Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6769
Peer-review started: October 26, 2021
First decision: December 27, 2021
Revised: January 10, 2022
Accepted: May 13, 2022
Article in press: May 13, 2022
Published online: July 16, 2022
Processing time: 251 Days and 13.2 Hours
Moderately severe and severe acute pancreatitis is characterized by local and systemic complications. Systemic complications predominate the early phase of acute pancreatitis while local complications are important in the late phase of the disease. Necrotic fluid collections represent the most important local com
Core Tip: Percutaneous catheter drainage is an important method for drainage of pancreatic fluid collections. In the early stage of the disease (2-4 wk), it is often the method of choice. It is shown to be effective alone in almost 50% of the patients. It is also an important part of the dual modality treatment that involves endoscopic drainage. It acts as a gateway for percutaneous endoscopic and minimally invasive surgical necrosectomy. There is evolving data regarding the indications and timing of drainage. Additionally, there are several recent studies describing methods to improve the percutaneous catheter drainage outcomes.
- Citation: Bansal A, Gupta P, Singh AK, Shah J, Samanta J, Mandavdhare HS, Sharma V, Sinha SK, Dutta U, Sandhu MS, Kochhar R. Drainage of pancreatic fluid collections in acute pancreatitis: A comprehensive overview. World J Clin Cases 2022; 10(20): 6769-6783
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6769.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6769
Acute pancreatitis (AP) is an acute inflammatory condition of the pancreas and one of the common causes of acute abdomen presenting as a medical emergency. Severity ranges from mild to moderately severe AP (MSAP) and severe AP disease (SAP). Mild AP has an excellent prognosis with conservative treatment[1,2]. In patients having infected necrosis or organ failure (OF), mortality rises to about 30% despite invasive and surgical management[1].
According to the revised Atlanta classification (RAC), AP is divided into early (< 1 wk) and late (> 1 wk) phases. The early phase is characterised by systemic inflammatory response and the late phase characterized by persistent OF or systemic complications[1]. Based on imaging findings, AP is divided into interstitial edematous pancreatitis (IEP) and acute necrotizing pancreatitis depending on the presence of necrosis (pancreatic, peripancreatic or both)[1,2].
Pancreatic fluid collections (PFCs) represent important local complications of AP. Not all PFCs are necrotic and not all necrotic collections are infected. Therefore, RAC provided an important distinction between the collections which contained purely fluid contents and those which also contained necrotic debris. PFCs associated with IEP are called acute peripancreatic fluid collections (APFCs; ≤ 4 wk) and pseudocysts (> 4 wk); and those associated with necrosis are called acute necrotic collections (ANCs ≤ 4 wk) and walled off necrosis (WON; > 4 wk) (Figure 1). Any of the above mentioned collections can get infected and require intervention[1,2]. Recent studies have highlighted that 4 wk threshold for classification of PFCs is imprecise. In fact, many collections have partial/clinically significant encapsulation in the 3rd week of illness[3-5].
Management of AP and its complications requires a multidisciplinary approach involving gastroenterologists, interventional radiologists, and surgeons. Conservative management is the rule in the early phase of AP, which includes fluid resuscitation, pain control, prophylactic antibodies, and oxygen and nutritional support. Antibiotic treatment should be initiated only in culture proven infection or when there is strong clinical suspicion of infection. They should not be given prophylactically[6]. Enteral feeding may become necessary to bypass the inflamed region of the bowel adjacent to the pancreas and may be achieved by nasojejunal tube or percutaneous jejunostomy[6,7]. PFCs can be treated conservatively or by percutaneous, endoscopic, or surgical methods depending on their nature[7,8].
There has been a paradigm shift in the treatment of necrotizing pancreatitis from open necrosectomy to a minimally invasive step-up approach after the publication of results of a large randomized controlled trial (PANTER trial), in which it was seen that 35% of the patients did not require any further intervention and patients managed with the step-up approach had a significantly lower incidence of new onset organ failure and diabetes[9]. This step-up approach is thus now the standard of care for all pancreatitis patients, with upgradations done if there was no clinical improvement or catheter displacement was seen (Figure 2)[9,10].
In this review, we outline the various minimally invasive intervention techniques (predominantly percutaneous with few salient endoscopic techniques), their indications, timing, image guidance, complications, and factors predicting response, so that an interventional radiologist can take an informed decision on when and why to drain a PFC.
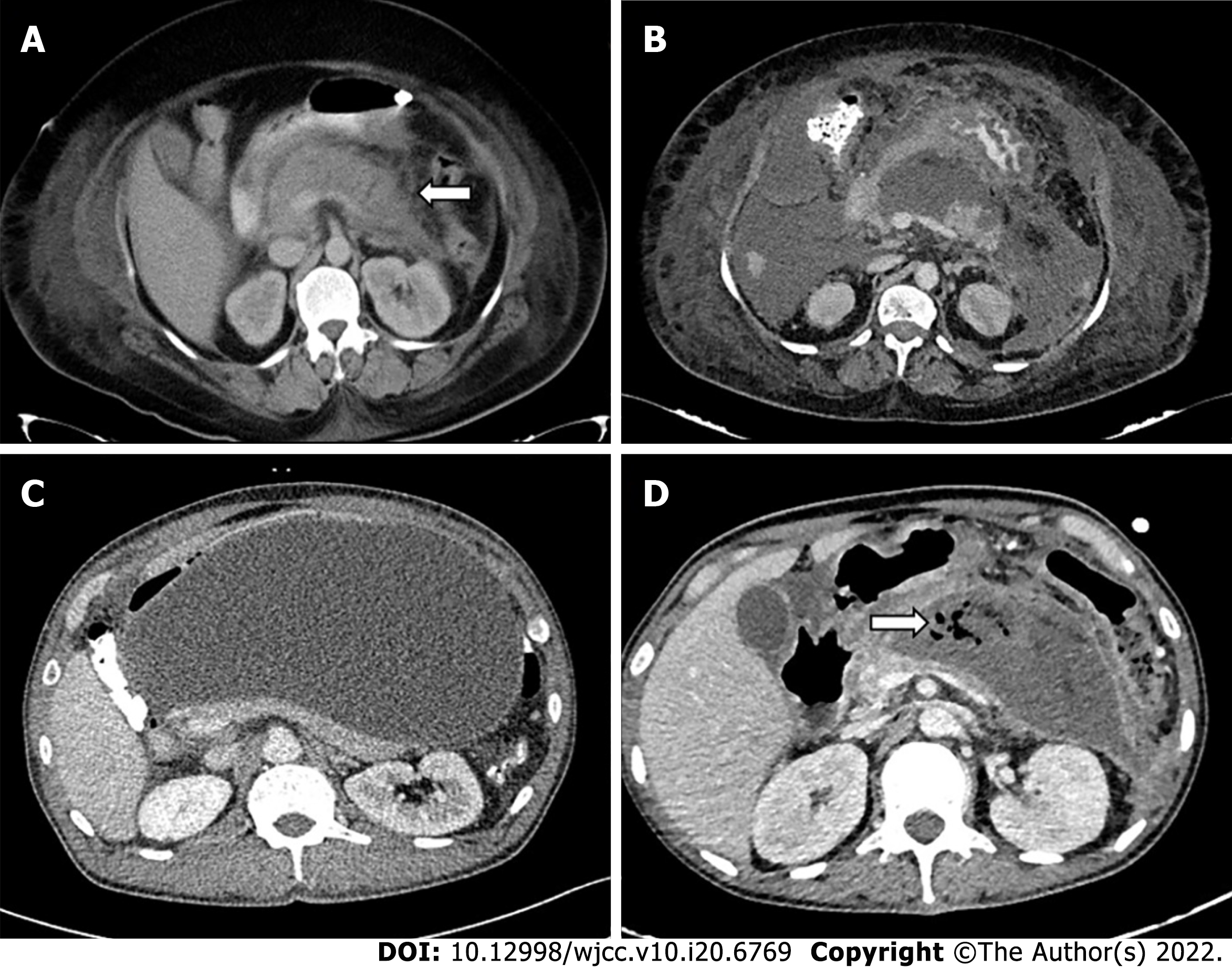
The standard indication of drainage of a PFC is infected necrosis, preferably at the stage of WON (usually after 3-4 wk)[11,12]. The drainage can be either percutaneous catheter drainage (PCD), endoscopic drainage (ED), or surgical debridement. Infection can be documented by the presence of gas on computed tomography (CT) (Figure 1D) or microbial growth on fine needle aspiration (FNA). The latter is now not commonly utilized as it has a high false negative rate (up to 25%)[13]. Additionally, there is a theoretical risk of introduction of infection into sterile collection[14]. According to a survey, most pancreatologists did not routinely perform FNA and 15% never performed FNA[15]. A strong suspicion of infected necrosis is based on clinical deterioration or fever in the absence of other sources of infection[7,12]. Persistent OF for several weeks in the presence of ANC or WON without evidence of infection is also an accepted indication for intervention[12].
However, there are several evolving indications to intervene either early, or in the absence of infection or persistent OF. These include mass effect caused by large collections causing either bowel or biliary obstruction, disconnected pancreatic syndrome, “persistent unwellness” (continued anorexia, intractable pain, and weight loss), or abdominal compartment syndrome (which is an emergency indication) (Figure 3)[12,14,16]. Most of these evolving indications would require a percutaneous intervention as endoscopic interventions generally require a walled off collection and a suitable location adjacent to the gastrointestinal tract, and cannot usually be undertaken in an emergency setting. Collections which do not require drainage include asymptomatic WONs, non-infected pseudocysts or APFCs and collections which drain through spontaneous gastrointestinal fistulas[12,17].
Any intervention of PFC, if performed in an acutely ill patient in the early phase of AP, is associated with high morbidity and mortality due to heightened systemic inflammation response at this time and an increased risk of haemorrhage[7]. Thus, current guidelines suggest delaying interventions (whether percutaneous, endoscopic, or surgical) to more than 3-4 wk after disease onset to decrease this morbidity, as well as to allow encapsulation of the collection[7,12]. This encapsulation allows necrotic parenchyma to be more clearly defined from normal viable parenchyma which leads to better patient outcomes and can avoid future pancreatic insufficiency[7,18]. According to the recent American Gastroenterology Association (AGA) clinical practice guidelines, PCD should be considered in patients with infected or symptomatic necrotic collections in the early, acute period[6]. In patients with abdominal compartment syndrome, emergent surgical or radiological intervention can be a life-saving procedure if medical management fails[12]. Early intervention at the ANC stage may also be required in patients with suspected or confirmed infected necrosis and persistent OF if medical management alone is insufficient[7,12].
This trial was conducted by the Dutch Pancreatitis Study group to compare early vs standard drainage in infected pancreatic necrosis. One-hundred and four patients with necrotizing AP were randomized into two groups-immediate drainage, i.e., within 24 h of diagnosis of infected necrosis, and postponed drainage group (after walled off stage). Necrosectomy, if needed, was postponed to walled-off stage in both arms. The primary outcome was comprehensive complication index (CCI) which included all complications occurring within 6 mo of follow-up, and secondary outcome included mortality, hospital stay, and intensive care unit (ICU) stay, number of interventions, and quality adjusted life-years. No difference was seen in CCI between the early and delayed drainage groups, and no difference was seen in mortality, hospital stay, ICU stay, or complication rates weighted for severity in the two groups. However, the median number of interventions was significantly higher in the immediate group as compared to the postponed group[19]. It is important to note that the indication for drainage in this trial was only infected necrosis and it did not consider other indications as discussed previously.
Recommendations for delayed intervention predominantly stem from an era of open surgical necrosectomy where early debridement in an acutely ill patient increased the morbidity and mortality by worsening OF and increasing physiological stress. On the contrary, new hypotheses have suggested that early and minimally invasive drainage (MID) of PFCs decreases systemic sepsis and allows maturation of the necrotic collections[7]. Several observational studies have suggested that encapsulation of collections for percutaneous or endoscopic interventions may not be as necessary as for open surgery, that early intervention (percutaneous or endoscopic) does not have a worse outcome than delayed intervention, and that some showed significant improvement in organ failure[3,20]. Complications between early and late interventions were more or less comparable, with late interventions leading to a significantly higher number of external pancreatic fistulae in one study[4].
Several other studies have evaluated the role of early drainage in indications other than infected necrosis, especially OF. Zhang et al[21] conducted a network meta-analysis to compare outcomes of MID (including ED, PCD, and minimally invasive surgery) and open surgical debridement with conservative management. It was found that MID decreased both mortality and multiple organ dysfunction syndrome (MODS) rate compared to conservative management. Early MID (defined as immediate or early intervention on diagnosis) as well as routine delayed MID also both significantly decreased mortality and MODS rate compared to conservative treatment. Another study reported improved outcomes in patients undergoing early PCD of sterile PFC in patients with SAP within 3 d of onset of OF[22]. The patients with MSAP did not exhibit similar benefit. A single arm retrospective study evaluated the role of early PCD (< 21 d), with a mean time of intervention of 14.3 ± 2.4 d, for multiple indications, which showed an overall survival rate of 73.1%. More than 50% of the patients survived on PCD alone, whereas 19.2% required additional necrosectomy[23].
A single centre randomized controlled trial also evaluated the role of early on-demand drainage vs standard drainage in ANC and persistent OF[24]. No significant difference was seen in the mortality, complication rate, length of hospital, or ICU stay between the two groups. However, the authors found a trend towards reduction in mortality and major complications as well as shorter duration of OF in the early drainage group.
Thus, early intervention is a feasible technique to treat septic or unstable patients if medical management alone is insufficient, with no adverse outcome in terms of morbidity and mortality.
Due to increasing use of minimally invasive interventions and step-up approach, multiple techniques and routes have been defined for management of PFC. The purpose of intervention could be drainage, lavage, fragmentation, debridement, or excision[16]. A multidisciplinary consensus conference categorized the available interventions into open surgical, minimally invasive surgical (laparoscopy or retroperitoneoscopy), and image-guided percutaneous, endoscopic, or hybrid procedures; and the routes as transperitoneal, retroperitoneal, or oral routes[16].
According to the recent AGA Clinical Practice Update 2020, percutaneous and endoscopic transmural interventions are both appropriate first-line management techniques for WON and organized collections. Endoscopic approach may be preferred as it does not lead to the risk of pancreatocutaneous fistula. Percutaneous drainage should be considered in patients with infected or symptomatic acute necrotic collections (< 2 wk) or with WON who are too ill to undergo endoscopic or surgical interventions. Percutaneous drainage can also be used as an adjunct to ED or as a monotherapy in collections with deep extensions[7]. Recent studies have shown that PCD alone can lead to successful treatment in a large number of patients with no further need of additional intervention[11,25].
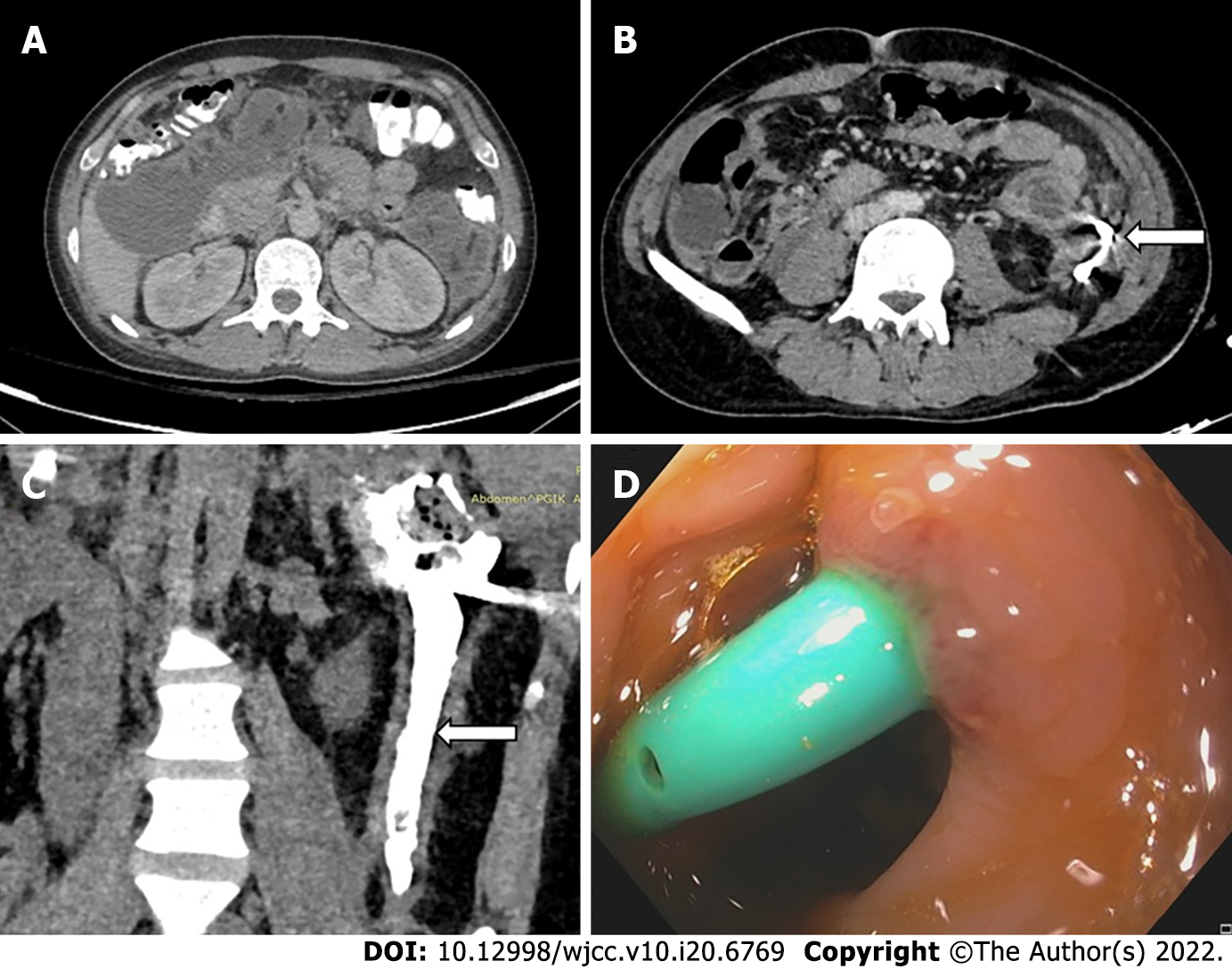
Endoscopic transmural drainage can be performed either by conventional endoscopic guidance or by using endoscopic ultrasound (EUS) and is accomplished by using stents (Figure 4A-C)[18]. Endoscopic stents can be either plastic or metallic with metallic stents showing a better outcome for drainage as they have larger lumens and permit endoscopic necrosectomy. Newer metallic stents like lumen apposing metal stents (LAMS) and bi-flanged metal stents prevent stent migration and are even better than earlier generation metal stents (Figure 4D and E)[6,18,26]. One meta-analysis showed endoscopic and surgical drainage to be superior to percutaneous catheter drainage in terms of length of hospital stay, recurrence, and clinical success; however, certain important co-variates were not considered in this study[27]. Sites of collection were not given due importance as ED can only be done in collections localized to the lesser sac and not extending to deep locations, where PCD is the initial step in management. The cost of re-interventions in both endoscopic and percutaneous interventions should also have been taken into account to describe the cost-effectiveness of either procedure. Also, the studies included in the meta-analysis only used catheter size between 8-16F, whereas endoscopic stents are far larger. Recent evidence of aggressive PCD upgradation shows higher success rates as an aggressive protocol of catheter upsizing every 4-6 d and drainage of all new collections leads to a significantly reduced hospital and ICU stay as compared to the standard protocol[28].
Thus, despite the increasing utilization of endoscopic and minimally invasive surgical techniques, PCD remains integral to the management of PFC.
PCD can be used as a monotherapy or as an adjunct to ED or as a bridge to surgical necrosectomy. PCD can be performed under ultrasound (US) or CT guidance. CT guidance is preferred for lesser sac collections as the bowel is avoided and retroperitoneal insertion is relatively easy. US guidance can be used in large and superficial collections or when a patient is in sepsis and requires emergent drainage. Due to its portable nature, it is also very useful in an ICU setting[7,28].
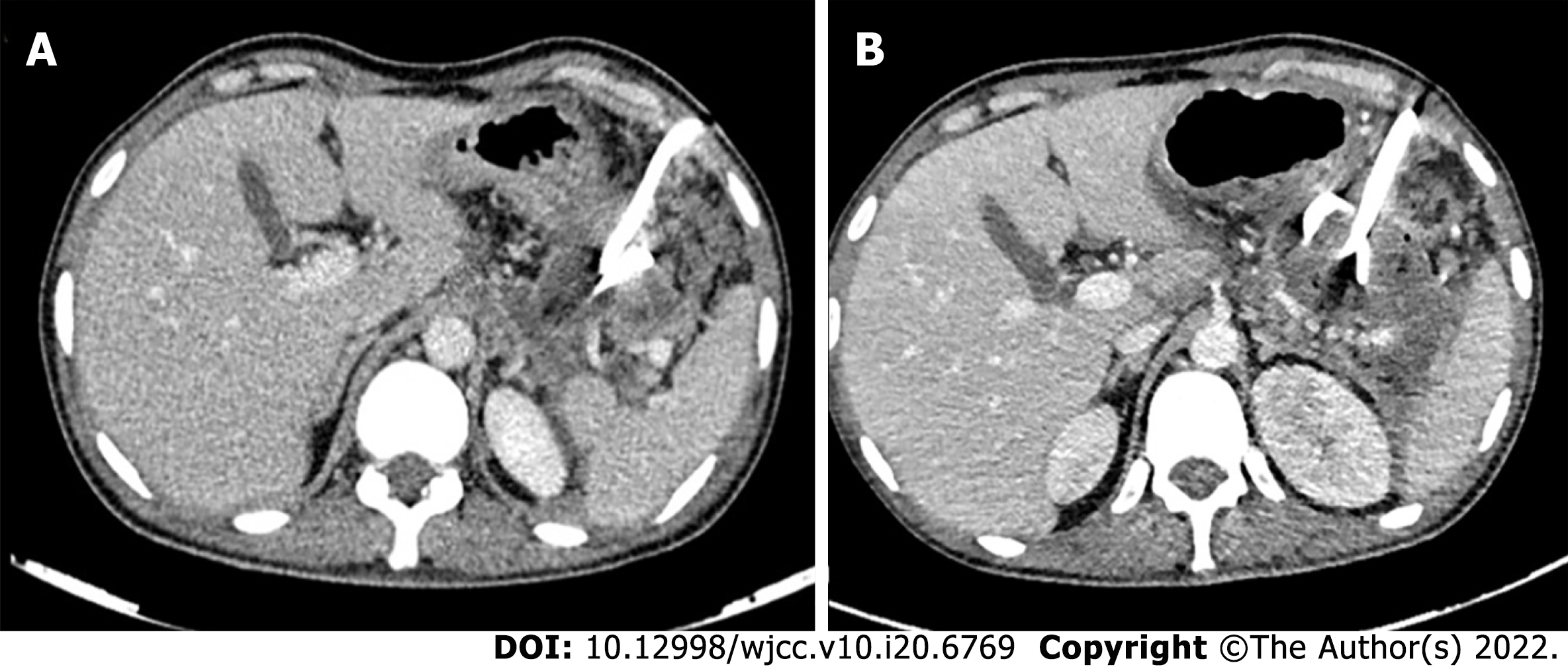
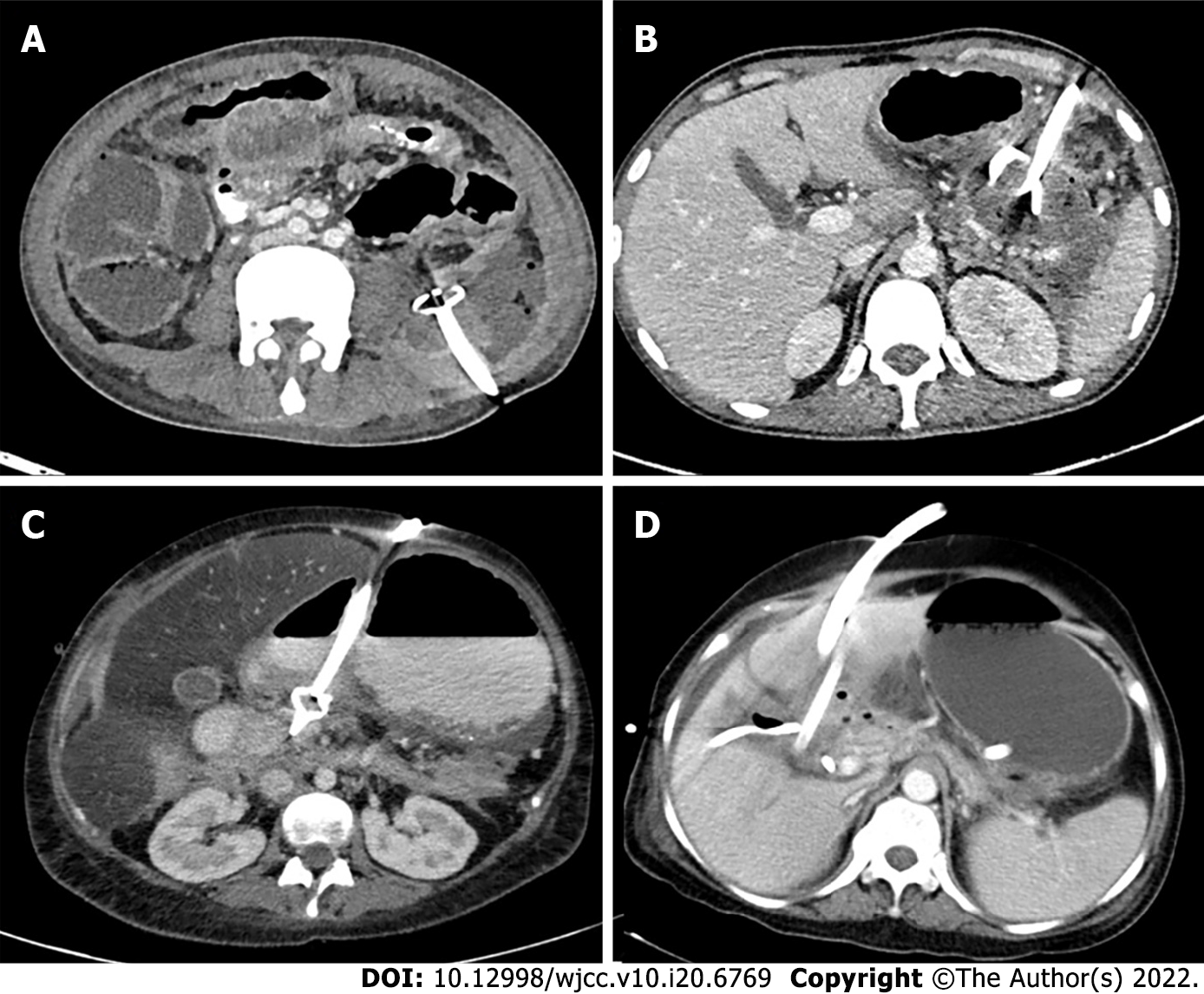
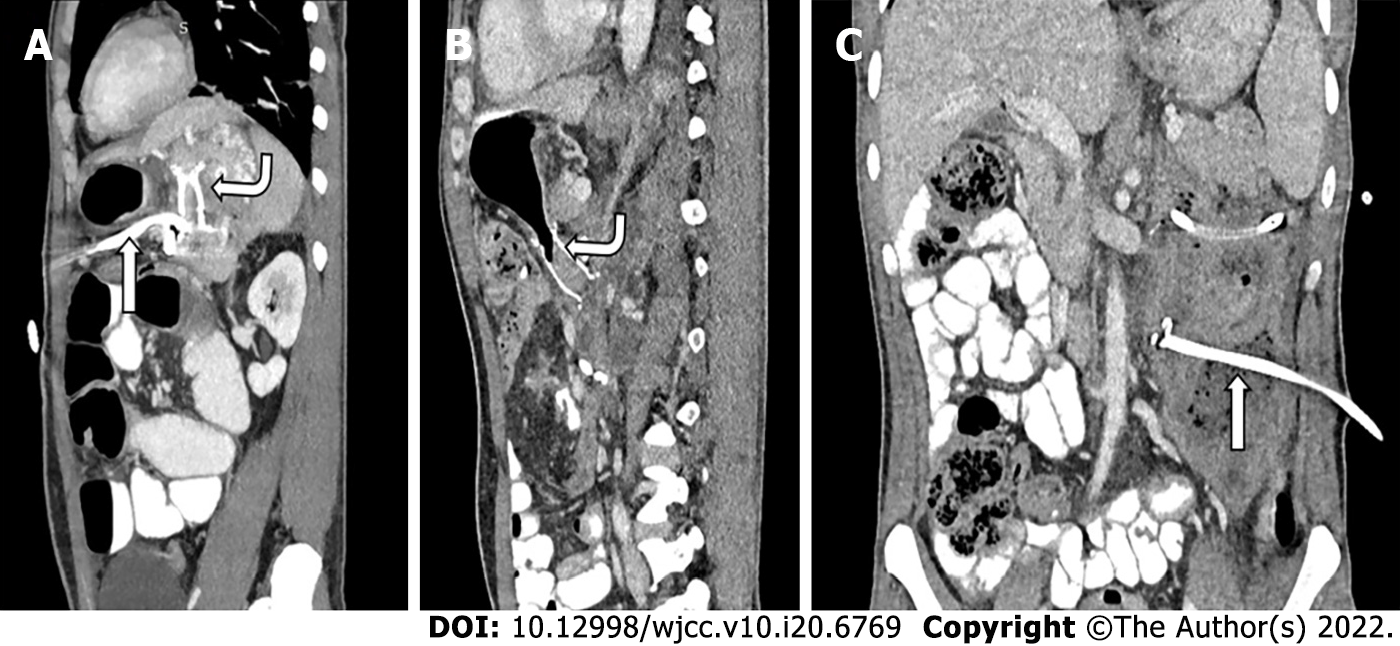
Access routes to the pancreatic collections are chosen to avoid the intestine (to prevent enteric leaks or contamination of potentially sterile collections) and vessels. Retroperitoneal route through the flank is the ideal route as it avoids the intestine, prevents disseminated spread of infection through the peritoneal cavity, and provides access for future minimally invasive surgeries (MIS) (Figure 5A)[8,12]. Transperitoneal route is the second preference in cases where retroperitoneal approach is not possible, and collections arising from pancreatic head and proximal body are located anteriorly (Figure 5B). Care should be taken to avoid vessels and other bowel loops when this approach is selected. Transgastric approach may be used when there is no bowel free approach to the lesser sac collection, and it is considered relatively safe due to bacteria free acidic gastric contents (Figure 5C)[8]. Very rarely trans-hepatic route may have to be used when there is no other feasible route, but it should generally be avoided (Figure 5D).
PCD can be performed via the Seldinger or the trocar technique. Seldinger technique employs initial access of the collection by an 18G needle followed by insertion of a 0.035 inch stiff guidewire and serial dilatation of the tract thereafter. Drainage catheter is then inserted along the guidewire after adequate dilatation of the tract. Trocar technique employs advancing a co-axial combination of a sharp stylet, stiffening cannula, and the draining catheter[29]. Seldinger technique is more useful for deep collections but is more time-consuming as it involves multiple steps. Trocar technique is useful in large and superficial collections but is more painful[29].
Initial catheter size is an important factor that may determine the success of PCD as well as other outcomes but has been inadequately addressed in the literature. It is generally believed that liquefied collections with a small amount of solid debris may be treated initially with small bore catheters ranging from 8-12 F, and organized, solid looking collections be treated with larger bore catheters but high level evidence is lacking[29,30]. Few retrospective studies and meta-analyses showed no significant difference in outcome with respect to mean catheter size[30,31]. However, a recent study has reported a significant reduction in ICU stay and number of re-admissions when patients were treated with an initial catheter size of > 12 and overall mortality was not significantly lower in patients undergoing initial large bore catheter drainage[11].
This technique involves placement of two catheters side by side through the same puncture site into the collection after serial dilatation of the tract[32]. The kissing catheters were deployed only when patients failed to respond to serial upgradations of a single catheter or mean CT density of the collection was > 30 HU, and the purpose of the kissing catheters was to provide one catheter for flushing and another for aspiration. Flushing, aspiration, and/or upsizing were done till there was no residual collection left. With this technique, eight out of ten treated patients did not require a surgical necrosectomy[32].
Liu et al[33] described a double catheter technique in which an inlet catheter for flushing and an aspirator catheter for drainage were inserted inside a large aperture tube with multiple side holes. One group of 15 patients underwent this double catheter placement followed by 1-2 wk of lavage, after which patients underwent percutaneous flexible endoscopic debridement. The other group of 12 patients underwent standard PCD placement with open necrosectomy thereafter. It was found that the occurrence of major complications and/or death was significantly lower in the double catheter group than in the standard PCD group. There was a lower rate of occurrence of new onset OF and reduced length of ICU stay in the double catheter group.
Another group evaluated the role of a novel dual-lumen flushable drainage catheter in evacuation of complex fluid collections[34]. Two prototype catheters of 20 and 28 F size were created by incorporating a customized infusion lumen within the wall of a large bore standard catheter and these were compared with standard 20 and 28 F catheters in in vitro models. Drainage rate of double lumen catheters was significantly superior to standard catheters in purulent, particulate, and haematoma models and complete drainage was achieved with double lumen catheters in the purulent model. Based on the promising results, these catheters may also improve outcomes in patients with necrotizing AP.
A randomized controlled trial compared patients undergoing lavage treatment (LT group) with dependent drainage (DD group) with primary end points being reversal of pre-existing OF, development of new onset OF, need for surgery, mortality, and hospital stay[35]. Lavage was initiated within 24 h of PCD insertion in the LT group and it was done with warmed isotonic saline solution. Initially, 250 mL was instilled through the catheter over 1-2 h and the catheter left on dependent drainage for the rest of the day. In patients with lavage return ≥ 70% of infused volume, lavage volume was gradually increased over 3-4 d and this fluid was infused slowly. Lavage was done for a period of 2 wk. The DD group did not undergo any lavage treatment and was left for standard drainage. It was shown that lavage treatment resulted in a significant reversal of OF and reduction in acute physiology and chronic health evaluation II scores as compared to the DD group. No difference was reported in the development of new onset OF, catheter related complications, or number of catheters between the two groups.
Werge et al[36] retrospectively evaluated patients treated with endoscopic transmural drainage and necrosectomy who underwent local instillation of antibiotics depending on microbial findings. Both intravenous and local antibiotics did not show eradication of bacteria between the first and second culture; however, local antibiotics were associated with eradication of microbes between the second and third culture which was not seen with intravenous antibiotics[36]. Thus, local instillation of antibiotics depending on microbial culture report can lead to early eradication of infection in MID. Although there are no published studies reporting the use of local antibiotics through the percutaneous catheters, there seems to be a potential role for local antibiotics to improve outcomes in patients undergoing PCD.
Streptokinase: Streptokinase acts on the surface of the necrosum and causes fibrinolysis which leads to its dissolution. This leads to better drainage of solid contents of the PFCs[37]. A retrospective study demonstrated that streptokinase irrigation of the PFC through percutaneous catheters resulted in a significantly higher sepsis reversal and reduced need for surgery in the streptokinase group as compared to saline irrigation[37]. Two doses of streptokinase were used (50000 IU/150000IU diluted in 100 mL saline infused over 60 min) and higher dose resulted in lower rates of necrosectomy, bleeding, and mortality.
Streptokinase vs hydrogen peroxide: Another study by the same group compared streptokinase irrigation (50000 IU in 100 mL saline) with hydrogen peroxide irrigation (3% diluted in 100 mL saline)[38]. Bleeding complications, need for surgery, mortality, and post-irrigation hospital stay were higher in the hydrogen peroxide group than in the streptokinase group; however, the difference was not statistically significant. Streptokinase, thus, appears to be safer compared with hydrogen peroxide.
Abdominal paracentesis drainage (APD) prior to PCD as part of the modified step-up approach or to relieve increased intra-abdominal pressure has shown encouraging results in a few studies. One study compared outcomes in patients managed with APD preceding PCD and with PCD alone and demonstrated that the reduction of peripancreatic fluid collection by < 50% after APD alone was an independent predictor of the subsequent need for PCD[33]. A similar study showed that the mortality in the APD plus PCD group was significantly lower than that with PCD alone, and mean interval between onset of disease to further intervention was also decreased in the first group[39]. Another prospective cohort study demonstrated a significant reduction in the severity scores and laboratory variables in the APD-PCD group. However, no relevant factors could be identified to predict the need of APD[40].
A prospective study evaluated the role of APD in infectious complications in MSAP and SAP and it was seen that patients who underwent APD had significantly lesser infectious complications as well as need for further PCD than patients with PCD alone[41]. No significant difference was seen in microbial spectrum or mortality between the two groups. Wang et al[42] evaluated the role of APD in decreasing the intra-abdominal pressure in patients with severe AP with sterile fluid collections. Patients were divided into a sterile collection group, a secondary infection group, and a primary infection group and it was seen that intra-abdominal hypertension was an independent risk factor for secondary infection, and a significant reduction in intra-abdominal pressure following APD (> 6.5 mmHg) led to a lower incidence of infection and better alleviation of OF[43]. A systematic review and meta-analysis evaluated the efficacy and safety of APD in patients with AP, and the pooled results suggested that APD significantly reduced the length of hospital stay and mortality of all causes during hospitalization. There was no increase in infectious complications following APD[44]. Thus, early application of APD prior to PCD can improve outcomes, reduce infective complications, and reduce intra-abdominal pressure in patients with MSAP and SAP.
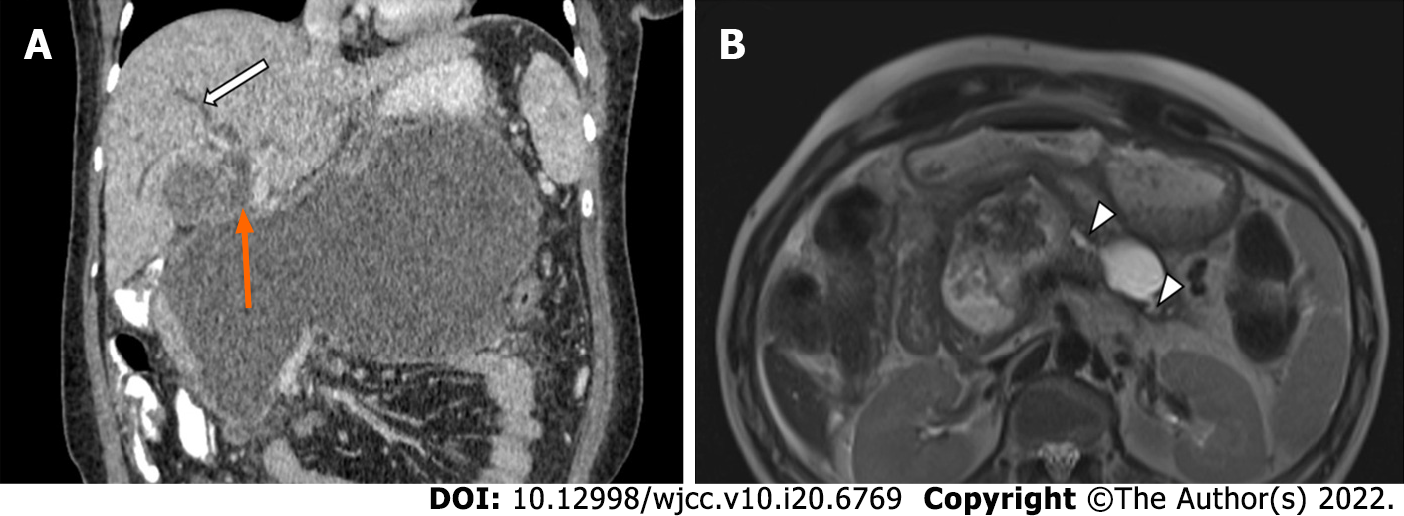
ED alone may not be adequate in treating deep collections and may not be feasible in extremely ill patients. Similarly, PCD alone leads to patient discomfort and risk of external pancreatic fistula. Thus, a combined drainage, called dual modality drainage (DMD), can be used to combine the advantages of both these modalities (internal drainage of ED and drainage of deep collections by PCD) (Figure 6). Irrigation of the collection with PCD as the ingress and internal drainage through the ED stent can result in debridement of the majority of the solid necrosum[16]. The newer LAMS have a large bore which is advantageous in draining solid component if irrigation and flushing are done using this technique. This can result in early removal of PCD, which may prevent formation of cutaneous fistula[16,45]. Improved outcomes were seen with addition of ED to PCD in a few studies[44,45]. One of these studies compared DMD to standard PCD and it was seen that the DMD cohort had a shorter hospital stay, shorter time to removal of PCD, fewer CT scans, and fewer endoscopic retrograde cholangiopancreatography procedures as compared to standard PCD. Additionally, none of the patients required surgery in the DMD group[45]. Another study comprising patients undergoing DMD demonstrated that none of the patients required surgical necrosectomy, and all patients who had completed treatment had their catheters removed, without any formation of pancreatocutaneous fistula[45].
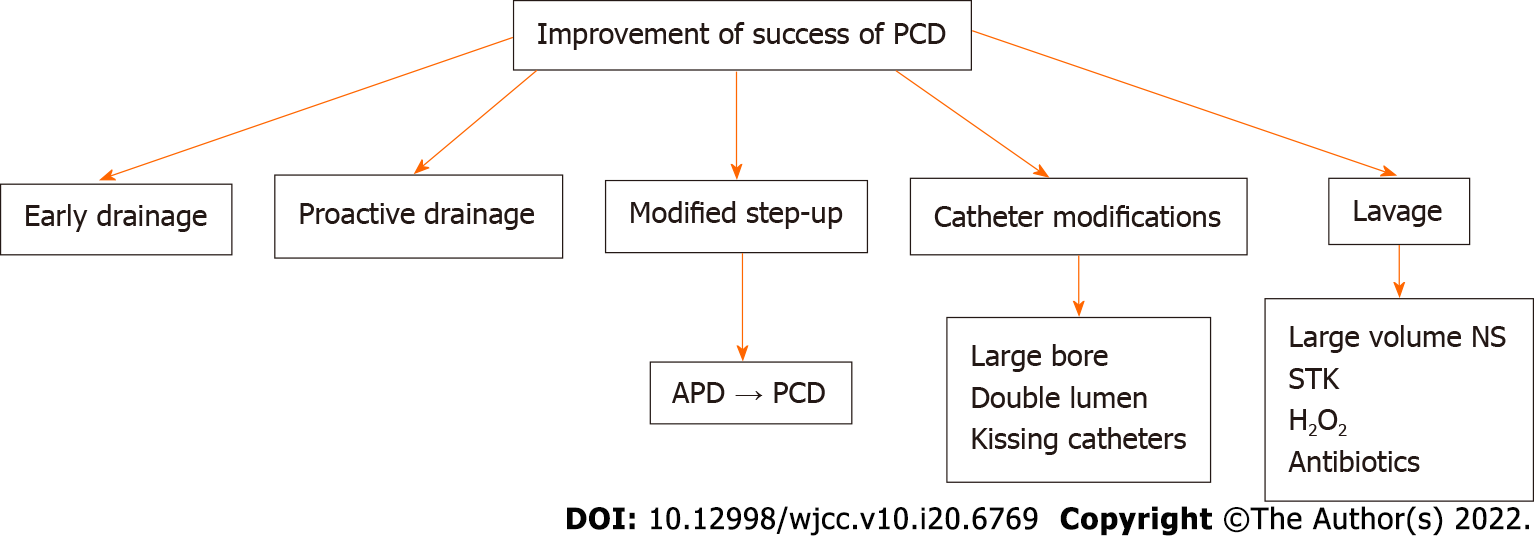
Success of PCD is defined as recovery of patients without need for surgery and resolution of OF[11,32,46]. Multiple factors affect the success of PCD. These can be grouped into pre-PCD or post-PCD.
One study reported a significant difference in the baseline values of C-reactive protein (CRP) and interleukin (IL)-6 between the PCD success and PCD failure groups, where resolution of OF, sepsis, and pressure symptoms was defined as success of PCD[46]. The mean baseline CRP values in the PCD success and failure groups were 146.48 ± 111.6 mg/L vs 189.10 ± 55.5 mg/L, respectively, and for IL-6, they were 166.09 ± 51.21 pg/mL vs 215.81 ± 52.40 pg/mL. Another study showed that pre-PCD CT density of the necrotic collection was significantly lower in the PCD success group compared to the PCD failure group[47]. Male sex, multiple OF, higher percentage of parenchymal necrosis, and heterogeneous attenuation of the collection were associated with a poorer outcome in another study[48].
CRP, IL-6, and IL-10 were seen to significantly decrease 7 d after PCD insertion in the PCD success group compared to the PCD failure group, and the percentage of decrease of IL-6 on day 3 and CRP on day 7 correlated with the outcomes[46].
Secondary infection of sterile necrosis or pseudocyst is a frequent complication and occurs in about 8% of the patients[29]. Conversely, peritoneal spill of infected pancreatic necrosis can occur in transperitoneal PCD or through intestinal leakage[29]. Haemorrhage is usually self-limited and venous in origin. Haemorrhage can occur within the pancreatic parenchyma, or inside the PFC or the gastrointestinal tract depending on the site of involvement[7]. Haemorrhage into the GI tract may present with upper or lower GI bleeding depending on etiology and the rate of haemorrhage. Bleeding into the peritoneal cavity may lead to abdominal distension and haemodynamic instability, whereas haemorrhage into the collection may not present with any outward signs except for haemodynamic instability. Sometimes, it can be due to rupture of an arterial branch during access, or formation of pseudoaneurysm during PCD insertion or LAMS placement[8,30,31]. These are usually managed by endovascular embolization. Formation of external pancreato-cutaneous fistula is common and is defined as persistent measurable drainage of clear pancreatic fluid (usually > 100 mL) through the percutaneous drain or the PCD tract more than 3 wk after PCD insertion[12]. Incidence can vary between 5 to 35% according to different studies[49-51]. In one study, these fistulae closed after a median period of 70 d[52]. In case of non-resolution, pancreatic duct stenting is indicated[12]. Internal gastrointestinal fistulae can also develop post PCD and they may be spontaneous, or caused by erosion of catheter into bowel wall or by iatrogenic injury of the wall during catheter placement (Figure 7). The most common site of these fistulae is the splenic flexure of the colon[17,53]. Management of GI fistulae depends on their location. Upper GI fistulae close spontaneously over time, whereas colonic fistula may be treated conservatively with continuous PCD drainage in stable patients. However, if GI fistulisation is associated with frank haemorrhage or sepsis, surgical management may be required.
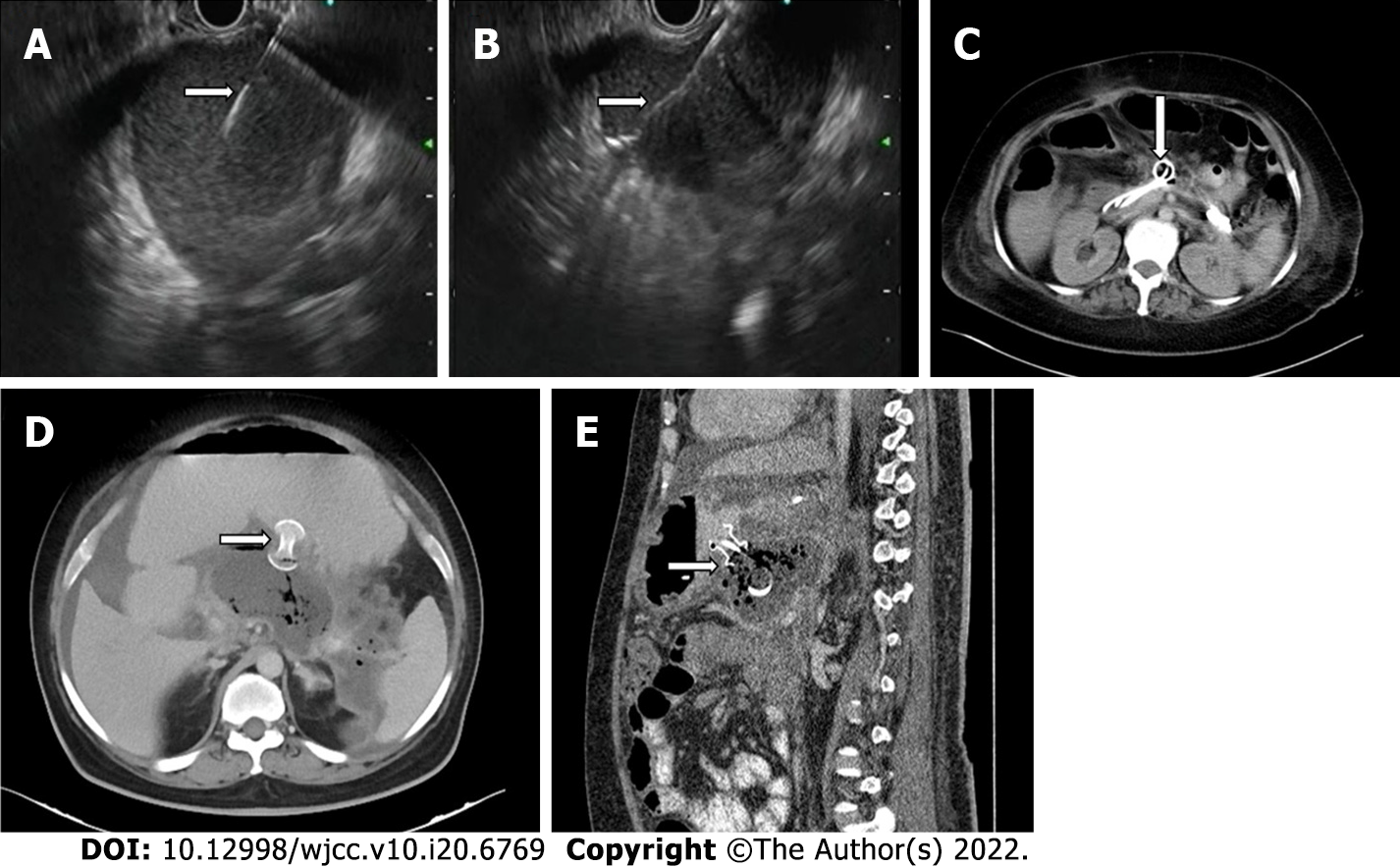
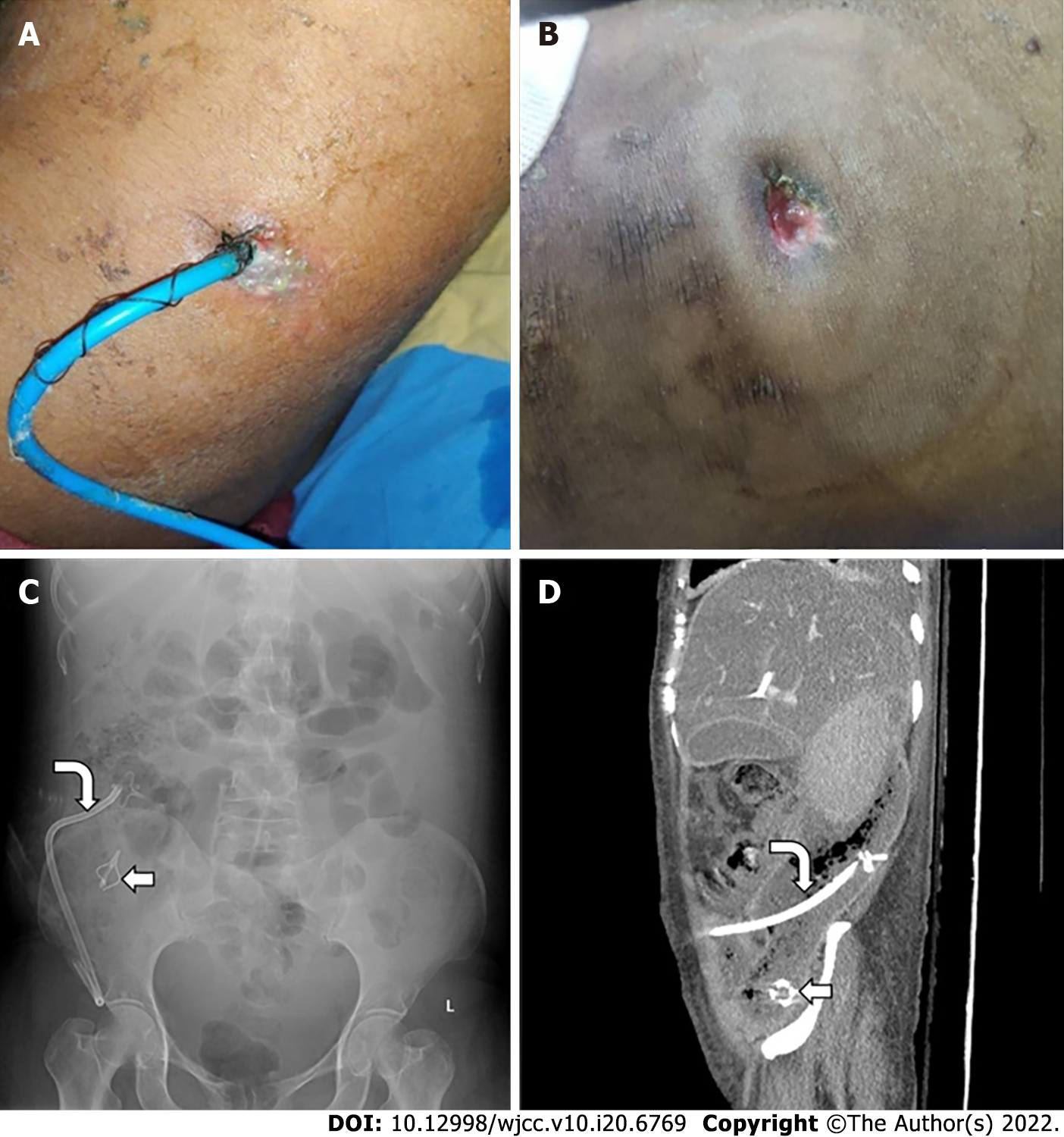
Catheter displacement, blockage, and peri-catheter leak are common but under-reported. Depending on the patients’ clinical status and presence of residual collection, these complications are managed by removal, re-insertion, or upgradation of the catheter. Peri-catheter leak and sutures and dressing can lead to skin erosion and bleeding (Figure 8A and B). Skin care thus becomes necessary in these patients. Very rarely, the catheter tip may get fractured and remain within the necrotic cavity (Figure 8C and D).
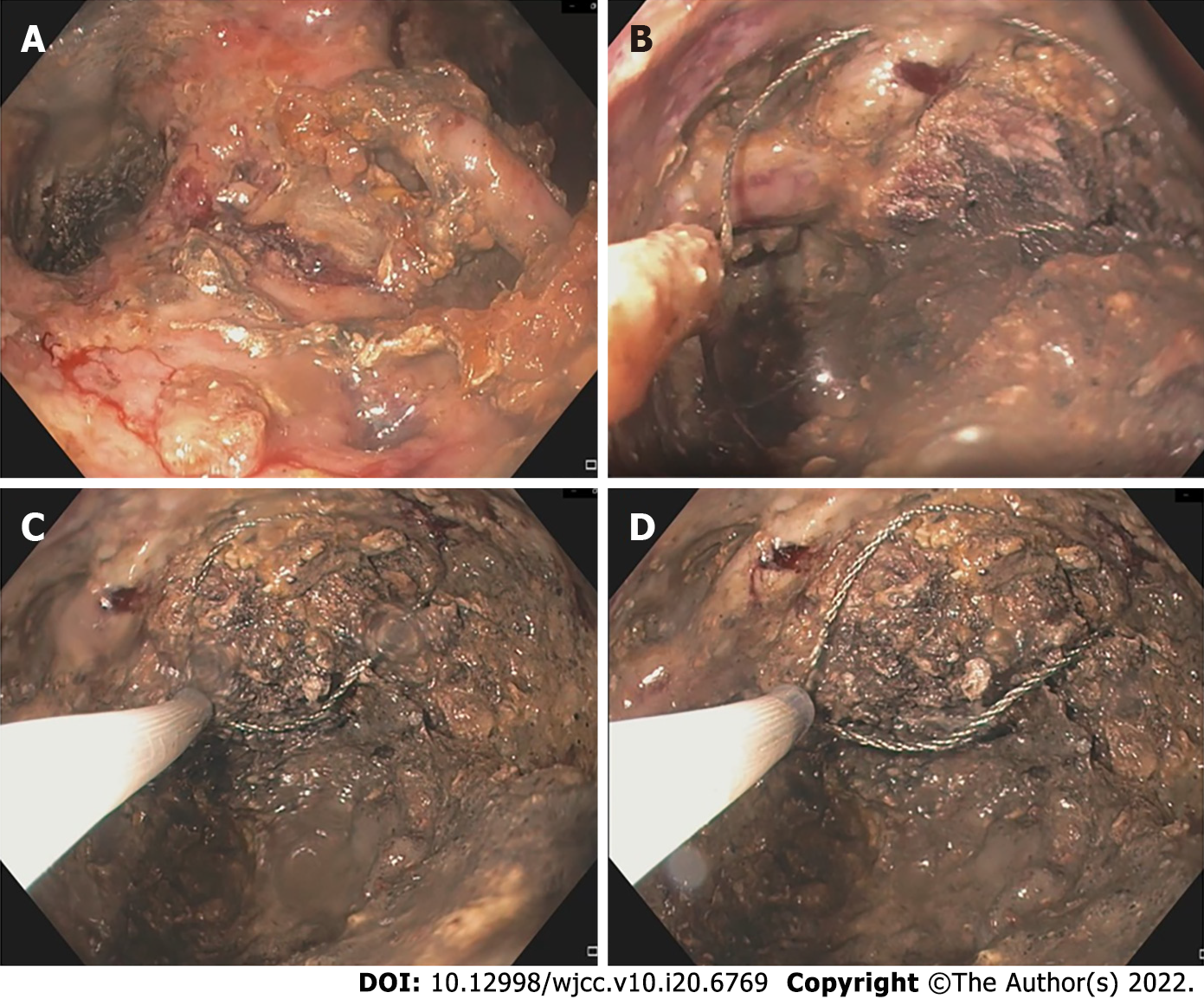
Necrosectomy can be endoscopic, MIS, laparoscopic, or open surgical. Step-up approach is used in both endoscopic and percutaneous MIS techniques with a randomized controlled trial showing no difference in mortality or complication rate between the two[10]. Endoscopic debridement consists of direct endoscopic necrosectomy (DEN) or transpapillary drainage (TPD)[18]. DEN involves debridement of necrotic tissue by passing an endoscope into the cavity, which can be done with fluid irrigation, or by snares and baskets (Figure 9)[6,16,18]. TPD involves placement of pancreatic ductal stents in cases with small collections (< 6 cm) communicating with the pancreatic duct[18].
Percutaneous minimally invasive techniques include percutaneous EN (PEN) which involves insertion of endoscope along the PCD tract and debridement by snares and baskets; and video assisted retroperitoneal debridement (VARD) which involves insertion of a zero-degree videoscope and debridement. Both these techniques require PCD insertion through the left lateral position via the retroperitoneal access route. Complete debridement is not the aim, and only loose necrosum is removed[6,16,54]. However, VARD is associated with formation of a significant number of pancreatic fistulae. Laparoscopic debridement can also be done which allows access to all abdominal compartments and successful single session debridement is feasible in most patients[16].
Open surgical necrosectomy was the standard of care before the advent of MIS techniques. However, due to its high morbidity, mortality, and complication rate, it has been superseded by minimally invasive techniques[16]. These days, open surgical necrosectomy is reserved for patients who do not respond to MIS or those who have an emergency indication for open surgery, which includes abdominal compartment syndrome, perforation of hollow viscus, ischemic bowel infarction, or uncontrolled haemorrhage which is not amenable for endovascular embolization[12].
PFC represent important complications of AP. Despite the increasing utilization of endoscopic and minimally invasive surgical techniques, interventional radiologists and PCD remain integral to the management of patients with PFC. They must be aware of the evolving indications and complementary role of PCD, ED, and MIS and factors influencing success of PCD (Figure 10).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Angelico R, Italy; Zhao CF, China S-Editor: Guo XR L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Foster BR, Jensen KK, Bakis G, Shaaban AM, Coakley FV. Revised Atlanta Classification for Acute Pancreatitis: A Pictorial Essay. Radiographics. 2016;36:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 2. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4269] [Article Influence: 355.8] [Reference Citation Analysis (44)] |
| 3. | Trikudanathan G, Tawfik P, Amateau SK, Munigala S, Arain M, Attam R, Beilman G, Flanagan S, Freeman ML, Mallery S. Early (<4 Weeks) Versus Standard (≥ 4 Weeks) Endoscopically Centered Step-Up Interventions for Necrotizing Pancreatitis. Am J Gastroenterol. 2018;113:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Oblizajek N, Takahashi N, Agayeva S, Bazerbachi F, Chandrasekhara V, Levy M, Storm A, Baron T, Chari S, Gleeson FC, Pearson R, Petersen BT, Vege SS, Lennon R, Topazian M, Abu Dayyeh BK. Outcomes of early endoscopic intervention for pancreatic necrotic collections: a matched case-control study. Gastrointest Endosc. 2020;91:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | van Grinsven J, van Brunschot S, van Baal MC, Besselink MG, Fockens P, van Goor H, van Santvoort HC, Bollen TL; Dutch Pancreatitis Study Group. Natural History of Gas Configurations and Encapsulation in Necrotic Collections During Necrotizing Pancreatitis. J Gastrointest Surg. 2018;22:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology. 2020;158:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 402] [Article Influence: 80.4] [Reference Citation Analysis (2)] |
| 7. | Shyu JY, Sainani NI, Sahni VA, Chick JF, Chauhan NR, Conwell DL, Clancy TE, Banks PA, Silverman SG. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics. 2014;34:1218-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 8. | Lee MJ, Wittich GR, Mueller PR. Percutaneous intervention in acute pancreatitis. Radiographics. 1998;18:711-24; discussion 728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1024] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 10. | van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck CH, Erkelens WG, van Goor H, van Grevenstein WMU, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden KP, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P; Dutch Pancreatitis Study Group. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018;391:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 463] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 11. | Gupta P, Bansal A, Samanta J, Mandavdhare H, Sharma V, Gupta V, Yadav TD, Dutta U, Kochhar R, Singh Sandhu M. Larger bore percutaneous catheter in necrotic pancreatic fluid collection is associated with better outcomes. Eur Radiol. 2021;31:3439-3446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current Concepts in Severe Acute and Necrotizing Pancreatitis: An Evidence-Based Approach. Gastroenterology. 2019;156:1994-2007.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 244] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 13. | van Baal MC, Bollen TL, Bakker OJ, van Goor H, Boermeester MA, Dejong CH, Gooszen HG, van der Harst E, van Eijck CH, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. The role of routine fine-needle aspiration in the diagnosis of infected necrotizing pancreatitis. Surgery. 2014;155:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | ASGE Standards of Practice Committee; Early DS, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Hwang JH, Jue TL, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf RN, Shergill AK, Cash BD. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | van Grinsven J, van Brunschot S, Bakker OJ, Bollen TL, Boermeester MA, Bruno MJ, Dejong CH, Dijkgraaf MG, van Eijck CH, Fockens P, van Goor H, Gooszen HG, Horvath KD, van Lienden KP, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. Diagnostic strategy and timing of intervention in infected necrotizing pancreatitis: an international expert survey and case vignette study. HPB (Oxford). 2016;18:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Freeman ML, Werner J, van Santvoort HC, Baron TH, Besselink MG, Windsor JA, Horvath KD, vanSonnenberg E, Bollen TL, Vege SS; International Multidisciplinary Panel of Speakers and Moderators. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41:1176-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 17. | Jiang W, Tong Z, Yang D, Ke L, Shen X, Zhou J, Li G, Li W, Li J. Gastrointestinal Fistulas in Acute Pancreatitis With Infected Pancreatic or Peripancreatic Necrosis: A 4-Year Single-Center Experience. Medicine (Baltimore). 2016;95:e3318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Nabi Z, Basha J, Reddy DN. Endoscopic management of pancreatic fluid collections-revisited. World J Gastroenterol. 2017;23:2660-2672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Boxhoorn L, van Dijk SM, van Grinsven J, Verdonk RC, Boermeester MA, Bollen TL, Bouwense SAW, Bruno MJ, Cappendijk VC, Dejong CHC, van Duijvendijk P, van Eijck CHJ, Fockens P, Francken MFG, van Goor H, Hadithi M, Hallensleben NDL, Haveman JW, Jacobs MAJM, Jansen JM, Kop MPM, van Lienden KP, Manusama ER, Mieog JSD, Molenaar IQ, Nieuwenhuijs VB, Poen AC, Poley JW, van de Poll M, Quispel R, Römkens TEH, Schwartz MP, Seerden TC, Stommel MWJ, Straathof JWA, Timmerhuis HC, Venneman NG, Voermans RP, van de Vrie W, Witteman BJ, Dijkgraaf MGW, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. Immediate versus Postponed Intervention for Infected Necrotizing Pancreatitis. N Engl J Med. 2021;385:1372-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 20. | van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG; Dutch Pancreatitis Study Group. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2011;98:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 21. | Zhang Y, Yu WQ, Zhang J, Fu SQ, Fu QH, Liang TB. Efficacy of Early Percutaneous Catheter Drainage in Acute Pancreatitis of Varying Severity Associated With Sterile Acute Inflammatory Pancreatic Fluid Collection. Pancreas. 2020;49:1246-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Mukund A, Singla N, Bhatia V, Arora A, Patidar Y, Sarin SK. Safety and efficacy of early image-guided percutaneous interventions in acute severe necrotizing pancreatitis: A single-center retrospective study. Indian J Gastroenterol. 2019;38:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Ke L, Dong X, Chen T, Doig GS, Li G, Ye B, Zhou J, Xiao X, Tong Z, Li W; Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG). Early on-demand drainage or standard management for acute pancreatitis patients with acute necrotic collections and persistent organ failure: A pilot randomized controlled trial. J Hepatobiliary Pancreat Sci. 2021;28:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Qu C, Zhang H, Chen T, Zhu Y, Feng Q, Guo F, Liu Z, Cao L, Yang J, Li G, Ye B, Zhou J, Ke L, Tong Z, Windsor J, Li W; Chinese Acute Pancreatitis Clinical Trials Group(CAPCTG). Early on-demand drainage versus standard management among acute necrotizing pancreatitis patients complicated by persistent organ failure: The protocol for an open-label multi-center randomized controlled trial. Pancreatology. 2020;20:1268-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Mehta V, Kumar R, Parkash S, Singla S, Singh A, Chaudhary J, Bains H. Role of percutaneous catheter drainage as primary treatment of necrotizing pancreatitis. Turk J Gastroenterol. 2019;30:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Tyberg A, Karia K, Gabr M, Desai A, Doshi R, Gaidhane M, Sharaiha RZ, Kahaleh M. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol. 2016;22:2256-2270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 27. | Szakó L, Mátrai P, Hegyi P, Pécsi D, Gyöngyi Z, Csupor D, Bajor J, Erőss B, Mikó A, Szakács Z, Dobszai D, Meczker Á, Márta K, Rostás I, Vincze Á. Endoscopic and surgical drainage for pancreatic fluid collections are better than percutaneous drainage: Meta-analysis. Pancreatology. 2020;20:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Wroński M, Cebulski W, Karkocha D, Słodkowski M, Wysocki L, Jankowski M, Krasnodębski IW. Ultrasound-guided percutaneous drainage of infected pancreatic necrosis. Surg Endosc. 2013;27:2841-2848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Sharma V, Gorsi U, Gupta R, Rana SS. Percutaneous Interventions in Acute Necrotizing Pancreatitis. Trop Gastroenterol. 2016;37:4-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Ke L, Li J, Hu P, Wang L, Chen H, Zhu Y. Percutaneous Catheter Drainage in Infected Pancreatitis Necrosis: a Systematic Review. Indian J Surg. 2016;78:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Bruennler T, Langgartner J, Lang S, Wrede CE, Klebl F, Zierhut S, Siebig S, Mandraka F, Rockmann F, Salzberger B, Feuerbach S, Schoelmerich J, Hamer OW. Outcome of patients with acute, necrotizing pancreatitis requiring drainage-does drainage size matter? World J Gastroenterol. 2008;14:725-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Gupta P, Koshi S, Samanta J, Mandavdhare H, Sharma V, K Sinha S, Dutta U, Kochhar R. Kissing catheter technique for percutaneous catheter drainage of necrotic pancreatic collections in acute pancreatitis. Exp Ther Med. 2020;20:2311-2316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Liu WH, Wang T, Yan HT, Chen T, Xu C, Ye P, Zhang N, Liu ZC, Tang LJ. Predictors of percutaneous catheter drainage (PCD) after abdominal paracentesis drainage (APD) in patients with moderately severe or severe acute pancreatitis along with fluid collections. PLoS One. 2015;10:e0115348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Roberts DG, Goudie MJ, Kim AJ, Kim H, Khademhosseini A, McWilliams JP. Novel Dual-Lumen Drainage Catheter to Enhance the Active Evacuation of Complex Fluid Collections. J Vasc Interv Radiol. 2021;32:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Kohli P, Gupta V, Kochhar R, Yadav TD, Sinha SK, Lal A. Lavage through percutaneous catheter drains in severe acute pancreatitis: Does it help? Pancreatology. 2019;19:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 36. | Werge M, Novovic S, Roug S, Knudsen JD, Feldager E, Gluud LL, Schmidt PN. Evaluation of local instillation of antibiotics in infected walled-off pancreatic necrosis. Pancreatology. 2018;18:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Bhargava V, Gupta R, Vaswani P, Jha B, Rana SS, Gorsi U, Kang M. Streptokinase irrigation through a percutaneous catheter helps decrease the need for necrosectomy and reduces mortality in necrotizing pancreatitis as part of a step-up approach. Surgery. 2021;170:1532-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Bhargava MV, Rana SS, Gorsi U, Kang M, Gupta R. Assessing the Efficacy and Outcomes Following Irrigation with Streptokinase Versus Hydrogen Peroxide in Necrotizing Pancreatitis: A Randomized Pilot Study. Dig Dis Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Liu WH, Ren LN, Chen T, Liu LY, Jiang JH, Wang T, Xu C, Yan HT, Zheng XB, Song FQ, Tang LJ. Abdominal paracentesis drainage ahead of percutaneous catheter drainage benefits patients attacked by acute pancreatitis with fluid collections: a retrospective clinical cohort study. Crit Care Med. 2015;43:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Zerem E, Kunosić S, Zerem D, Boloban A, Zerem O, Zlomužica E. Benefits of abdominal paracentesis drainage performed ahead of percutaneous catheter drainage as a modification of the step-up approach in acute pancreatitis with fluid collections. Acta Gastroenterol Belg. 2020;83:285-293. [PubMed] |
| 41. | Liu L, Yan H, Liu W, Cui J, Wang T, Dai R, Liang H, Luo H, Tang L. Abdominal Paracentesis Drainage Does Not Increase Infection in Severe Acute Pancreatitis: A Prospective Study. J Clin Gastroenterol. 2015;49:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Wang T, Liu LY, Luo H, Dai RW, Liang HY, Chen T, Yan HT, Cui JF, Li NL, Yang W, Liu WH, Tang LJ. Intra-Abdominal Pressure Reduction After Percutaneous Catheter Drainage Is a Protective Factor for Severe Pancreatitis Patients With Sterile Fluid Collections. Pancreas. 2016;45:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Lu Z, Zhu X, Hua T, Zhang J, Xiao W, Jia D, Yang M. Efficacy and safety of abdominal paracentesis drainage on patients with acute pancreatitis: a systematic review and meta-analysis. BMJ Open. 2021;11:e045031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Gluck M, Ross A, Irani S, Lin O, Gan SI, Fotoohi M, Hauptmann E, Crane R, Siegal J, Robinson DH, Traverso LW, Kozarek RA. Dual modality drainage for symptomatic walled-off pancreatic necrosis reduces length of hospitalization, radiological procedures, and number of endoscopies compared to standard percutaneous drainage. J Gastrointest Surg. 2012;16:248-56; discussion 256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 45. | Ross AS, Irani S, Gan SI, Rocha F, Siegal J, Fotoohi M, Hauptmann E, Robinson D, Crane R, Kozarek R, Gluck M. Dual-modality drainage of infected and symptomatic walled-off pancreatic necrosis: long-term clinical outcomes. Gastrointest Endosc. 2014;79:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 46. | Mallick B, Tomer S, Arora SK, Lal A, Dhaka N, Samanta J, Sinha SK, Gupta V, Yadav TD, Kochhar R. Change in serum levels of inflammatory markers reflects response of percutaneous catheter drainage in symptomatic fluid collections in patients with acute pancreatitis. JGH Open. 2019;3:295-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Guo Q, Li A, Hu W. Predictive factors for successful ultrasound-guided percutaneous drainage in necrotizing pancreatitis. Surg Endosc. 2016;30:2929-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Hollemans RA, Bollen TL, van Brunschot S, Bakker OJ, Ahmed Ali U, van Goor H, Boermeester MA, Gooszen HG, Besselink MG, van Santvoort HC; Dutch Pancreatitis Study Group. Predicting Success of Catheter Drainage in Infected Necrotizing Pancreatitis. Ann Surg. 2016;263:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 49. | Gomatos IP, Halloran CM, Ghaneh P, Raraty MG, Polydoros F, Evans JC, Smart HL, Yagati-Satchidanand R, Garry JM, Whelan PA, Hughes FE, Sutton R, Neoptolemos JP. Outcomes From Minimal Access Retroperitoneal and Open Pancreatic Necrosectomy in 394 Patients With Necrotizing Pancreatitis. Ann Surg. 2016;263:992-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Horvath KD, Kao LS, Wherry KL, Pellegrini CA, Sinanan MN. A technique for laparoscopic-assisted percutaneous drainage of infected pancreatic necrosis and pancreatic abscess. Surg Endosc. 2001;15:1221-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Raraty MG, Halloran CM, Dodd S, Ghaneh P, Connor S, Evans J, Sutton R, Neoptolemos JP. Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg. 2010;251:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 52. | Bakker OJ, van Baal MC, van Santvoort HC, Besselink MG, Poley JW, Heisterkamp J, Bollen TL, Gooszen HG, van Eijck CH; Dutch Pancreatitis Study Group. Endoscopic transpapillary stenting or conservative treatment for pancreatic fistulas in necrotizing pancreatitis: multicenter series and literature review. Ann Surg. 2011;253:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Hua Z, Su Y, Huang X, Zhang K, Yin Z, Wang X, Liu P. Analysis of risk factors related to gastrointestinal fistula in patients with severe acute pancreatitis: a retrospective study of 344 cases in a single Chinese center. BMC Gastroenterol. 2017;17:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | van Santvoort HC, Besselink MG, Horvath KD, Sinanan MN, Bollen TL, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford). 2007;9:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |