Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6759
Peer-review started: January 6, 2022
First decision: March 9, 2022
Revised: April 10, 2022
Accepted: May 13, 2022
Article in press: May 13, 2022
Published online: July 16, 2022
Processing time: 179 Days and 9.4 Hours
Metabolically associated fatty liver disease (MAFLD) is a liver manifestation of metabolic syndrome potentially related to unfavorable hepatic and extrahepatic outcomes and progression to cirrhosis. Up to date, there are no approved pharmacotherapies for the treatment of MAFLD, so management focused on lifestyle interventions to encourage weight loss, and treatment of coexisting conditions is the only available option. Unfortunately, the aforementioned is often not potent enough to offer reversal or slow down hepatic inflammation and fibrosis. Glucagon-like peptide-1 receptor agonists have a favorable effect on glycemic management and weight loss of patients with type 2 diabetes mellitus and recently published data suggest their potential in MAFLD treatment. In addition, some of the agents have proven cardiovascular and renal benefits in dedicated cardiovascular outcome trials, making them an interesting therapeutic option. In this opinion review, we discuss the role of semaglutide in MAFLD.
Core Tip: The pathogenesis of metabolically associated fatty liver disease (MAFLD) is closely interrelated to type 2 diabetes mellitus (T2DM), with insulin resistance and hyperinsulinemia as key characteristics. Glucagon-like peptide-1 receptor agonists have a favorable effect on glycemic management and weight loss in T2DM patients. Semaglutide is an especially interesting agent with favorable metabolic actions in patients sharing T2DM and MAFLD (but also sole MAFLD) phenotype, available in injectable and oral formulation, thus more attractive for a broader spectrum of patients.
- Citation: Cigrovski Berkovic M, Rezic T, Bilic-Curcic I, Mrzljak A. Semaglutide might be a key for breaking the vicious cycle of metabolically associated fatty liver disease spectrum? World J Clin Cases 2022; 10(20): 6759-6768
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6759.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6759
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease that includes a broad spectrum of clinical and histopathological conditions, from simple steatosis (non-alcoholic fatty liver) to liver inflammation and injury with or without fibrosis [non-alcoholic steatohepatitis (NASH)] that can further progress to cirrhosis and hepatocellular carcinoma (HCC)[1]. Exclusion of patients with alcohol intake, or other chronic liver diseases is mandatory for the diagnosis. Nowadays, NAFLD is the leading cause of liver disease worldwide. Its prevalence is rising, becoming a major cause of liver disease-related deaths and liver transplantation[2,3]. Additionally, it carries an increased risk for cardiovascular disease (CVD) morbidity and mortality[4]. The condition is strongly associated with obesity and type 2 diabetes mellitus (T2DM) and is considered a liver manifestation of metabolic syndrome. The definition of NAFLD is relatively narrow and based on exclusion. Thus, in recent years, a new concept has emerged, better represented by the term metabolically associated fatty liver disease (MAFLD). The diagnosis of MAFLD is based on the presence of hepatic fat (diagnosed by histology, imaging, or blood biomarkers) along with at least one of these three metabolic conditions: overweight/obesity, T2DM, or evidence of metabolic dysregulation[5]. The latter is defined by at least two criteria in patients with normal body mass index (BMI): enlarged waist circumference; hypertension or anti-hypertensive treatment; increased triglycerides or treatment with hypolipemic drugs; low high-density lipoprotein cholesterol; prediabetes; high Homeostatic Model Assessment of Insulin Resistance score; and high-sensitivity C-reactive protein[6]. In addition, MAFLD diagnosis does not exclude excessive alcohol consumption and other causes of liver disease.
The pathogenesis of MAFLD is multifactorial and closely interrelated to the pathogenesis of T2DM, with insulin resistance (IR) and hyperinsulinemia as key shared characteristics of both conditions. Moreover, individuals with MAFLD are more insulin resistant than those without MAFLD, irrespective of glucose tolerance and BMI[7]. IR acts on adipose tissue, worsens adipocyte dysfunction, induces lipolysis, and releases adipokines and proinflammatory cytokines. IR increases de novo lipogenesis in the liver, resulting in elevated free fatty acids and lipid accumulation within hepatocytes, predisposing to liver injury and inflammation[8]. Proinflammatory environment further contributes to CVD[9].
The relationship between T2DM and NAFLD/MAFLD is bidirectional; T2DM is a risk factor for the progression of NAFLD/MAFLD to fibrosis[10,11], as well as HCC[12], and conversely, NAFLD/ MAFLD increases the risk of developing T2DM[13]. In addition, patients with NAFLD are known to have high cardiovascular (CV) risk and CVD is the leading cause of death in NAFLD patients[4]. Furthermore, given its broader definition, it should be expected that MAFLD is associated with higher CVD morbidity and mortality compared to NAFLD. However, the data comparing the two are inconclusive and scarce. In consideration that MAFLD is inclusive of patients with alcohol consumption and other liver disease, and it is relatively new concept additional studies are needed to define group of patients that are especially at risk of CVD morbidity and mortality[14].
Nevertheless, a treatment that addresses all of the above conditions would be strongly recommended.
Currently, no specific therapies alter the natural history of MAFLD and its progression to more severe forms of steatohepatitis ending in liver cirrhosis and/or liver cancer. Lifestyle modification remains the cornerstone of treatment[15,16].
Considering that IR is the pathogenetic factor involved in MAFLD, antihyperglycemic agents, especially insulin sensitizers, emerged as the potential therapeutic option. Pioglitazone is currently the only pharmacological agent recommended in patients with biopsy-proven NASH as it improves liver histology, both in patients with and without T2DM[15-17].
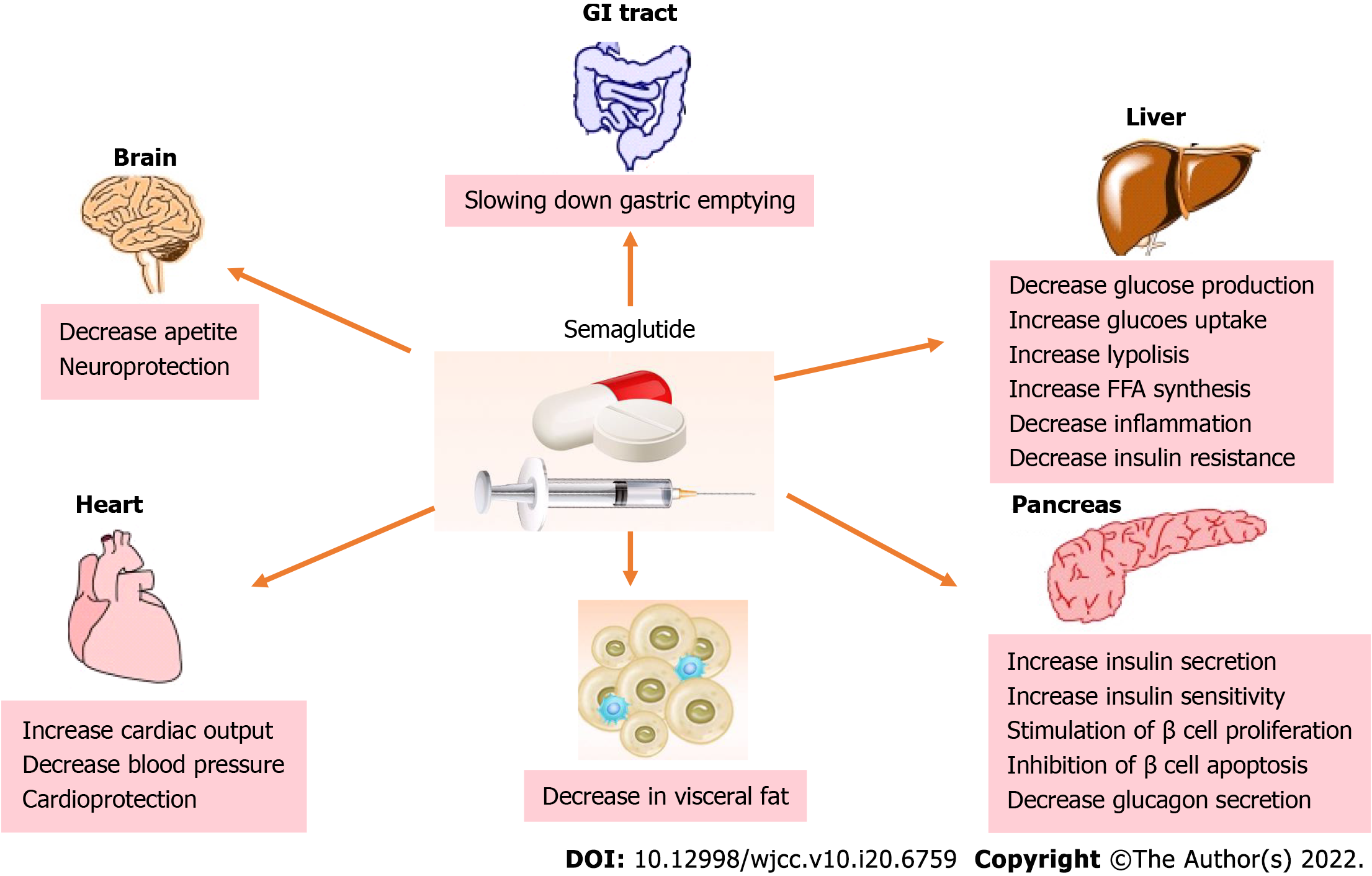
In recent years, newer antihyperglycemic agents, glucagon-like peptide-1 receptor agonists (GLP-1RAs), have exhibited beneficial direct and indirect effects on metabolism and weight loss, raising the interest as a new drug class with potential in MAFLD prevention and treatment. Moreover, some of these agents showed CV protection in dedicated CV outcome trials, placing them in the spotlight for broader use in additional indications, particularly suitable for patients sharing diabetes and MAFLD phenotype (Figure 1).
GLP-1RAs are agents available to treat T2DM patients, especially those with atherosclerotic CVD and obesity. Either daily (liraglutide, lixisenatide, exenatide) or weekly injectable GLP1-RA preparations (dulaglutide, semaglutide, exenatide once weekly) have been available, and recently, a daily oral formulation (semaglutide) was approved[18]. GLP-1RAs have many beneficial effects, including stimulating glucose-dependent insulin secretion, inhibition of glucagon secretion and stimulation of β-cell proliferation, delay of gastric emptying, and increasing satiety via central nervous system pathways[18]. In dedicated randomized control trials (RCTs), including T2DM patients all over the diabetes spectrum, GLP-1RAs have proven glucose-lowering and significant weight-lowering effects alongside their cardio- and renoprotective properties[19,20]. Also, GLP-1RAs can improve serum transaminase levels in patients with MAFLD[21]. Additionally, patients with MAFLD have exhibited a decrease in endogenous GLP-1 secretion, highlighting GLP-1RAs as a potential treatment[22]. Given their multifactorial effects and targeting many pathways involved in MAFLD, including IR, inflammation, obesity, and offering cardiovascular protection, GLP-1 RAs are emerging as a promising treatment for MAFLD patients.
The hepatic effects of GLP-1 RAs are mostly evident indirectly by reducing body weight, IR and improving fatty acid metabolism. Obese patients with NAFLD are insulin resistant at the level of adipose tissue, liver, and skeletal muscle. They exhibit a progressive deterioration in metabolic parameters, hepatic IR, and liver fibrosis as adipose tissue IR worsens[23]. The liver acts as a metabolic sensor of dysfunctional adipose tissue, and insulin resistant adipose tissue is closely connected to intrahepatic triglyceride accumulation[24]. By acting favorably on body weight, GLP1-RAs decrease adipose tissue (primarily visceral) and indirectly reduce intrahepatic fat content and lead to MAFLD prevention/amelioration. Additionally, GLP-1RAs show a beneficial effect on lipoprotein metabolism, modulating reverse cholesterol transport, reducing triglyceride production rate from the liver and intrahepatic triglyceride content, and consequently reducing fasting and postprandial concentration of triglycerides[25].
But what about their direct effects on the liver? We know that GLP-1RAs exert their effects by binding to receptors found in islet cells and other extrapancreatic tissues (lung, kidney, brain, nervous system, gastrointestinal system, etc.). Gupta et al[26] found GLP-1 receptors on human hepatocytes in vitro, showing a direct role in improving hepatic steatosis by modulating insulin signaling pathways and decreasing hepatic IR and fatty acid synthesis. Furthermore, GLP-1 RAs improved hepatocyte survival and reduced hepatic steatosis by inhibiting endoplasmic reticulum stress response and reducing fatty acid accumulation by inducing autophagy[27,28]. Still, the direct effects of GLP-1 RAs on the liver remain not fully understood, and large-scale RCTs are needed to investigate the efficacy and safety of GLP-1-based therapies in treating patients with MAFLD.
In recent years, several studies have examined the efficacy of GLP-1 RAs in managing MAFLD in patients with and without T2DM. These studies mainly evaluated exenatide and liraglutide in the treatment of MAFLD/NASH, primarily in patients with concomitant T2DM. Liraglutide was the most widely studied among GLP-1 RAs and, until recently, the only one that showed improvement in liver histology for patients with biopsy-proven NASH. The LEAN study (liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis)[29] included patients with and without T2DM and showed the histological resolution of NASH in patients treated with liraglutide. In addition to improvements in histological steatosis and hepatocyte ballooning, fewer patients had fibrosis progression. Other trials with liraglutide were not conducted in biopsy-proven NASH. Few studies compared liraglutide to other antihyperglycemic agents in NAFLD and T2DM. Ohki et al[30] conducted a retrospective study evaluating the efficacy of liraglutide vs sitagliptin and pioglitazone. A significant decrease in serum aminotransferase levels for all groups was reported, while the aspartate aminotransferase (AST)-to-platelet counts ratio index was significantly reduced only for the liraglutide and pioglitazone groups[30]. Another trial by Feng et al[31] randomized T2DM patients with NAFLD to receive liraglutide, metformin, or gliclazide. The liraglutide group showed the greatest reduction in intrahepatic steatosis and liver enzymes[31]. Few trials compared exenatide to other hypoglycaemic agents in NAFLD patients with T2DM. Shao et al[32] compared exenatide plus insulin glargine U-100 (exenatide group) with insulin glargine U-100 plus insulin aspart (intensive insulin group). The liver enzymes were significantly lower, and the reversal rate of liver steatosis was higher in the exenatide group than in the intensive insulin group[32]. Another RCT compared the efficacy of exenatide vs metformin in patients with NAFLD and T2DM, concluding that exenatide was more effective than metformin in reducing body weight and improving liver enzymes[33]. Exenatide has not been studied in RCTs with liver histology outcomes in NASH patients. Nevertheless, a recent meta-analysis of eight studies with exenatide and liraglutide in patients with T2DM and MAFLD found significant improvements in hepatic fat content, liver biochemistry, body composition, metabolic parameters (glucose parameters, lipid parameters, insulin sensitivity), and inflammatory markers following GLP-1 RAs treatment. Moreover, GLP-1RAs also improved fibrosis markers without statistical significance[34]. The mentioned meta-analysis did not include studies that examined liver histology. The data regarding dulaglutide and NAFLD are limited and primarily based on retrospective studies[35]. Only one RCT evaluated the effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD (D-LIFT trial). This study compared patients receiving dulaglutide (add-on to usual care) vs the usual care. The dulaglutide group showed a significant reduction in liver fat content and gamma-glutamyl transferase levels in participants with NAFLD. The dulaglutide group showed non-significant reductions in pancreatic fat content, liver stiffness, serum AST, and serum alanine aminotransferase (ALT) levels[36]. Lastly, an updated meta-analysis included eleven placebo-controlled or active-controlled phase-2 RCTs that used liraglutide (n = 6 RCTs), exenatide (n = 3 RCTs), dulaglutide (n = 1 RCT) or semaglutide (n = 1 RCT) to specifically treat NAFLD or NASH, detected by liver biopsy (n = 2 RCTs) or imaging techniques (n = 9 RCTs). Compared to placebo or reference therapy, treatment with GLP-1 RAs was associated with significant reductions in liver fat content on magnetic resonance-based techniques and serum aminotransferase levels, as well as with the greater histological resolution of NASH without worsening of liver fibrosis (for liraglutide and semaglutide only)[37].
Semaglutide is a novel GLP-1 receptor agonist that has been recently approved for the treatment of T2DM and obesity. Two formulations are currently available, once-weekly subcutaneous semaglutide and once-daily oral semaglutide, the subcutaneous form in different dose ranges depending on the indication (for T2DM subcutaneous semaglutide up to 1 mg weekly and oral semaglutide up to 14 mg daily; for obesity subcutaneous semaglutide 2.4 mg weekly). Currently completed studies with subcutaneous in T2DM[38-56] and oral semaglutide[57-67] are presented in Table 1, Table 2, and Table 3.
| Study | Ref. | Main conclusion |
| SUSTAIN 1 | Sorli et al[38], 2017 | Semaglutide significantly improved HbA1c and bodyweight in T2DM patients compared to placebo |
| SUSTAIN 2 | Ahren et al[39], 2017 | Semaglutide is superior to sitagliptin at improving glycemia and bodyweight when added to metformin+/-pioglitazon |
| SUSTAIN 3 | Ahmann et al[40], 2018 | Semaglutide is superior to exenatide ER in glycemic control and body weight reduction |
| SUSTAIN 4 | Aroda et al[41], 2017 | semaglutide is superior to insulin glargine U100 in glycemic control and bodyweight reduction |
| SUSTAIN 5 | Rodbar et al[42], 2018 | Semaglutide, added to basal insulin, significantly reduced HbA1c and body weight in patients with uncontrolled T2D vs placebo |
| SUSTAIN 61 | Marso et al[43], 2016 | In T2DM patients at high cardiovascular risk, semaglutide was significantly better compared to placebo in reduction of 3 point MACE |
| SUSTAIN 7 | Pratley et al[44], 2018 | At low and high doses, semaglutide was superior to dulaglutide in improving glycaemic control and reducing body weight of T2DM patients |
| SUSTAIN 8 | Lingway et al[45], 2019 | Once-weekly semaglutide 1.0 mg was superior to daily canagliflozin 300 mg in reducing HbA1c and bodyweight in patients with type 2 diabetes uncontrolled on metformin therapy |
| SUSTAIN 8 substudy | McCrimmon et al[46], 2019 | In individuals with uncontrolled T2DM on stable-dose metformin, the changes in body composition with semaglutide and canagliflozin were not significantly different |
| SUSTAIN 9 | Zinman et al[47], 2019 | Adding semaglutide to SGLT-2 inhibitor therapy significantly improves glycaemic control and reduces bodyweight in patients with inadequately controlled T2DM |
| SUSTAIN 10 | Capehorn et al[48], 2020 | Semaglutide was superior to liraglutide in reducing HbA1c and body weight |
| SUSTAIN (Japan) | Kaku et al[49], 2018 | Semaglutide treatment significantly reduced HbA1c and body weight vs additional OAD treatment in Japanese people with T2D |
| SUSTAIN Forte | Frias et al[50], 2021 | Semaglutide 2.0 mg was superior to 1.0 mg in reducing HbA1c, with additional body weight loss and a similar safety profile in poorly controlled T2DM |
| SUSTAIN China MRCT | Ji et al[51], 2020 | Once-weekly semaglutide was superior to sitagliptin in improving glycaemic control and reducing body weight in Chinese T2DM patients inadequately controlled on metformin |
| Study | Ref. | Main conclusion |
| Step 1 | Wilding et al[52], 2021 | In participants with overweight or obesity, 2.4 mg of semaglutide once weekly plus lifestyle intervention was associated with sustained, clinically relevant reduction in body weight |
| Step 2 | Davies et al[53], 2021 | In adults with overweight or obesity, and type 2 diabetes, semaglutide 2.4 mg once a week achieved a superior and clinically meaningful decrease in body weight compared with placebo |
| Step 3 | Wadden et al[54], 2021 | Among adults with overweight or obesity, once-weekly subcutaneous semaglutide compared with placebo, used as an adjunct to intensive behavioral therapy and initial low-calorie diet, resulted in significantly greater weight loss during 68 wk |
| Step 4 | Rubino et al[55], 2022 | Among adults with overweight or obesity without diabetes, once-weekly subcutaneous semaglutide compared with once-daily subcutaneous liraglutide, added to counseling for diet and physical activity, resulted in significantly greater weight loss during 68 wk |
| Step 6 | Kadowaki et al[56], 2022 | Adults from east Asia with obesity, with or without type 2 diabetes, given semaglutide 2.4 mg once a week had superior and clinically meaningful reductions in body weight, and greater reductions in abdominal visceral fat area compared with placebo |
| Study | Ref. | Main conclusion |
| PIONEER 1 | Aroda et al[57], 2019 | Oral semaglutide monotherapy demonstrated superior and clinically relevant improvements in HbA1c (all doses) and body weight loss (14 mg dose) versus placebo |
| PIONEER 2 | Rodbard et al[58], 2019 | Oral semaglutide was superior to empagliflozin in reducing HbA1c but not body weight at 26 wk in T2DM patients uncontrolled on metformin. At week 52, HbA1c and body weight (trial product estimand) were significantly reduced versus empagliflozin |
| PIONEER 3 | Rosenstock et al[59], 2019 | Oral semaglutide, 7 mg/d and 14 mg/d, compared with sitagliptin, resulted in significantly greater reductions in HbA1c over 26 wk |
| PIONEER 4 | Pratley et al[60], 2019 | Oral semaglutide was non-inferior to subcutaneous liraglutide and superior to placebo in decreasing HbA1c, and superior in decreasing body weight compared with both liraglutide and placebo at week 26 |
| PIONEER 5 | Mosenzon et al[61], 2019 | Oral semaglutide was effective in patients with type 2 diabetes and moderate renal impairment |
| PIONEER 61 | Husain et al[62], 2019 | The cardiovascular risk profile of oral semaglutide was not inferior to that of placebo in high CV risk T2DM patients |
| PIONEER 7 | Pieber et al[63], 2019 | Superior glycemic control and weight loss with once-daily oral semaglutide with flexible dose adjustment versus sitagliptin 100 mg in type 2 diabetes |
| PIONEER 7 EXTENSION | Buse et al[64], 2020 | Switching from sitagliptin to flexibly dosed oral semaglutide maintained HbA1c reductions, helped more patients achieve HbA1c targets with less use of additional glucose-lowering medication, and offers the potential for additional reductions in body weight |
| PIONEER 8 | Zinman et al[65], 2019 | Oral semaglutide was superior to placebo in reducing HbA1c and body weight when added to insulin with or without metformin in patients with T2DM |
| PIONEER 9 | Yamada et al[66], 2020 | Oral semaglutide provides significant reductions in HbA1c compared with placebo in a dose-dependent manner in Japanese patients with T2DM |
| PIONEER 10 | Yabe et al[67], 2020 | Once-daily oral semaglutide reduced HbA1c and bodyweight vs weekly dulaglutide 0.75 µg in Japanese T2DM patients |
Across the SUSTAIN program, once-weekly subcutaneous semaglutide showed more pronounced metabolic effects than active comparators (including liraglutide, a widely used GLP-1RA)[68]. Semaglutide was associated with reduced CV risk among patients with T2DM at high CV risk[43]. Recently published data from STEP RCTs, on patients receiving subcutaneous semaglutide in dose 2.4 mg once weekly for treatment of obesity suggest its favorable and prolonged effect on weight reduction (twice as many patients reduced more than 5% of initial weight compared to placebo, with a range of weight loss of 10% to 20% in the majority of patients on semaglutide), which is associated with clinically meaningful improvements in cardiovascular and metabolic risk factors and more pronounced when compared to reduction achieved on liraglutide 3.0 mg sc daily[69]. In addition, a new oral formulation is available, with similar efficacy and safety profile to the subcutaneous formulation, confirmed across the PIONEER program. Furthermore, oral semaglutide offers an alternative for patients with concerns regarding injectable treatment and creates an opportunity to expand the utilization of GLP-1 RAs[68].
Semaglutide has been shown to significantly reduce ALT and markers of inflammation[70]. Recently, a RCT comparing subcutaneous semaglutide vs placebo in subjects with NAFLD assessed by MRI was conducted. The trial investigated the effects of subcutaneous semaglutide on liver stiffness, a marker of fibrosis, and liver steatosis in subjects with NAFLD, using non-invasive MRI methods after 24, 48, and 72 wk of treatment. Significant improvement in liver steatosis was found, accompanied by improvements in liver enzymes and metabolic parameters. In addition, more participants receiving semaglutide achieved > 15% reduction in liver stiffness compared to placebo, although the difference was not significant[71].
For now, only two RCTs were conducted with GLP-1 RAs in patients with biopsy-proven NASH, the already mentioned liraglutide[29] and semaglutide. A 72-wk phase 2 trial evaluated the effect of semaglutide on the histologic resolution of NASH in patients with biopsy-proven NASH and fibrosis. Patients were randomized to receive 0.1 mg, 0.2 mg, or 0.4 mg once daily semaglutide or placebo. The semaglutide 0.4 mg was superior to placebo regarding NASH resolution without worsening liver fibrosis. However, a significant between-group difference in improving at least one fibrosis stage was not shown[70]. A much longer duration may be required for improvements in the fibrosis stage to become apparent, especially since most patients in the current study had advanced fibrosis. The most reported adverse events were gastroenterological disorders (nausea, constipation, decreased appetite, vomiting, and abdominal pain), which are already known from RCTs and real-world data. They were dose-dependent and mainly occurred during the dose-escalation period in the first 20 wk of the trial.
Semaglutide is a promising treatment for patients with NASH. Additional studies are needed to evaluate the optimal dosage and formulation for MAFLD treatment. The approved doses of injectable semaglutide for treatment of T2DM are 0.5 mg and 1.0 mg once weekly and for obesity 2.4 mg once weekly, which is different from the once daily 0.1 mg, 0.2 mg, and 0.4 mg doses used in the previously mentioned study. Future dedicated trials enrolling MAFLD patients to receive subcutaneous semaglutide 2.4 mg and development of oral semaglutide for treatment of obesity, almost an inseparable condition from MAFLD, is holding promise as a new therapeutic option.
Semaglutide efficacy in the treatment of NASH was undoubtedly confirmed in the recent RCT in patients with and without T2DM. Even though improvement in the fibrosis stage was not shown in this study, a longer duration of treatment may be needed, especially for advanced-stage fibrosis. Furthermore, semaglutide is currently the only GLP-1RA available in an injectable and oral formulation. Thus, the dosage and formulation of semaglutide in NASH treatment need to be further established. Given its definite potency, it is a promising drug for the treatment of NASH, offering the benefit of the choice of the formulation to best suit individual patients’ preferences.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Giacomelli L, Italy; Li Z, China; Patoulias D, Greece S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2288] [Article Influence: 163.4] [Reference Citation Analysis (0)] |
| 2. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7489] [Article Influence: 832.1] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation. 2019;103:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 4. | Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69:1691-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 502] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 5. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2189] [Article Influence: 437.8] [Reference Citation Analysis (1)] |
| 6. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2792] [Article Influence: 558.4] [Reference Citation Analysis (1)] |
| 7. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1097] [Article Influence: 42.2] [Reference Citation Analysis (1)] |
| 8. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2104] [Article Influence: 233.8] [Reference Citation Analysis (1)] |
| 9. | Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 798] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 10. | Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 659] [Article Influence: 33.0] [Reference Citation Analysis (1)] |
| 11. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 791] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 12. | Reeves HL, Zaki MY, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig Dis Sci. 2016;61:1234-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1316] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 14. | Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential Clinical Characteristics and Mortality Outcomes in Persons With NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19:2172-2181.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 15. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4914] [Article Influence: 702.0] [Reference Citation Analysis (9)] |
| 16. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3169] [Article Influence: 352.1] [Reference Citation Analysis (4)] |
| 17. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2457] [Article Influence: 163.8] [Reference Citation Analysis (2)] |
| 18. | Brunton SA, Wysham CH. GLP-1 receptor agonists in the treatment of type 2 diabetes: role and clinical experience to date. Postgrad Med. 2020;132:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 19. | Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 831] [Article Influence: 207.8] [Reference Citation Analysis (0)] |
| 20. | Sachinidis A, Nikolic D, Stoian AP, Papanas N, Tarar O, Rizvi AA, Rizzo M. Cardiovascular outcomes trials with incretin-based medications: a critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors. Metabolism. 2020;111:154343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Dhir G, Cusi K. Glucagon like peptide-1 receptor agonists for the management of obesity and non-alcoholic fatty liver disease: a novel therapeutic option. J Investig Med. 2018;66:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Bernsmeier C, Meyer-Gerspach AC, Blaser LS, Jeker L, Steinert RE, Heim MH, Beglinger C. Glucose-induced glucagon-like Peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. PLoS One. 2014;9:e87488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 23. | Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:1389-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 339] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 24. | Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711-725.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 657] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 25. | Patel VJ, Joharapurkar AA, Shah GB, Jain MR. Effect of GLP-1 based therapies on diabetic dyslipidemia. Curr Diabetes Rev. 2014;10:238-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 406] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 27. | Wang XC, Gusdon AM, Liu H, Qu S. Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation. World J Gastroenterol. 2014;20:14821-14830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One. 2011;6:e25269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 29. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1448] [Article Influence: 160.9] [Reference Citation Analysis (1)] |
| 30. | Ohki T, Isogawa A, Iwamoto M, Ohsugi M, Yoshida H, Toda N, Tagawa K, Omata M, Koike K. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. ScientificWorldJournal. 2012;2012:496453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Feng W, Gao C, Bi Y, Wu M, Li P, Shen S, Chen W, Yin T, Zhu D. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes. 2017;9:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 32. | Shao N, Kuang HY, Hao M, Gao XY, Lin WJ, Zou W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 33. | Fan H, Pan Q, Xu Y, Yang X. Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq Bras Endocrinol Metabol. 2013;57:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Wong C, Lee MH, Yaow CYL, Chin YH, Goh XL, Ng CH, Lim AYL, Muthiah MD, Khoo CM. Glucagon-Like Peptide-1 Receptor Agonists for Non-Alcoholic Fatty Liver Disease in Type 2 Diabetes: A Meta-Analysis. Front Endocrinol (Lausanne). 2021;12:609110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 35. | Sofogianni A, Filippidis A, Chrysavgis L, Tziomalos K, Cholongitas E. Glucagon-like peptide-1 receptor agonists in non-alcoholic fatty liver disease: An update. World J Hepatol. 2020;12:493-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Kuchay MS, Krishan S, Mishra SK, Choudhary NS, Singh MK, Wasir JS, Kaur P, Gill HK, Bano T, Farooqui KJ, Mithal A. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D-LIFT trial). Diabetologia. 2020;63:2434-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 37. | Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Targher G. Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 190] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 38. | Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, Bain SC. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 448] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 39. | Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, Chow F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 345] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 40. | Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, Holst AG, Annett MP, Aroda VR. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care. 2018;41:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 403] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 41. | Aroda VR, Bain SC, Cariou B, Piletič M, Rose L, Axelsen M, Rowe E, DeVries JH. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 328] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 42. | Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, Araki E, Chu PL, Wijayasinghe N, Norwood P. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial. J Clin Endocrinol Metab. 2018;103:2291-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 43. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3025] [Cited by in RCA: 4021] [Article Influence: 446.8] [Reference Citation Analysis (1)] |
| 44. | Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, Viljoen A; SUSTAIN 7 investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 512] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 45. | Lingvay I, Catarig AM, Frias JP, Kumar H, Lausvig NL, le Roux CW, Thielke D, Viljoen A, McCrimmon RJ. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:834-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 46. | McCrimmon RJ, Catarig AM, Frias JP, Lausvig NL, le Roux CW, Thielke D, Lingvay I. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia. 2020;63:473-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 47. | Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, Thrasher J, Woo V, Philis-Tsimikas A. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:356-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 48. | Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, Vergès B, Marre M. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 49. | Kaku K, Yamada Y, Watada H, Abiko A, Nishida T, Zacho J, Kiyosue A. Safety and efficacy of once-weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: A randomized trial. Diabetes Obes Metab. 2018;20:1202-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 50. | Frías JP, Auerbach P, Bajaj HS, Fukushima Y, Lingvay I, Macura S, Søndergaard AL, Tankova TI, Tentolouris N, Buse JB. Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021;9:563-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 51. | Ji L, Dong X, Li Y, Lim S, Liu M, Ning Z, Rasmussen S, Skjøth TV, Yuan G, Eliaschewitz FG. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes Metab. 2021;23:404-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 52. | Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384:989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 2124] [Article Influence: 531.0] [Reference Citation Analysis (0)] |
| 53. | Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, Rosenstock J, Shimomura I, Viljoen A, Wadden TA, Lingvay I; STEP 2 Study Group. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 646] [Article Influence: 161.5] [Reference Citation Analysis (0)] |
| 54. | Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, Lingvay I, O'Neil PM, Rubino DM, Skovgaard D, Wallenstein SOR, Garvey WT; STEP 3 Investigators. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA. 2021;325:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 572] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 55. | Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, Lingvay I, Mosenzon O, Rosenstock J, Rubio MA, Rudofsky G, Tadayon S, Wadden TA, Dicker D; STEP 4 Investigators. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021;325:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 656] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 56. | Kadowaki T, Isendahl J, Khalid U, Lee SY, Nishida T, Ogawa W, Tobe K, Yamauchi T, Lim S; STEP 6 investigators. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10:193-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 163] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 57. | Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, Jeppesen OK, Christiansen E, Hertz CL, Haluzík M; PIONEER 1 Investigators. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes Care. 2019;42:1724-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 58. | Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, Lingvay I, Søndergaard AL, Treppendahl MB, Montanya E; PIONEER 2 Investigators. Oral Semaglutide Versus Empagliflozin in Patients With Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care. 2019;42:2272-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 59. | Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, Serusclat P, Violante R, Watada H, Davies M; PIONEER 3 Investigators. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA. 2019;321:1466-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 60. | Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ; PIONEER 4 investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 61. | Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, Pratley R, Sathyapalan T, Desouza C; PIONEER 5 Investigators. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 62. | Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC; PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1163] [Article Influence: 193.8] [Reference Citation Analysis (0)] |
| 63. | Pieber TR, Bode B, Mertens A, Cho YM, Christiansen E, Hertz CL, Wallenstein SOR, Buse JB; PIONEER 7 investigators. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:528-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 64. | Buse JB, Bode BW, Mertens A, Cho YM, Christiansen E, Hertz CL, Nielsen MA, Pieber TR; PIONEER 7 investigators. Long-term efficacy and safety of oral semaglutide and the effect of switching from sitagliptin to oral semaglutide in patients with type 2 diabetes: a 52-week, randomized, open-label extension of the PIONEER 7 trial. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 65. | Zinman B, Aroda VR, Buse JB, Cariou B, Harris SB, Hoff ST, Pedersen KB, Tarp-Johansen MJ, Araki E; PIONEER 8 Investigators. Efficacy, Safety, and Tolerability of Oral Semaglutide Versus Placebo Added to Insulin With or Without Metformin in Patients With Type 2 Diabetes: The PIONEER 8 Trial. Diabetes Care. 2019;42:2262-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 66. | Yamada Y, Katagiri H, Hamamoto Y, Deenadayalan S, Navarria A, Nishijima K, Seino Y; PIONEER 9 investigators. Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52-week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020;8:377-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 67. | Yabe D, Nakamura J, Kaneto H, Deenadayalan S, Navarria A, Gislum M, Inagaki N; PIONEER 10 Investigators. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2020;8:392-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 68. | Meier JJ. Efficacy of Semaglutide in a Subcutaneous and an Oral Formulation. Front Endocrinol (Lausanne). 2021;12:645617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 69. | Kushner RF, Calanna S, Davies M, Dicker D, Garvey WT, Goldman B, Lingvay I, Thomsen M, Wadden TA, Wharton S, Wilding JPH, Rubino D. Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5. Obesity (Silver Spring). 2020;28:1050-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 70. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 1174] [Article Influence: 293.5] [Reference Citation Analysis (0)] |
| 71. | Flint A, Andersen G, Hockings P, Johansson L, Morsing A, Sundby Palle M, Vogl T, Loomba R, Plum-Mörschel L. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2021;54:1150-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |