Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.717
Peer-review started: August 18, 2021
First decision: November 1, 2021
Revised: November 11, 2021
Accepted: December 11, 2021
Article in press: December 11, 2021
Published online: January 14, 2022
Processing time: 146 Days and 12.4 Hours
Varicella-zoster virus (VZV) generally causes chickenpox at first infection in childhood and then establishes latent infection in the dorsal root ganglia of the spinal cord or other nerves. Virus reactivation owing to an impaired immune system causes inflammation along spinal nerves from the affected spinal segment, leading to skin manifestations (herpes zoster). Viremia and subsequent hematogenous transmission and nerve axonal transport of the virus may lead to meningitis, encephalitis, and myelitis. One such case is described in this study.
A 64-year-old man presented with dysuria, pyrexia, and progressive disturbance in consciousness. He had signs of meningeal irritation, and cerebrospinal fluid (CSF) analysis revealed marked pleocytosis with mononuclear predominance and a CSF/serum glucose ratio of 0.64. Head magnetic resonance imaging revealed hyperintense areas in the frontal lobes. He had four isolated blisters with papules and halos on his right chest, right lumbar region, and left scapular region. Infected giant cells were detected using the Tzanck test. Degenerated epidermal cells with intranuclear inclusion bodies and ballooning degeneration were present on skin biopsy. Serum VZV antibody titers suggested previous infection, and the CSF tested positive for VZV-DNA. He developed paraplegia, decreased temperature perception in the legs, urinary retention, and fecal incontinence. The patient was diagnosed with meningitis, encephalitis, and myelitis and was treated with acyclovir for 23 days and prednisolone for 14 days. Despite gradual improvement, the urinary retention and gait disturbances persisted as sequelae.
VZV reactivation should be considered in differential diagnoses of patients with sporadic blisters and unexplained central nervous system symptoms.
Core Tip: We describe a rare case of multiple central nervous system (CNS) complications following varicella zoster virus (VZV) reactivation in an immunocompetent patient with mild diabetes. He had four isolated blisters with encephalitis, meningitis, and myelitis. VZV reactivation should be considered in the differential diagnosis of patients with sporadic blisters and unexplained CNS symptoms.
- Citation: Takami K, Kenzaka T, Kumabe A, Fukuzawa M, Eto Y, Nakata S, Shinohara K, Endo K. Varicella-zoster virus-associated meningitis, encephalitis, and myelitis with sporadic skin blisters: A case report. World J Clin Cases 2022; 10(2): 717-724
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/717.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.717
The varicella-zoster virus (VZV) generally causes chickenpox at its first infection in childhood and then establishes latent infection in the dorsal root ganglia of the spinal cord or other nerves[1]. When reactivated owing to impaired systemic immune function, the virus causes inflammation along the spinal nerves from the spinal segment affected by the latent infection, leading to skin manifestations (herpes zoster)[1]. Viremia and subsequent hematogenous transmission and nerve axonal transport of the virus may lead to meningitis, encephalitis, and myelitis[2].
Upon VZV reactivation, viremia is usually absent[3], and viral particles are transported via the nerves. However, in immunocompromised hosts, skin lesions can be more extensive and severe, and viral replication in epithelial cells may result in viremia[4]. In severely immunocompromised hosts, VZV can be disseminated to the lungs, liver, central nervous system (CNS), and other tissues. Myelitis is a relatively common complication of herpes zoster[5] and is considered to be caused by the spread of VZV from the dorsal root ganglia to the spinal cord at the spinal level affected by skin rashes[6].
Here, we report about a very rare case of concurrent meningitis, encephalitis, and myelitis caused by VZV reactivation in a patient with just a few blisters.
Fever, progressive disturbance in consciousness.
The patient was a 64-year-old man. He was transported by ambulance with urinary retention and pyrexia of > 38 °C that persisted for several days as well as progressive disturbance in consciousness.
He did not have regular health checkups and there was no significant medical history. He had an uncertain history of chickenpox in childhood and no history of varicella vaccination. He tested negative for human immunodeficiency virus (HIV) and had no history of bone marrow transplantation or other known conditions associated with immunosuppression.
He had consumed 540 mL of Japanese sake (60 g of alcohol) and smoked 20 cigarettes per day since the age of 20 years.
The patient’s body temperature was 37.2 °C, blood pressure was 182/107 mmHg, heart rate was 106 beats/min and regular, and his respiratory rate was 21 breaths/min; and SpO2 of 99% in room air. Neurological examination showed a Glasgow Coma Scale score of E4V3M5, pupils equal in size (3 mm/3 mm), round, and reactive to light (+/+), positive nuchal rigidity, and negative Kernig’s sign. The tendon reflexes were markedly increased, predominantly in the lower extremities, with a positive Babinski sign bilaterally. There was no obvious quadriplegia.
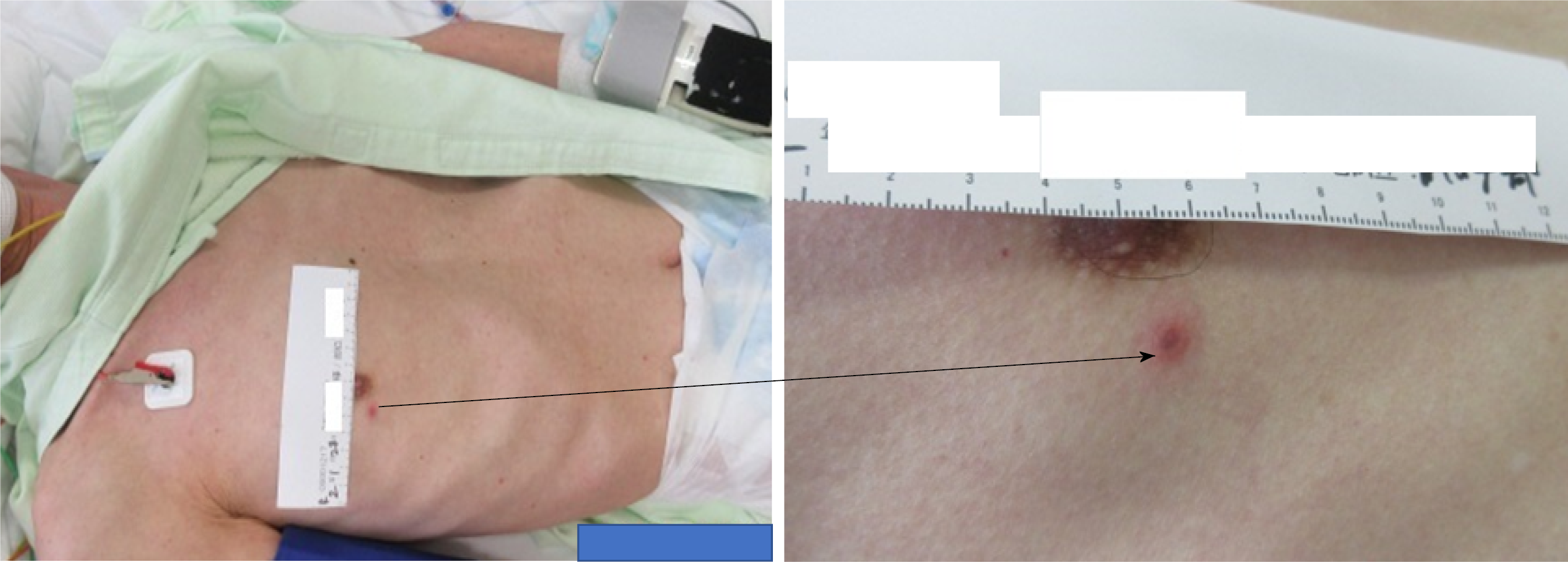
Skin findings included four isolated and sporadic blisters with red papules and a red halo in the right anterior/Lateral chest, right lumbar region, and left scapular region (Figure 1).
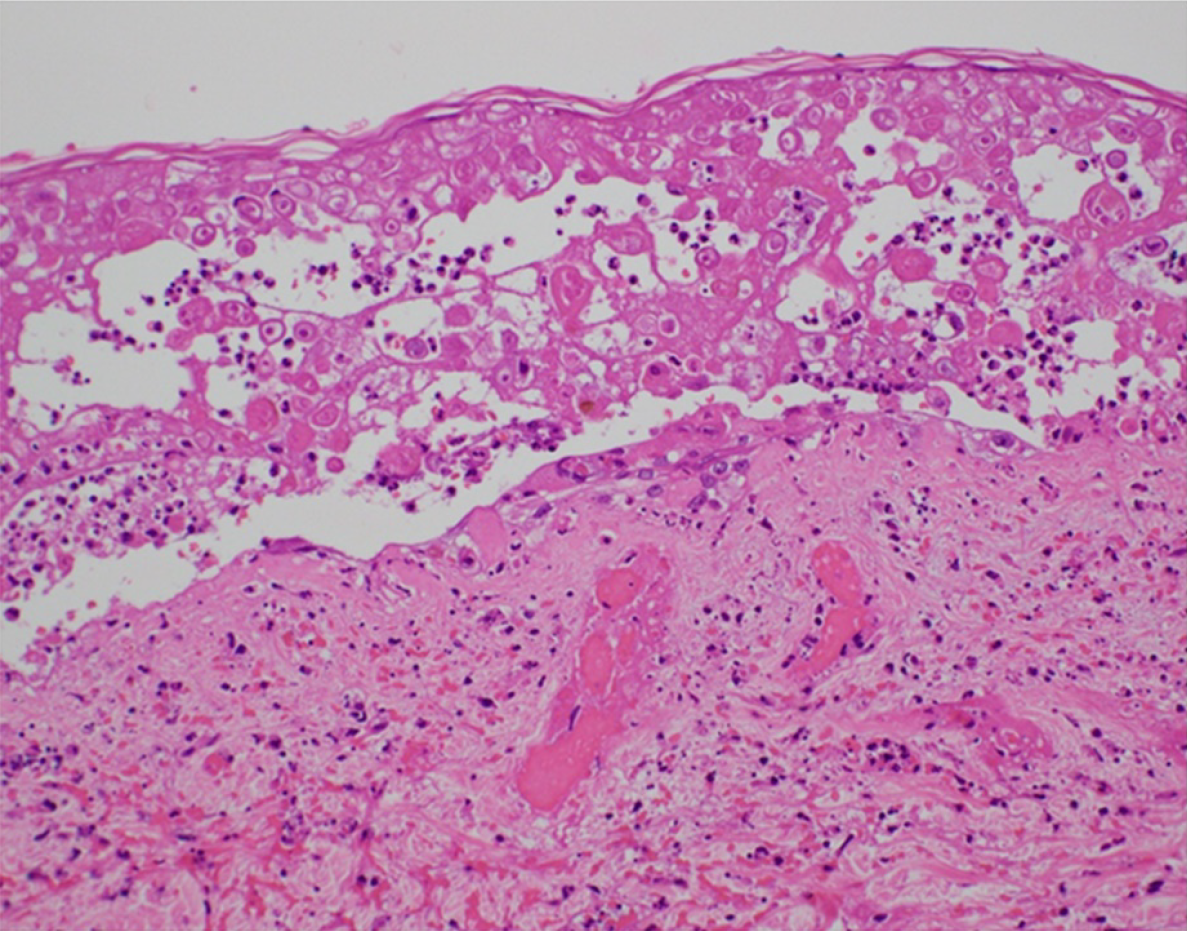
We detected infected giant cells using the Tzanck test and degenerated epidermal cells with intranuclear inclusion bodies and ballooning degeneration on skin biopsy of blisters in the right chest (Figure 2); these findings were consistent with VZV infection.
Mild increase in blood glucose levels (174 mg/dL) and HbA1c level (6.8%) suggestive of diabetes were noted. No increase in inflammatory response was observed (White blood cell count 5800 cells/µL, C-reactive protein 0.15 mg/L). There was no evidence of impaired humoral immunity with a negative HIV antibody test. Serum VZV antibody titers were 75.9 (+) for VZV immunoglobulin G (IgG) (EIA) and 0.49 (−) for VZV immunoglobulin M (IgM) (EIA), which is a pattern indicative of a previous infection. Cerebrospinal fluid (CSF) analysis (Table 1) showed marked pleocytosis with mononuclear predominance, with no decrease in the CSF/serum glucose ratio (0.64). The CSF tested positive for VZV-DNA with 1.4×106 copies/mL.
| Parameter | Patient’s value | Reference value |
| Color | Light yellow | Clear |
| Xanthochromia | Positive | Negative |
| Cell count (cells/μL) | 484 | 0–3 |
| Monocyte count (cells/μL) | 448 | 0–3 |
| Neutrophil count (cells/μL) | 36 | 0–3 |
| Total protein (mg/dL) | 788 | 10–40 |
| Glucose (mg/dL) | 111 | 50–75 |
| HSV antibody titer (CFT) | Negative | Negative |
| HSV-DNA (PCR) (copies/mL) | Negative | Negative |
| VZV antibody titer (CFT) | 4 | Negative |
| VZV-DNA (PCR) (copies/mL) | 1.4 × 106 | Negative |
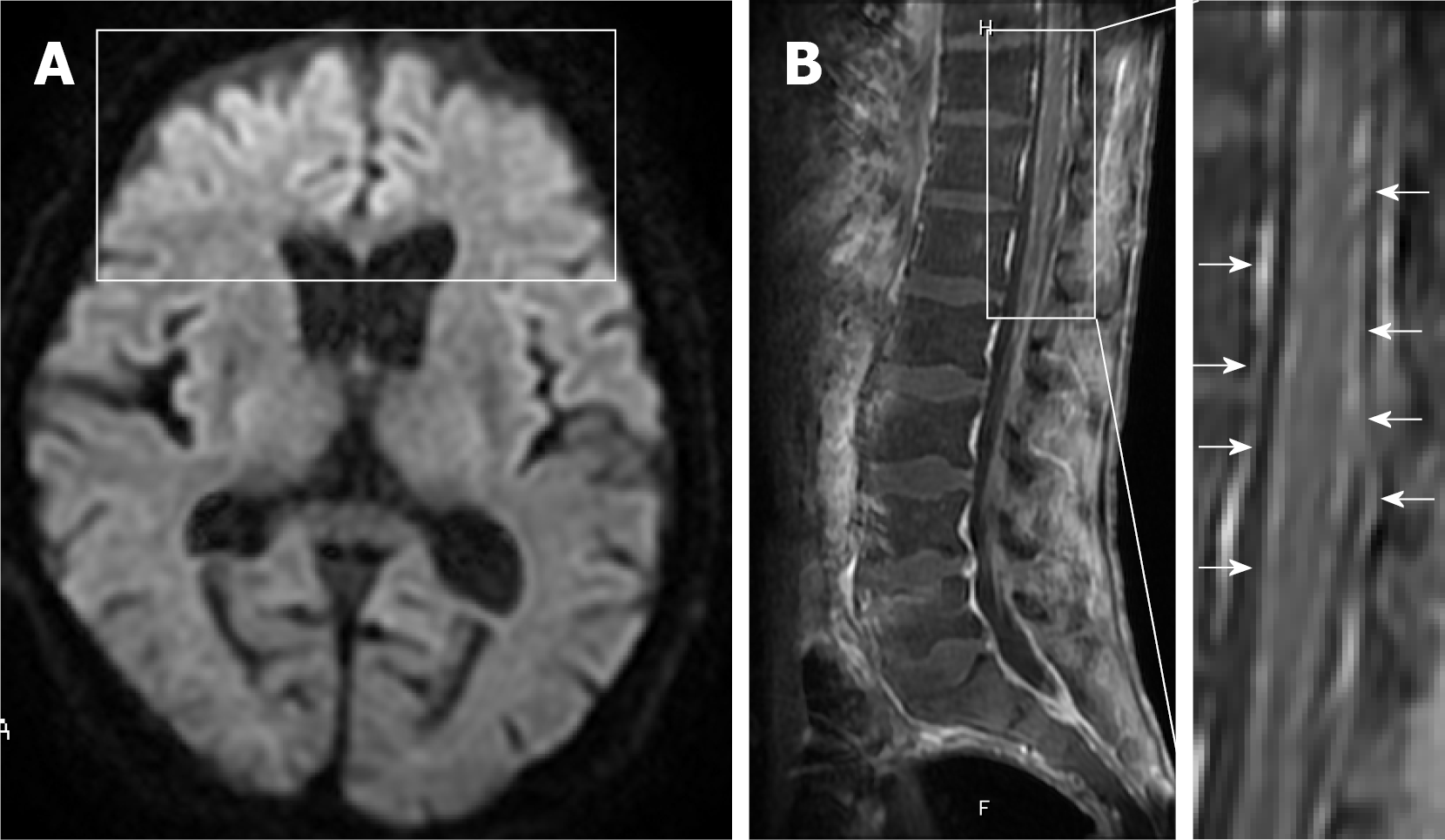
For symptoms associated with frontal lobe disorders such as altered personality and talkativeness that occurred after admission, the patient underwent plain head magnetic resonance imaging (MRI) on hospital day 3 (Figure 3). Diffusion-weighted imaging revealed hyperintensities in the bilateral frontal lobes. Although not evident immediately after admission, paraplegia became apparent after private room isolation was discontinued and rehabilitation was started. Decreased thermal nociception and urinary retention and rectal disturbances were also noted.
Contrast-enhanced thoracolumbar MRI (Figure 3) performed on hospital day 14 showed diffuse enhancement of the meninges at Th9 to L5 with lumbar predominance and no enhancement of the cord itself. Concurrent myelitis was diagnosed based on the diagnostic criteria proposed by the Transverse Myelitis Consortium Working Group[7] although contrast-enhanced MRI of the thoracolumbar spine showed no clear enhancement of the spinal cord.
Based on these findings, we diagnosed meningitis, encephalitis, and myelitis caused by VZV reactivation.
Treatment was initiated with 500 mg acyclovir intravenously three times a day (for 23 days) and prednisolone at 60 mg (1.5 mg/kg)/d (tapered, for 14 days), resulting in a gradual improvement of symptoms.
Changes in the serum VZV antibody titer and CSF analysis results are shown in Table 2. Results of the CSF analysis showed a steady decrease in cell count, and on hospital day 16, the CSF was confirmed to be negative for VZV-DNA. The CSF VZV antibody titer increased more than 8-fold. Regarding serum VZV antibody titers, a significant increase in IgG was noted, whereas no apparent IgM seroconversion was observed. Symptoms of frontal lobe disorders improved with treatment. Plain head MRI performed on hospital day 39 revealed the disappearance of the frontal lobe hyperintensities. The patient continued rehabilitation and showed a slight improve
| Day | 1 | 9 | 16 | 23 |
| CSF cell count (cells/μL) | 484 | 112 | 119 | 29 |
| CSF VZV antibody titer (CFT) | 4 | 32 | ||
| CSF VZV-DNA (PCR) (copies/mL) | 1.4 × 106 | 3.2 × 102 | (-) | (-) |
| Serum VZV-IgM (EIA) | 0.49 (-) | 0.95 (±) | 0.44 (-) | |
| Serum VZV-IgG (EIA) | 75.9 (+) | > 128 (+) | > 128 (+) |
On hospital day 60, the patient was transferred to another hospital for continued rehabilitation. Two months after the transfer, the patient developed lacunar infarction which was treated conservatively. Thereafter, he continued rehabilitation and was discharged home with an activities of daily living grade corresponding to independent wheelchair operation.
In this case report, the patient with mild diabetes but no apparent immunodeficiency developed meningitis, encephalitis, and myelitis owing to VZV reactivation. This was a very rare case with only sporadic blisters accompanied by multiple CNS complications. CNS involvement has been reported as a rare complication of VZV infection, with an incidence of 0.1%–0.3% and 35% in immunocompetent and immunocompromised individuals, respectively[8]. Our literature search found only a few cases of multiple CNS complications caused by VZV, including eight cases of concurrent meningitis, encephalitis, and myelitis[8-14].
The early detection of blisters on emergency arrival led to a strong suspicion and early diagnosis of VZV meningitis in our case. Concurrent myelitis was diagnosed based on the diagnostic criteria proposed by the Transverse Myelitis Consortium Working Group[7] although contrast-enhanced MRI of the thoracolumbar spine showed no clear enhancement of the spinal cord. Despite the prompt initiation of treatment with acyclovir and steroid, gait disturbance and urinary retention associated with myelitis persisted as sequelae.
The reported risk factors for herpes zoster include advanced age, cellular immunodeficiency (an immunocompromised host), diabetes, female sex, genetic susceptibility, mechanical trauma, recent mental stress, and white race, with the most important risk factor being advanced age[16]. A study examining the correlation between age, herpes zoster incidence, and VZV-specific cellular immunity showed a gradual decline in VZV-specific cellular immunity and increased incidence of herpes zoster with increasing age[17]. Studies also showed that diabetes patients have lower VZV-specific cellular immunity than healthy individuals[18]. NK cells[19], ADCC activity[20], and cytotoxic T lymphocytes[21] are considered to play more important roles than humoral immunity in suppressing viral growth. A decrease in these cellular immunity components appears to contribute to the development of severe infection[22]. In our case, it is also likely that decreased cellular immunity owing to aging and mild diabetes played a role in the development of severe illness.
VZV reaches the nervous system either via the bloodstream or by spreading directly from the sensory ganglia, where the virus establishes latent infection, causing CNS complications[23]. In herpes zoster, VZV that is reactivated in the dorsal root ganglia can spread to the spinal cord at the level affected by skin rashes, resulting in myelitis[6]. In our case, the skin rash did not occur along the dermatome as it does in herpes zoster. It appeared in a similar way to chickenpox where the skin areas innervated by the spinal cord, and the level of rash did not match.
Table 3 compares the eight previously reported cases[8-14] of concurrent meningitis, encephalitis, and myelitis caused by VZV reactivation with our case. Although CNS complications of VZV infection are more common in immunocompromised individuals, no underlying disease was identified in three cases. These patients had no predisposing factors for immunosuppression, such as acquired immunodeficiency syndrome, prior organ transplant, or use of oral immunosuppressants. Therefore, it is likely that decreased immunity caused by aging and other factors led to the reactivation of VZV. Among the previously reported cases, only one patient had a skin rash with C8-Th3 distribution[9]. The other seven patients presented neurological complications of VZV without skin symptoms, which is consistent with zoster sine herpete (ZSH)[24]. Our patient had only four sporadic blisters with no band-like cluster of skin eruptions. Bilateral blisters are an uncommon dermic manifestation of VZV in geriatrics, but his disseminated herpes zoster caused bilateral sporadic skin eruptions. The simultaneous onset of meningitis, encephalitis, and myelitis could occur in immunocompetent patients, with none or a few eruptions rather than typical herpes zoster.
| Case | First Author (yr) | Ref | Age (yr) | Sex | Underlying diseases | Rash | Treatment | Outcome |
| 1 | Mancardi (1987) | 8 | 32 | F | None | None | Acyclovir | Survived |
| 2 | Kleinschmidt-DeMasters (1996) | 9 | 69 | F | Steroid dependent asthma | C8-Th3 distribution zoster | Not reported | Died |
| 3 | Kleinschmidt-DeMasters (1996) | 9 | 36 | M | HIV | None | Not reported | Died |
| 4 | Cinque (1997) | 10 | Not reported | Not reported | HIV | None | Acyclovir | Died |
| 5 | Sissoko (1998) | 11 | 75 | M | None | None | Acyclovir | Survived |
| 6 | Russman (2003) | 12 | 51 | F | Steroid dependent CREST syndrome | None | Acyclovir | Survived |
| 7 | Tavazzi (2008) | 13 | 85 | F | None | None | Acyclovir | Died |
| 8 | Tizazu (2020) | 14 | 53 | F | Steroid dependent SLE | None | Acyclovir | Survived |
| 9 | Takami (2021) | Present case | 64 | M | Mild diabetes mellitus | Four sporadic blisters | Acyclovir | Survived |
The skin rash in our patient appeared like a chickenpox rash, and the possibility of reinfection with VZV was also considered. However, a high serum VZV-IgG level on admission and the absence of VZV-IgM seroconversion over time suggested that VZV reactivation was more likely the cause. Generally, herpes zoster does not manifest itself in an immunocompetent host, and whether lesions are formed depends on the host’s immune status. In the event of virus reactivation, individuals with an active immune system may develop ZSH with only prodromal pain and no skin lesions. A previous report documented that 44% of patients with VZV infection in the CNS had no skin rash[25]. In ZSH, CD4+ T cells and CD8+ memory T cells expressing skin-homing receptors are considered to provide innate immunity to suppress the spread of VZV between skin cells, thereby preventing skin rash manifestation[26]. The number of skin eruptions caused by VZV correlates with the amount of virus in circulation before their manifestation[27], and therefore, delayed occurrence of an immune response can lead to the deeper and broader spread of the viral infection and severe lesions. The number and severity of skin eruptions were low in our patient, probably owing to the low viral load in circulation because the skin eruptions were caused by reactivation, not reinfection, of the virus from the previous infection.
Patients with mild skin rash may develop severe illness, similar to our patient. This highlights the importance of early recognition of blisters through skin examination of the entire body and considering VZV infection as a differential diagnosis even if the patient has isolated and sporadic blisters. It is also important to consider VZV infection when diagnosing a patient with unexplained CNS symptoms.
We reported a rare case of concurrent meningitis, encephalitis, and myelitis owing to VZV reactivation in a 64-year-old patient with mild diabetes but with no apparent immunodeficiency. The patient had a small number of blisters. Based on our case findings, VZV infection should be considered a differential diagnosis in patients with sporadic blisters or unexplained CNS symptoms.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sotelo J S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 444] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 2. | Nagel MA, Gilden D. Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol. 2014;27:356-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Kimura H, Kido S, Ozaki T, Tanaka N, Ito Y, Williams RK, Morishima T. Comparison of quantitations of viral load in varicella and zoster. J Clin Microbiol. 2000;38:2447-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Vafai A, Wroblewska Z, Graf L. Antigenic cross-reaction between a varicella-zoster virus nucleocapsid protein encoded by gene 40 and a herpes simplex virus nucleocapsid protein. Virus Res. 1990;15:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Steiner I, Steiner-Birmanns B, Levin N, Hershko K, Korn-Lubetzki I, Biran I. Spinal cord involvement in uncomplicated herpes zoster. Clin Diagn Lab Immunol. 2001;8:850-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | De La Blanchardiere A, Rozenberg F, Caumes E, Picard O, Lionnet F, Livartowski J, Coste J, Sicard D, Lebon P, Salmon-Cèron D. Neurological complications of varicella-zoster virus infection in adults with human immunodeficiency virus infection. Scand J Infect Dis. 2000;32:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Mancardi GL, Melioli G, Traverso F, Tabaton M, Farinelli M, Terragna A. Zoster sine herpete causing encephalomyelitis. Ital J Neurol Sci. 1987;8:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Kleinschmidt-DeMasters BK, Amlie-Lefond C, Gilden DH. The patterns of varicella zoster virus encephalitis. Hum Pathol. 1996;27:927-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 85] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Cinque P, Bossolasco S, Vago L, Fornara C, Lipari S, Racca S, Lazzarin A, Linde A. Varicella-zoster virus (VZV) DNA in cerebrospinal fluid of patients infected with human immunodeficiency virus: VZV disease of the central nervous system or subclinical reactivation of VZV infection? Clin Infect Dis. 1997;25:634-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Sissoko D, Bellagra N, Dewilde A, Rogelet P, Hober D, Wattré P. [Varicella-zoster virus meningo-encephalomyelitis without skin eruption]. Ann Biol Clin (Paris). 1998;56:211-212. [PubMed] |
| 12. | Russman AN, Lederman RJ, Calabrese LH, Embi PJ, Forghani B, Gilden DH. Multifocal varicella-zoster virus vasculopathy without rash. Arch Neurol. 2003;60:1607-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Tavazzi E, Minoli L, Ferrante P, Scagnelli P, Del Bue S, Romani A, Ravaglia S, Marchioni E. Varicella zoster virus meningo-encephalo-myelitis in an immunocompetent patient. Neurol Sci. 2008;29:279-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Tizazu EF, Chang G, Berger JR. Varicella zoster virus (VZV) oculomeningoencephalomyeloradiculitis: a previously undescribed constellation. J Neurovirol. 2020;26:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 542] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 16. | Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48:S2-S7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Takao Y, Miyazaki Y, Onishi F, Kumihashi H, Gomi Y, Ishikawa T, Okuno Y, Mori Y, Asada H, Yamanishi K, Iso H; SHEZ Study group. The Shozu Herpes Zoster (SHEZ) study: rationale, design, and description of a prospective cohort study. J Epidemiol. 2012;22:167-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Okamoto S, Hata A, Sadaoka K, Yamanishi K, Mori Y. Comparison of varicella-zoster virus-specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis. 2009;200:1606-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Ito M, Bandyopadhyay S, Matsumoto-Kobayashi M, Clark SC, Miller D, Starr SE. Interleukin 2 enhances natural killing of varicella-zoster virus-infected targets. Clin Exp Immunol. 1986;65:182-189. [PubMed] |
| 20. | Ihara T, Ito M, Starr SE. Human lymphocyte, monocyte and polymorphonuclear leucocyte mediated antibody-dependent cellular cytotoxicity against varicella-zoster virus-infected targets. Clin Exp Immunol. 1986;63:179-187. [PubMed] |
| 21. | Diaz PS, Smith S, Hunter E, Arvin AM. T lymphocyte cytotoxicity with natural varicella-zoster virus infection and after immunization with live attenuated varicella vaccine. J Immunol. 1989;142:636-641. [PubMed] |
| 22. | Ihara T, Oitani K, Torigoe S, Kitamura K, Ito M, Kamiya H, Sakurai M. Cytotoxicity against varicella zoster virus infected targets in children with acute leukemia. Acta Paediatr Jpn. 1994;36:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Echevarría JM, Casas I, Martínez-Martín P. Infections of the nervous system caused by varicella-zoster virus: a review. Intervirology. 1997;40:72-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | LEWIS GW. Zoster sine herpete. Br Med J. 1958;2:418-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Koskiniemi M, Piiparinen H, Rantalaiho T, Eränkö P, Färkkilä M, Räihä K, Salonen EM, Ukkonen P, Vaheri A. Acute central nervous system complications in varicella zoster virus infections. J Clin Virol. 2002;25:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Drago F, Herzum A, Ciccarese G, Broccolo F, Rebora A, Parodi A. Acute pain and postherpetic neuralgia related to Varicella zoster virus reactivation: Comparison between typical herpes zoster and zoster sine herpete. J Med Virol. 2019;91:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Asano Y, Itakura N, Kajita Y, Suga S, Yoshikawa T, Yazaki T, Ozaki T, Yamanishi K, Takahashi M. Severity of viremia and clinical findings in children with varicella. J Infect Dis. 1990;161:1095-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |