8.10) | 0.244 | ||
| Albumin (g/dL) | 4.15 (3.10-4.80) | 4.15 (3.50-4.90) | 0.895 | ||
| Total bilirubin (mg/dL) | 0.75 (0.20-2.30) | 0.70 (0.20-1.20) | 0.198 | ||
| Aspartate transaminase (IU/L) | 25 (14-134) | 24 (9-52) | 0.546 | ||
| Alanine transaminase (IU/L) | 22 (10-125) | 21 (9-50) | 0.593 | ||
| Platelet (× 105/μL) | 19 (8.8-84) | 23 (10.9-93) | 0.204 | ||
| Prothrombin time (%) | 95.6 (49.1-127.5) | 99.7 (64.9-112.3) | 0.375 | ||
| ICG-R15 (%) | 12 (3-27) | 12.5 (2-29) | 0.722 | ||
| ICG (K) | 0.15 (0.06-0.24) | 0.15 (0.10-0.25) | 0.543 | ||
| Child-Pugh grade (5/6/7 or more) | 34/6/0 | 22/2/0 | 0.699 | ||
| Liver damage (A/B/C) | 39/1/0 | 23/1/0 | 0.999 | ||
| Intra-operative | |||||
| Operative procedure | |||||
| Approach (open/laparoscopic) | 24/16 | 24/0 | < 0.001 | ||
| Hr (0 + S, 1, 2) | 23/7/10 | 8/8/8 | 0.151 | ||
| Extrahepatic bile duct resection (yes/no) | 6/34 | 1/23 | 0.241 | ||
| Operation time (min) | 235 (76-980) | 298 (187-543) | 0.061 | ||
| Blood loss (mL) | 227.5 (0-1820) | 432 (95-1165) | 0.047 | ||
| Post-operative | |||||
| Clavien Dindo classification (0 or I/II/III/IV) | 32/4/4/0 | 14/3/7/0 | 0.118 | ||
| Biliary fistula grade (none or A/B/C) | 38/2/0 | 22/2/0 | 0.627 | ||
| Blood transfusion (include intraoperative) | |||||
| Red blood cell (yes/no) | 1/39 | 2/22 | 0.551 | ||
| Fresh frozen plasma (yes/no) | 5/35 | 7/17 | 0.113 | ||
| Highest value of blood examination | |||||
| Aspartate transaminase (IU/L) | 368 (40-1653) | 188 (37-1084) | 0.315 | ||
| Alanine transaminase (IU/L) | 279.5 (37-2026) | 147 (33-1236) | 0.241 | ||
| In hospital mortality | 0 | 0 | NA | ||
| Post-operative hospital stay (d) | 8 (5-57) | 8.5 (6-190) | 0.949 | ||
| Histological diagnosis | 0.028 | ||||
| Hepatocellular carcinoma (%) | 18 (45) | 12 (50) | |||
| Intrahepatic cholangiocarcinoma (%) | 1 (2.5) | 6 (25) | |||
| Combined hepatocellular and cholangiocarcinoma (%) | 1 (2.5) | 1 (4.2) | |||
| Bile duct cancer (%) | 7 (30) | 0 (0) | |||
| Metastatic cancer (%) | 12 (2.5) | 4 (16.7) | |||
| Other (benign lesion) (%) | 1 (17.5) | 1 (4.2) | |||
| F (0, 1 or 2/3 or 4) | 28/12 | 18/6 | 0.778 | ||
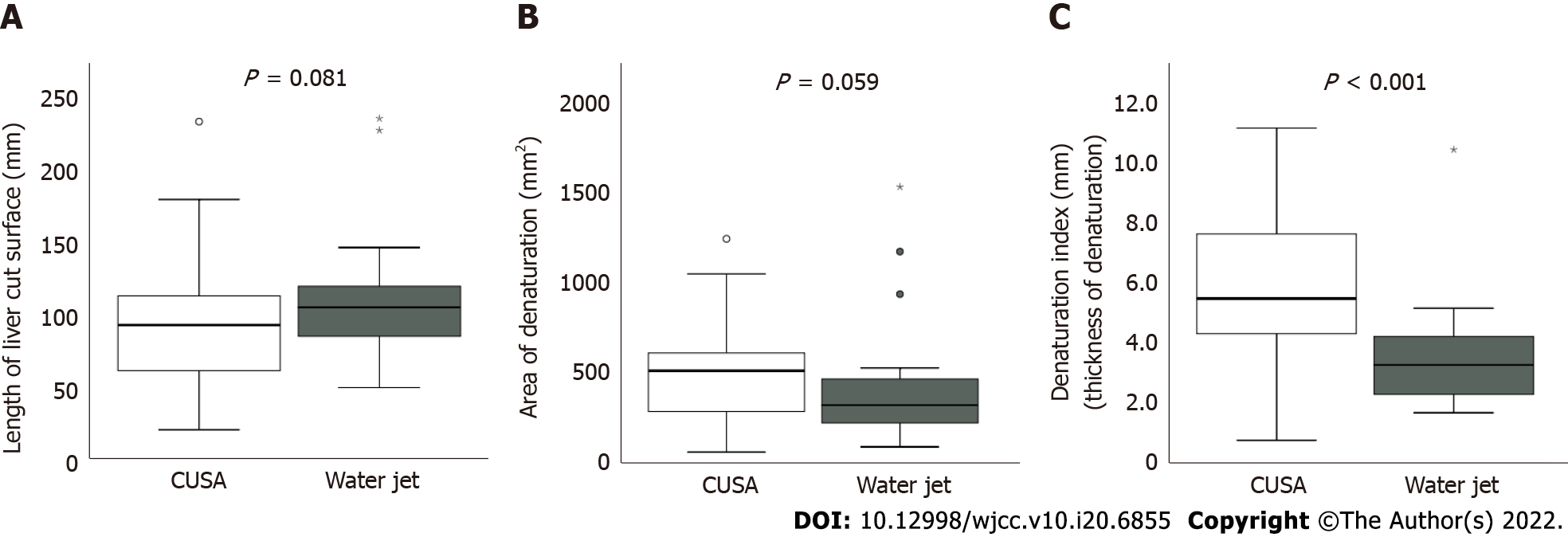
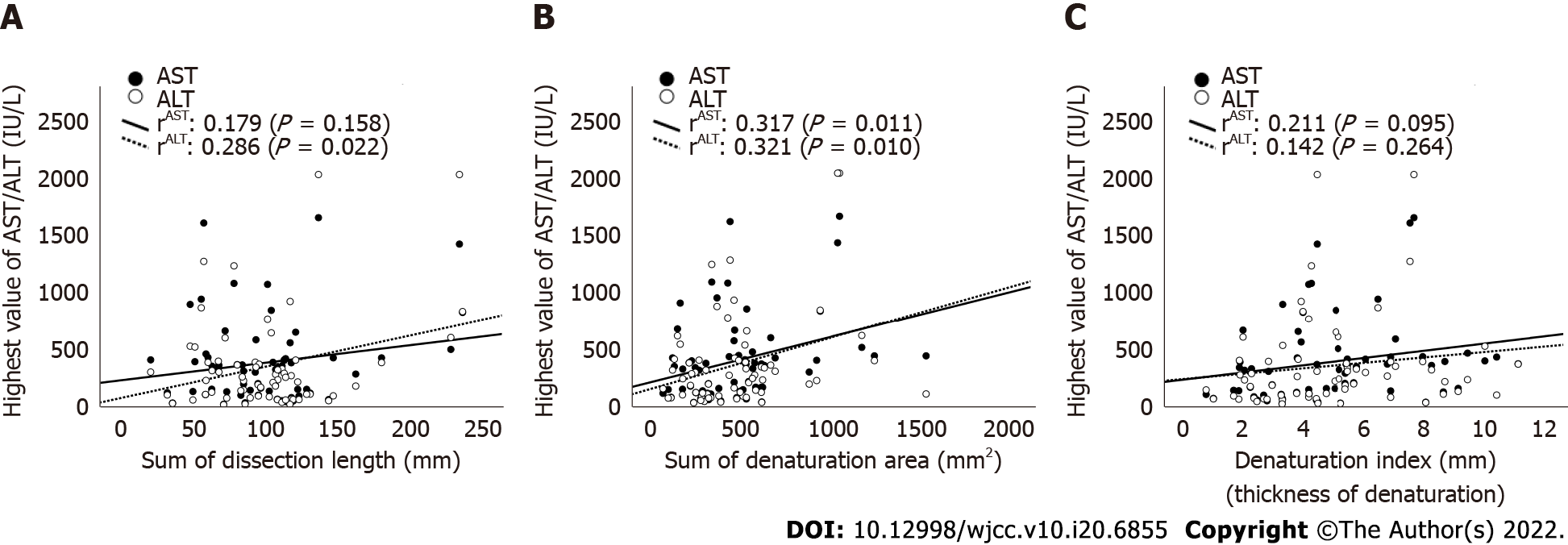
Table 2 depicts the results of the evaluation of CE-CT images. The median lengths of the liver dissection plane were 95.3 (range: 21.5-233.9) mm in the CUSA group and 105.9 (range: 50.6-236.1) mm in the WJ group. The median areas of denaturation on the liver dissection planes were 522 (range: 109.5-1242) mm2 in the CUSA group and 324 (range: 93.6-1529) mm2 in the WJ group. Both lengths and areas did not significantly differ between the two groups (P = 0.081, 0.059, respectively). However, the DI of the WJ group was significantly lower than that of the CUSA group (3.3 vs 5.8 mm, P < 0.001, Figure 3). The scatter plot of the CT values (length, area, and DI) obtained in this study was established to explore its implications in relation to the highest postoperative AST and ALT levels (Figure 4A-C). The scatter plots of the highest postoperative AST/ALT values and area of denaturation had a weak but significant positive correlation with these indices (rAST = 0.317, P = 0.011; rALT = 0.312, 0.010, Figure 4B).
| Post-operative CT values | CUSA (n = 40) | Water jet (n = 24) | P value |
| Sum of dissection length (mm) | 95.3 (21.5-233.9) | 106 (50.6-236.1) | 0.081 |
| Sum of denaturation area (mm2) | 522 (109.5-1242) | 324 (93.6-1529) | 0.059 |
| Denaturation index (denaturation thickness, mm) | 5.8 (0.7-11.1) | 3.3 (1.7-10.4) | < 0.001 |
This study showed that patients who underwent hepatectomy in the WJ group had a significantly lower denaturation thickness (DI) on the hepatic dissection plane than those in the CUSA group. To the best of our knowledge, this is the first study that evaluated the effect of transection methods on the hepatic resection plane based on the examination of a real human body.
Hepatectomy is routinely performed to resect benign lesions and primary and metastatic neoplasms in the liver and is the only curative therapy for malignancies[16-18]. Moreover, it is a complex surgical procedure. For ultimate simplicity, it can be divided into two steps: dissection of the liver parenchyma as well as ligation and dissection of the exposed vessels. These steps must be performed safely to secure the vessels safely. In this study, the WJ method could expose the small vessels without damaging them more than the CUSA method. Further, it first examined whether the WJ method cannot cause bleeding. Results showed that the WJ group had a higher volume of blood loss than the CUSA group (Table 1). However, this result did not differ from our intraoperative impression. This might be attributed to the fact that, in addition to the actual volume of intraoperative blood loss, the volume of saline used to dissect the liver parenchyma was calculated together with the volume of blood loss. In relation to this, there was no significant difference in terms of intra- and postoperative blood (fresh frozen plasma and red blood cell) transfusion rate between the CUSA and WJ groups. Furthermore, there was less degeneration of the liver parenchyma after hepatic incision using the WJ method (Figure 1B and C). This could be attributed to the possibility that the WJ group required less electrocautery and suturing procedures for minor bleeding. Moreover, the WJ group had a significantly higher DI or degenerative thickness of the hepatic dissection than the CUSA group based on the CT values examined in this study (Figure 3C). In addition, DI was positively correlated, although weakly, with the highest postoperative AST and ALT levels. Therefore, this index might have some biological significance in indicating damage in the remnant liver. The ability of the WJ method to expose the fine vessels in the liver parenchyma was better based on our experience. The WJ method had a better exposure ability in preserving these vessels. This reduces the need for electrocauterization for bleeding, resulting in a reduced DI in the hepatic dissection plane.
It is quite difficult to directly validate the relevance of histological changes to the site of CT changes in vivo and in clinical settings. However, thermal degeneration of liver sections caused by electrocautery is often observed on the cross-section of a specimen by hepato-biliary-pancreatic surgeons (Figure 5). Even in in vivo remnant livers, cauterization, or suturing for hemorrhage results in changes observed in the specimen. Hence, the contrast effect at the detachment site was reduced (Figure 2), which might have been observed as a lower DI in the liver resection surface.
The current study had several limitations. That is, it has a retrospective design, the sample size was small, and there was an unclear relationship between CT values and clinical data. Hence, a prospective study with cautious planning should be performed to determine whether a small area of denaturation in the liver section is associated with preventing post-hepatectomy liver failure.
WJ hepatectomy has a short learning curve and can be easily mastered[8]. In this study, in terms of safety, WJ hepatectomy was not inferior to CUSA hepatectomy, which is a conventional method. The WJ method can effectively reduce thermal degeneration during dissection of the preserved liver and can be one of the most effective liver dissection techniques.
The WJ method is as safe as CUSA. However, it is associated with a slightly higher volume of intraoperative blood loss and, possibly, lower thermal damage to the detached liver section. Moreover, it may be more advantageous in preserving the remnant functioning liver volume during massive hepa
Reports on the usefulness of the water jet (WJ) technique as a relatively new liver resection method have been increasing. Although there have been many reports on the effectiveness of WJ in improving blood loss and operation time, there has been no evaluation of its effect on the hepatic dissection plane.
Our department has used the cavitron ultrasonic surgical aspirator (CUSA) method for liver parenchymal sections for many years; however, we recently introduced the WJ for liver resection. Based on this experience, we observed the liver section planes of the WJ and CUSA methods, and we had the impression that the WJ method had less thermal degeneration on the dissected plane. Although less degeneration of the residual liver may lead to preservation of residual liver function and further contribute to avoiding postoperative liver failure, there are no previous reports evaluating the denaturation of the liver dissected cross-section by the method of liver dissection.
To investigate and compare the impact of the WJ and CUSA methods on the residual liver cut surface.
Forty cases of liver resection with CUSA and 24 cases of liver resection with WJ who underwent liver resection between 2019 and 2021 and had contrast-enhanced computed tomography (CT) during postoperative hospitalization were included in this retrospective study. Furthermore, the postoperative CT scans were used to assess the cut surface length of the remnant liver and the degenerative thickness of the areas with a reduced contrast effect in the dissected plane.
On CT scan, the median areas of denaturation in the liver dissection planes were 522 (range: 109.5-1242) mm2 in the CUSA group and 324 (range: 93.6-1529) mm2 in the WJ group. The area did not significantly differ between the two groups; however, the denaturation thickness of the WJ group was significantly lower than that of the CUSA group [5.8 (range: 0.7-11.1) mm vs 3.3 (range: 1.7-10.4), P < 0.001].
Hepatectomies using WJ showed significantly thinner low-contrast areas in the dissected plane than CUSA.
The WJ hepatectomy may contribute to safe liver resection in terms of minor thermal damage on the residual liver and avoiding postoperative liver failure.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li HL, China; Wang LM, China A-Editor: Yao QG, China S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Langenbuch C. Ein Fall von Resection eines linksseitigen Schnürlappens der Leber. Heilung. In: Heinz-Peter S, Winau R, Rudolf H. Erste Operationen Berliner Chirurgen 1817–1931. De Gruyter: Walter de Gruyter GmbH, 2015: 59-61. |
| 2. | Bismuth H, Houssin D, Castaing D. Major and minor segmentectomies "réglées" in liver surgery. World J Surg. 1982;6:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 168] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Kokudo N, Kimura H, Yamamoto H, Seki M, Ohta H, Matsubara T, Takahashi T. Hepatic parenchymal transection using ultrasonic coagulating shears: a preliminary report. J Hepatobiliary Pancreat Surg. 2000;7:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Gans H, Mori K, Matsumoto K, Tan BH. Evaluation of the effects of the finger fracture technique used in hepatic resection. Surg Gynecol Obstet. 1974;138:885-890. [PubMed] |
| 5. | Ishizaki Y, Yoshimoto J, Sugo H, Miwa K, Kawasaki S. Hepatectomy using traditional Péan clamp-crushing technique under intermittent Pringle maneuver. Am J Surg. 2008;196:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Rau HG, Duessel AP, Wurzbacher S. The use of water-jet dissection in open and laparoscopic liver resection. HPB (Oxford). 2008;10:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Papachristou DN, Barters R. Resection of the liver with a water jet. Br J Surg. 1982;69:93-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Vollmer CM, Dixon E, Sahajpal A, Cattral MS, Grant DR, Gallinger S, Taylor BR, Greig PD. Water-jet dissection for parenchymal division during hepatectomy. HPB (Oxford). 2006;8:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Tschan CA, Tschan K, Krauss JK, Oertel J. First experimental results with a new waterjet dissector: Erbejet 2. Acta Neurochir (Wien). 2009;151:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Itoh S, Fukuzawa K, Shitomi Y, Okamoto M, Kinoshita T, Taketomi A, Shirabe K, Wakasugi K, Maehara Y. Impact of the VIO system in hepatic resection for patients with hepatocellular carcinoma. Surg Today. 2012;42:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Ishiko T, Inomata Y, Beppu T, Asonuma K, Okajima H, Takeiti T, Tikamoto A, Yamamoto H, Baba H. An improved technique for liver transection using a new device for soft coagulation in living donor hepatectomy. Hepatogastroenterology. 2012;59:1907-1910. [PubMed] |
| 12. | Liu F, Wei Y, Li H, Wang W, Wen T, Wu H, Yang J, Xu M, Li B. LigaSure vs CUSA for parenchymal transection during laparoscopic hepatectomy in hepatocellular carcinoma patients with cirrhosis: a propensity score-matched analysis. Surg Endosc. 2018;32:2454-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Bodzin AS, Leiby BE, Ramirez CG, Frank AM, Doria C. Liver resection using cavitron ultrasonic surgical aspirator (CUSA) vs harmonic scalpel: a retrospective cohort study. Int J Surg. 2014;12:500-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Ichida F, Tsuji T, Omata M, Ichida T, Inoue K, Kamimura T, Yamada G, Hino K, Yokosuka O, Suzuki H. New Inuyama classification; new criteria for histological assessment of chronic hepatitis. Int Hepatol Commun. 1996;6:112-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 273] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Liver Cancer Study Group of Japan. Primary Liver Cancer in Japan. 3rd ed.Tokyo: Kanehara & Co., Ltd., 2015. |
| 16. | Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Farges O, Fuks D, Boleslawski E, Le Treut YP, Castaing D, Laurent A, Ducerf C, Rivoire M, Bachellier P, Chiche L, Nuzzo G, Regimbeau JM. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254:824-29; discussion 830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 278] [Article Influence: 21.4] [Reference Citation Analysis (0)] |