Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.671
Peer-review started: 23 June 2021
First decision: 16 July 2021
Revised: June 15, 2021
Accepted: December 8, 2021
Article in press: December 8, 2021
Published online: January 14, 2022
Processing time: 202 Days and 9 Hours
Myopic foveoschisis (MF) is a common complication of pathological myopia. A macular hole (MH) usually results from the natural progression of MF and is a common complication of vitrectomy. Vitrectomy combined with residual internal limiting membrane (ILM) covering and autologous blood was effective for closing a secondary MH.
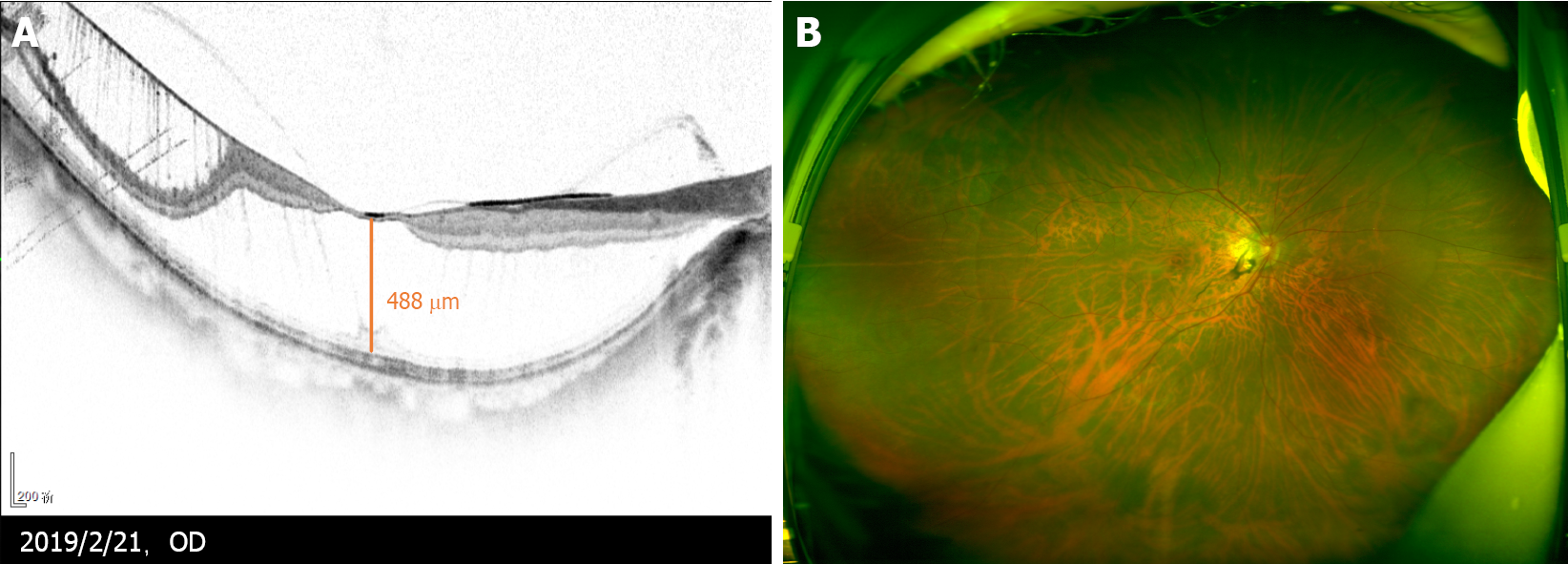
A 52-year-old woman presented to our clinic with a complaint of blurred vision in the right eye for 7 years. Her best corrected visual acuity (BCVA) was 20/100, axial length was 25.79 mm and standard equivalent refractive error was -10.5 dioptres. Preoperative optical coherence tomography revealed foveoschisis in the right eye. Vitrectomy with fovea-sparing ILM peeling was performed. An MH developed and gradually expanded 5 mo after the initial vitrectomy. Vitrectomy with residual ILM covering and autologous blood was performed. The MH closed 3 wk after the second vitrectomy.
Fovea-sparing ILM peeling can provide residual ILM for the treatment of MH secondary to vitrectomy for MF. Vitrectomy combined with residual ILM covering and autologous blood is effective for closing secondary MH and improving BCVA.
Core Tip: A macular hole (MH) is a common complication after vitrectomy for myopic foveoschisis (MF). This report describes a case of an MH secondary to vitrectomy with fovea-sparing internal limiting membrane (ILM) peeling for MF. We found that the repair process of MF may be centripetal, gradually moving from the peripheral retina to the macula. Second vitrectomy with residual ILM covering and autologous blood is effective for closing secondary MH and improving best corrected visual acuity.
- Citation: Ying HF, Wu SQ, Hu WP, Ni LY, Zhang ZL, Xu YG. Vitrectomy with residual internal limiting membrane covering and autologous blood for a secondary macular hole: A case report. World J Clin Cases 2022; 10(2): 671-676
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/671.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.671
Myopic foveoschisis (MF) is a common complication of pathological myopia and is characterised by intraretinal splitting in the macular region, which can eventually develop into macular hole (MH) or even retinal detachment[1,2]. It can result from the combined action of tangential and longitudinal vitreoretinal tractions, and surgery can release these two types of traction[3].
Vitrectomy is the mainstay of treatment for MF, but it is still disputable whether internal limiting membrane (ILM) peeling should be performed[4]. A recent meta-analysis concluded that vitrectomy with ILM peeling may lead to a better anatomic outcome of MF than that without peeling. However, vitrectomy with ILM peeling did not significantly improve the best corrected visual acuity (BCVA) or lead to fewer complications[5]. Qi et al[6] reported that vitrectomy with fovea-sparing ILM peeling may lead to a better anatomic outcome of MF and fewer complications and similar visual function than that without peeling. A meta-analysis by Azuma et al[3] suggested that vitrectomy with fovea-sparing ILM peeling could achieve similar anatomic outcomes in the treatment of MF, with greater visual benefits and a lower risk of MH than complete ILM peeling. Furthermore, Lai et al[7] reported that vitrectomy combined with ILM repositioning and autologous blood clot was significantly effective for closing MHs, reattaching the retina and improving BCVA in patients with MH retinal detachment.
In the present case, we found that fovea-sparing ILM peeling was advantageous for providing residual ILM to treat MH secondary to vitrectomy for MF. Vitrectomy combined with residual ILM covering and autologous blood was effective for closing secondary MH and improving BCVA.
A 52-year-old woman presented to our hospital with a complaint of blurred vision in the right eye for 7 years.
The patient had no other positive symptoms.
The patient had no previous medical history.
The patient was married and had a son. There was no family history of ocular disease.
At the initial examination, her BCVA was 20/100 in the right eye and 20/60 in the left eye. The standard equivalent refractive error was -10.5 dioptres in the right eye and -12.5 dioptres in the left eye. The intraocular pressures were 12.2 and 12.3 mmHg and axial lengths were 25.79 and 27.1 mm in the right and left eyes, respectively. In both eyes, the crystalline lens demonstrated opacification, and the anterior chamber was deep and clear, with tessellated fundus.
Her prothrombin time and blood analysis, blood chemistry and urinalysis results were normal. The electrocardiographic findings were normal, and chest computed tomography showed pulmonary nodules.
Preoperative optical coherence tomography (OCT) (Figure 1A) showed that vitreomacular traction was present in the right eye, the retinal nerve fibre layer was split on the temporal side and the outer nuclear layer was split extensively on the entire macula. Preoperative optomap (Figure 1B) revealed tessellated fundus in the right eye.
The final diagnosis of the case was binocular cataract, binocular MF, binocular high myopia and pulmonary nodules.
Initial vitrectomy with fovea-sparing ILM peeling for MF was performed on 10 September 2020. Standard 25-G three-port pars plana vitrectomy combined with phacoemulsification and intraocular lens implantation was performed in the right eye under retrobulbar anaesthesia. After cataract surgery, the central vitreous core was removed, and the posterior hyaloid membrane was removed from the macular surface. The ILM was stained with indocyanine green (0.5 mg/mL). The ILM was removed in the macular area (fovea-sparing, 2 PD). Fluid–gas exchange was conducted with gas tamponade by room air at the end of the surgery. The patient was instructed to remain in the prone position for 3 d and avoid the supine position during the follow-up period until the gas was absorbed.
The secondary MH was detected after 1 wk, and second vitrectomy combined with residual ILM covering and autologous blood was performed 5 mo after the first surgery. Standard 25-G three-port pars plana vitrectomy was performed in the right eye under retrobulbar anaesthesia. The residual vitreous was removed, and the residual ILM was stained with indocyanine green (0.5 mg/mL). The residual ILM flap was inverted and covered the MH. Fluid–gas exchange was conducted. Then, fresh blood obtained from the patient’s vein was injected gently to cover the macula. The patient was instructed to remain in the supine position for 30 min and then change to the prone position for 3 d and avoid the supine position thereafter until the gas was absorbed.
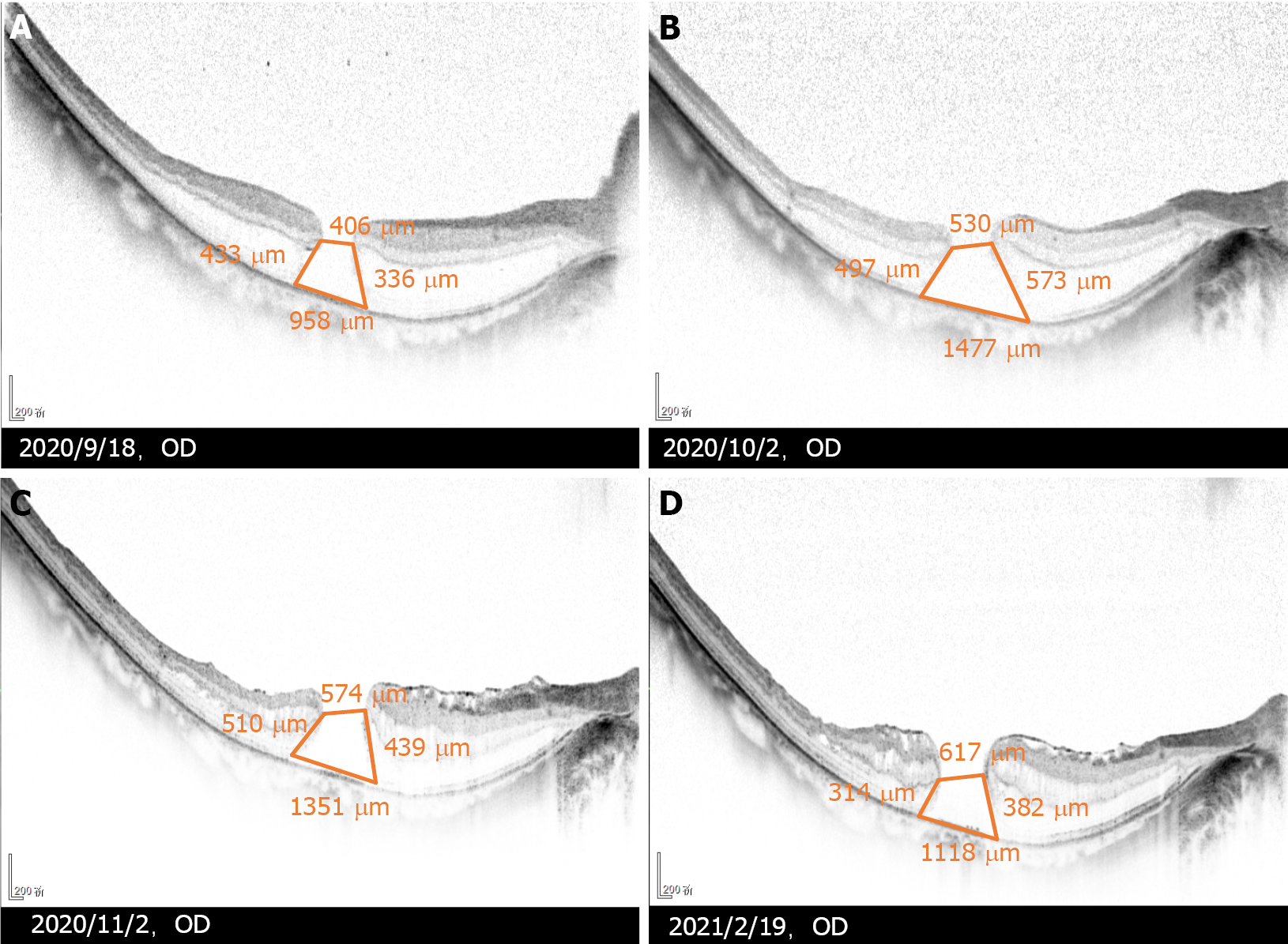
Five months after the initial vitrectomy, postoperative OCT (Figure 2) demonstrated that the split range of the outer nuclear layer gradually reduced, a new split of the inner nuclear layer appeared, and the MH gradually expanded. The MH diameter was 406 μm at 1 wk after the initial vitrectomy and expanded to 617 μm at 5 mo after the initial vitrectomy. The epiretinal membrane proliferated on the residual ILM, wrinkled and caused the disordered shape of the nasal retinal nerve fibre layer.
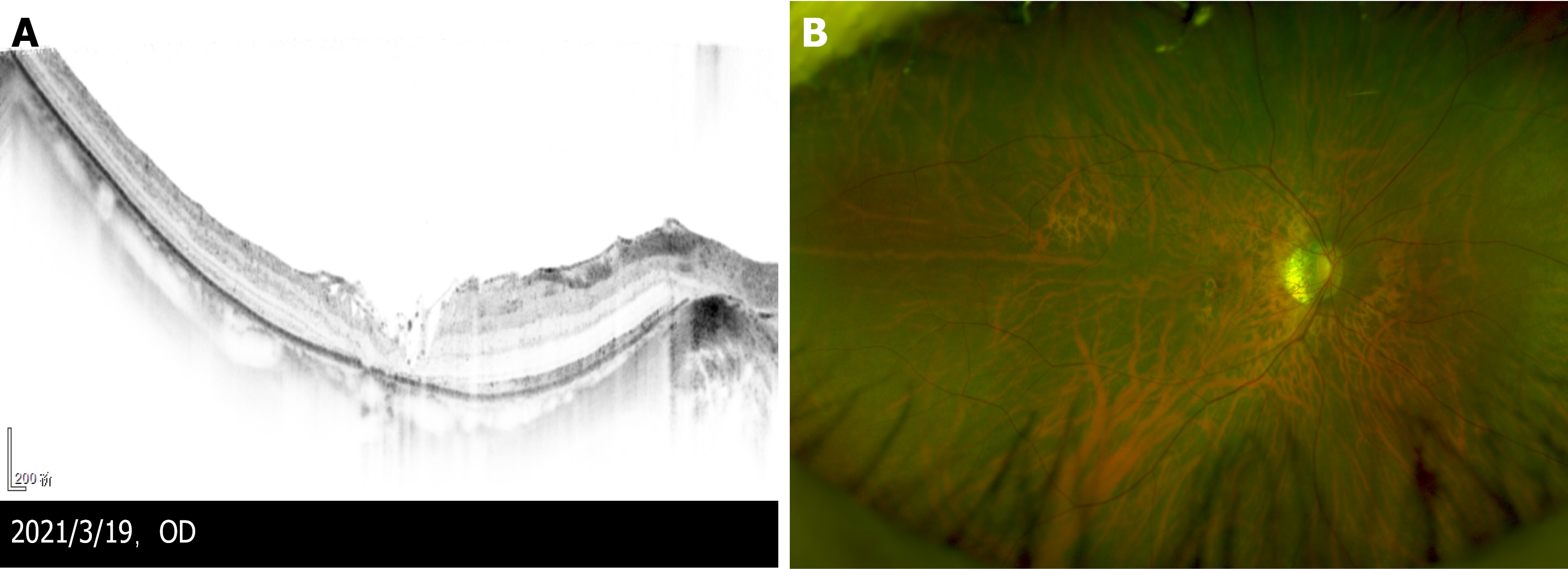
Postoperative OCT 3 wk after the second vitrectomy revealed that the MH closed, covered by flocculent ILM (Figure 3A). The split of each layer was significantly reduced, and photoreceptor cells were absent in the macular area. Optomap (Figure 3B) revealed tessellated fundus only. The patient’s BCVA was 25/100 in the right eye, but she complained of central scotoma.
A recent meta-analysis reported that compared with complete ILM peeling, fovea-sparing ILM peeling may lead to similar morphological effects and may contribute to greater visual benefits and a lower risk of postoperative MH formation[3]. Fovea-sparing ILM peeling could release tangential traction force and preserve the integrity of Müller cells in the macular fovea. Thus, the traction force over the extremely thinned foveal tissue can be minimised, and less irritation and injury are helpful to maintain the stability of the macular area and avoid postoperative MH formation.
The residual ILM may contract 2-10 mo postoperatively. Although it does not affect vision in the short term, it can still cause pathological changes similar to that of epiretinal membrane formation. The ILM is composed of type IV, type VI and type VIII collagen, which is the basement membrane of Müller cells[2]. Removal of the ILM can affect the adhesion of Müller cells, leading to its dysfunction[8]. Therefore, the size of the preserved ILM in the macular region warrants further study.
The secondary MH was detected 1 wk after the initial vitrectomy. MH usually results from the natural progression of MF and is a common complication of vitrectomy[4]. The stiffness of Müller cells might considerably increase in MF, which might translate to higher mechanical force transmission to photoreceptors[9]. The inner retinal surface of the MF was vulnerable to developing a break, and tangential and anteroposterior traction between the vitreous and retina might induce MH formation[10]. Gao et al[11] investigated the possible mechanisms and risk factors for the development of secondary MH in MF based on preoperative OCT findings and found that a preoperative inner segment/outer segment (IS/OS) junction defect can be a risk factor for MH development. Kumar et al[12] found that vitrectomy with intraoperative OCT (I-OCT)-guided fovea-sparing ILM peeling helps in complete removal of traction, resolution of retinoschisis and good functional recovery, with fewer intraoperative and postoperative complications. Therefore, if preoperative OCT reveals an IS/OS junction defect, we should pay more attention to MH formation. Vitrectomy should be performed centripetal rather than centrifugal near the macula, and anteroposterior traction should be avoided to protect Müller cell processes and photoreceptor axons from damage. I-OCT may be used as it is associated with few intraoperative and postoperative complications.
In the present case, 5 mo after the initial vitrectomy, we observed a gradual reduction in the split range of the outer nuclear layer, a new split of the inner nuclear layer and gradual enlargement of the MH. The repair process of MF may be centripetal, gradually moving from the peripheral retina to macula. However, due to the expansion of the sclera in high myopia, the MH could not be closed. A new split was formed in the inner nuclear layer due to the longitudinal traction caused by the repair process of the outer nuclear layer and tangential traction caused by the epiretinal membrane in the residual ILM against retinal extension.
Vitrectomy combined with various auxiliary methods have been used to treat MH[7,13]. Lai et al[7] repaired MH with retinal detachment in patients with high myopia by vitrectomy combined with ILM repositioning and autologous blood. Moreover, 96% of the MH was closed with the retina reattached after the first surgery, and 100% of the MH was closed with the retina reattached after the second surgery. Morizane et al[14] used a free autologous flap of the ILM to fill the MH and then covered it with low-molecular-weight viscoelastic material to repair the refractory MH, and the hole closure rate was 90%. Faria et al[15] reported that the external limiting membrane and ellipsoid zone were better repaired with inverted ILM covering in the MH than with inverted ILM filling the MH.
We treated the secondary MH successfully using 25-G vitrectomy combined with residual ILM inversion and covering of the MH and autologous blood. Fresh blood forms a blood clot within minutes, keeping the residual ILM flap inverted and covering the MH, thereby reducing the risk of dislocation after surgery[7]. The inverted ILM flap and blood clot mixture covering on the MH might seal the hole and provide a smooth surface for glial cell proliferation. The components and growth factors present in the blood could facilitate the healing processes of the MH. Additionally, the serum may reduce the toxic effects of indocyanine green staining of the ILM[16].
Our case report suggests that fovea-sparing ILM peeling provides a better chance to repair the secondary MH after initial vitrectomy. Additional cases need to be evaluated.
We consider that fovea-sparing ILM peeling can treat MF with fewer surgical complications effectively. When secondary MH develops after initial vitrectomy, the residual ILM in the macula can also provide better repair of the MH.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cimen SG S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1315] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 2. | Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, García-Layana A, Ruiz-Moreno JM. Myopic maculopathy: Current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 3. | Azuma K, Hirasawa K, Araki F, Shiraya T, Yashiro S, Kato S, Nagahara M, Ueta T. Fovea-Sparing as Opposed to Total Peeling of Internal Limiting Membrane for Myopic Foveoschisis: A Systematic Review and Meta-analysis. Ophthalmol Retina. 2021;5:670-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Dolar-Szczasny J, Święch-Zubilewicz A, Mackiewicz J. A Review of Current Myopic Foveoschisis Management Strategies. Semin Ophthalmol. 2019;34:146-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Meng B, Zhao L, Yin Y, Li H, Wang X, Yang X, You R, Wang J, Zhang Y, Wang H, Du R, Wang N, Zhan S, Wang Y. Internal limiting membrane peeling and gas tamponade for myopic foveoschisis: a systematic review and meta-analysis. BMC Ophthalmol. 2017;17:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Qi Y, Yan P, Fan W, Wang N, Duan AL. Comparison of fovea-sparing and non-internal limiting membrane peeling for retinoschisis with foveal detachment in highly myopic eyes. Eye (Lond). 2021;35:1467-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Lai CC, Chen YP, Wang NK, Chuang LH, Liu L, Chen KJ, Hwang YS, Wu WC, Chen TL. Vitrectomy with Internal Limiting Membrane Repositioning and Autologous Blood for Macular Hole Retinal Detachment in Highly Myopic Eyes. Ophthalmology. 2015;122:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Díaz-Valverde A, Wu L. TO PEEL OR NOT TO PEEL THE INTERNAL LIMITING MEMBRANE IN IDIOPATHIC EPIRETINAL MEMBRANES. Retina. 2018;38 Suppl 1:S5-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Govetto A, Hubschman JP, Sarraf D, Figueroa MS, Bottoni F, dell'Omo R, Curcio CA, Seidenari P, Delledonne G, Gunzenhauser R, Ferrara M, Au A, Virgili G, Scialdone A, Repetto R, Romano MR. The role of Müller cells in tractional macular disorders: an optical coherence tomography study and physical model of mechanical force transmission. Br J Ophthalmol. 2020;104:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Smiddy WE, Flynn HW Jr. Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol. 2004;137:525-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Gao X, Ikuno Y, Fujimoto S, Nishida K. Risk factors for development of full-thickness macular holes after pars plana vitrectomy for myopic foveoschisis. Am J Ophthalmol. 2013;155:1021-1027.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Kumar A, Ravani R, Mehta A, Simakurthy S, Dhull C. Outcomes of microscope-integrated intraoperative optical coherence tomography-guided center-sparing internal limiting membrane peeling for myopic traction maculopathy: a novel technique. Int Ophthalmol. 2018;38:1689-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Lim LS, Tsai A, Wong D, Wong E, Yeo I, Loh BK, Ang CL, Ong SG, Lee SY. Prognostic factor analysis of vitrectomy for retinal detachment associated with myopic macular holes. Ophthalmology. 2014;121:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Morizane Y, Shiraga F, Kimura S, Hosokawa M, Shiode Y, Kawata T, Hosogi M, Shirakata Y, Okanouchi T. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol. 2014;157:861-869.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Faria MY, Proença H, Ferreira NG, Sousa DC, Neto E, Marques-Neves C. INVERTED INTERNAL LIMITING MEMBRANE FLAP TECHNIQUES AND OUTER RETINAL LAYER STRUCTURES. Retina. 2020;40:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Nakamura H, Hayakawa K, Sawaguchi S, Gaja T. Removal of retinal indocyanine green dye by autologous serum irrigation in macular hole surgery. Retina. 2005;25:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |