Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.643
Peer-review started: June 13, 2021
First decision: July 16, 2021
Revised: August 1, 2021
Accepted: December 8, 2021
Article in press: December 8, 2021
Published online: January 14, 2022
Processing time: 212 Days and 17.5 Hours
Ewing’s sarcoma of the adrenal gland with inferior vena cava (IVC) and right atrium thrombus is extremely rare. Here, we report a case of giant adrenal Ewing’s sarcoma with IVC and right atrium tumor thrombus and summarize the anesthesia and perioperative management.
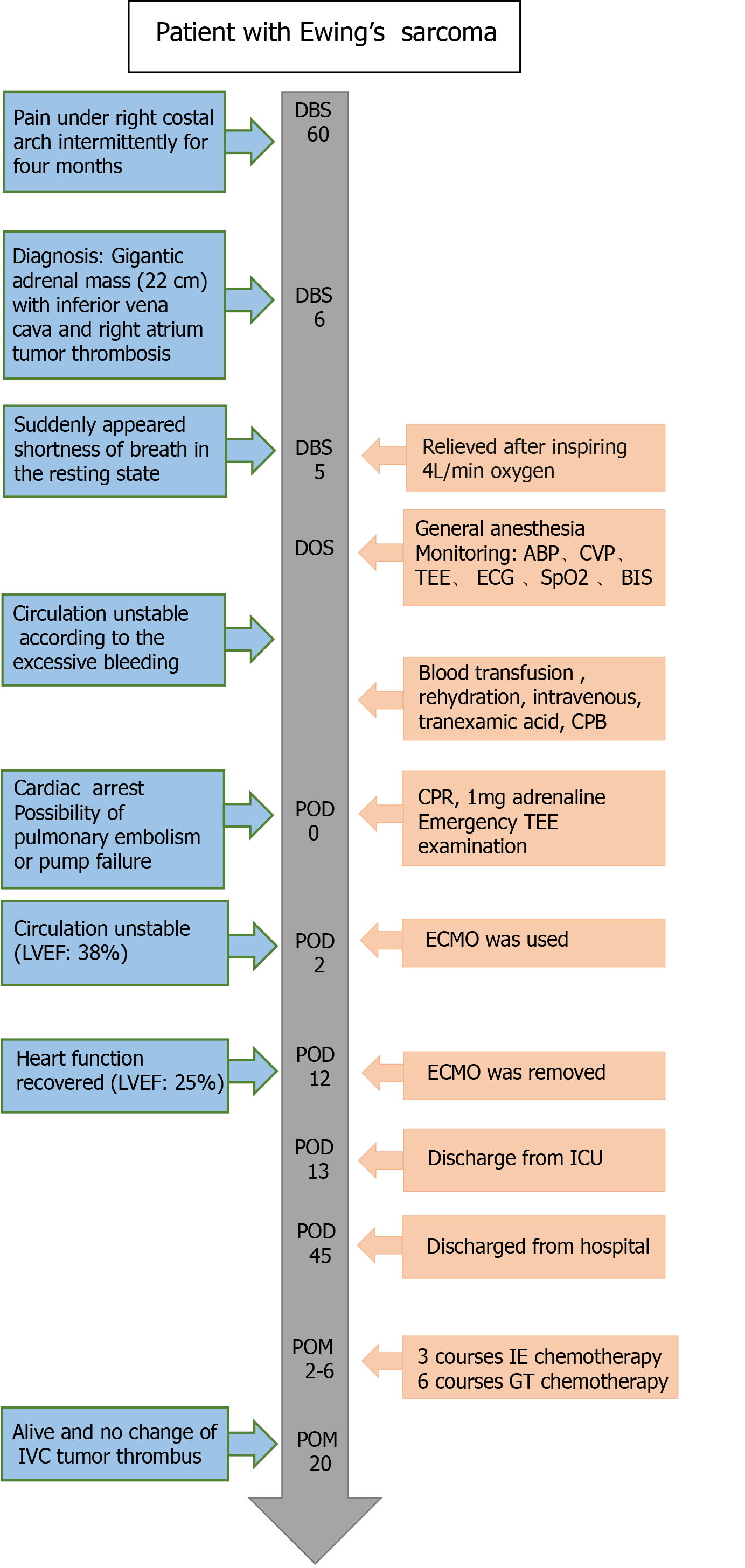
A young female was admitted to the Department of Urology with intermittent pain under the right costal arch for four months. Enhanced abdominal computed tomography revealed a large retroperitoneal mass (22 cm in diameter), which may have originated from the right adrenal gland and was closely related to the liver. Transthoracic echocardiography showed a strong echogenic filling measuring 70 mm extended from the IVC into the right atrium and ventricle. After preoperative preparation with cardiopulmonary bypass, sufficient blood products, transesophageal echocardiography and multiple monitoring, tumor and thrombus resection by IVC exploration and right atriotomy were successfully performed by a multidisciplinary team. Intraoperative hemodynamic stability was the major concern of anesthesiologists and the status of tumor thrombus and pulmonary embolism were monitored continuously. During transfer of the patient to the intensive care unit (ICU), cardiac arrest occurred without external stimulus. Cardiopulmonary resuscitation was performed immediately and cardiac function was restored after 1 min. In the ICU, extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT) were provided to maintain cardiac, liver and kidney function. Histopathologic examination confirmed the diagnosis of Ewing’s sarcoma. After postoperative treatments and rehabilitation, the patient was discharged from the urology ward.
An adrenal Ewing’s sarcoma with IVC and right atrium thrombus is extremely rare, and its anesthesia and perioperative management have not been reported. Thus, this report provides significant insights in the perioperative management of patients with adrenal Ewing’s sarcoma and IVC tumor thrombus. Intraoperative circulation fluctuations and sudden cardiovascular events are the major challenges during surgery. In addition, postoperative treatments including ECMO and CRRT provide essential support in critically ill patients. Moreover, this case report also highlights the importance of multidisciplinary cooperation during treatment of the disease.
Core Tip: An adrenal Ewing’s sarcoma with IVC and right atrium thrombus is extremely rare, and its anesthesia and perioperative management have not been reported. We report tumor resection and perioperative management in a case of adrenal Ewing’s sarcoma with IVC and right atrium thrombus. After surgery, cardiac arrest occurred and CPR was successfully performed. Following postoperative treatment and rehabilitation, the patient was discharged from the hospital and then survived for more than 17 mo. Therefore, this report provides insights for the perioperative management of adrenal Ewing’s sarcoma with distant vascular extension.
- Citation: Wang JL, Xu CY, Geng CJ, Liu L, Zhang MZ, Wang H, Xiao RT, Liu L, Zhang G, Ni C, Guo XY. Anesthesia and perioperative management for giant adrenal Ewing’s sarcoma with inferior vena cava and right atrium tumor thrombus: A case report. World J Clin Cases 2022; 10(2): 643-655
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/643.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.643
The Ewing’s sarcoma family is an aggressive group of childhood cancers, including classic Ewing’s sarcoma, Askin tumor, and peripheral primitive neuroectodermal tumor[1]. Extraosseous Ewing’s sarcoma is a rare, rapidly growing, round-cell, malignant tumor that starts anywhere in soft tissues. The average age of patients with extraosseous Ewing’s sarcoma is higher than those with bone cancer[2]. Nkx2.2 is a transcription factor that functions in neuronal and neuroendocrine differentiation, which is upregulated in Ewing’s sarcoma and required for its oncogenesis[3]. The tumor cells characteristically express CD99 (MIC2 antigen), a glycoprotein localized on cell membrane. The defining feature of Ewing’s sarcoma is a characteristic t(11;22)(q24;q12) translocation involving the Ewing’s sarcoma breakpoint region 1 (EWSR1) gene on chromosome 22 and the FLI1 gene on chromosome 11[4]. Currently, localized Ewing’s sarcoma has a relative survival rate of 75%, while the survival rate for patients with metastases is only 30%[5,6].
Thrombus of the inferior vena cava (IVC) is very rare in the absence of congenital abnormalities and is usually a result of hypercoagulable state along with acquired pathological changes in the IVC or its adjacent structures. There are two main causes of thrombus. One is the abnormal prothrombotic function caused by various diseases, and the other one relates to the changes in abdominal pathophysiology[7-9]. For renal cell carcinoma, the incidence of IVC tumor thrombus has been reported in 4% to 10%[10], and the incidence of tumor thrombus in the right atrium was 0.3% to 1.0%[11]. The prognosis is mainly related to the grade of tumor thrombus. The perioperative mortality rate ranges from 2.7% to 40% and the major complications include bleeding, pulmonary embolism, wound infection and acute renal failure[12]. According to research on adrenocortical carcinoma by the Mayo Clinic, IVC tumor thrombus is an independent prognostic factor that contributes to worse overall survival[13]. To date, there are five cases of adrenal Ewing’s sarcoma with IVC tumor thrombus focusing on the diagnosis and treatment (Table 1). However, due to the rarity of cases, anesthetic management of adrenal Ewing’s sarcoma has not been reported.
| No | Ref. | Age | Gender | Position | Tumor size, cm | Initial infiltration or metastasis | Surgical procedure | Outcome at time of report |
| 1 | Zhang et al[14] | 30 | M | R | 12 | IVC tumor thrombus | Adr | Dead (8 mo) |
| 2 | Zhang et al[14] | 22 | M | L | 17 | IVC tumor thrombus | Adr + Neph + Spl + IVCt | Alive |
| 3 | Abi-Raad et al[4] | 26 | F | L | 11.3 | IVC tumor thrombus | Adr + Neph + Spl + IVCt | Alive (8 mo) |
| 4 | Kim et al[15] | 25 | F | L | 15.2 | lung, IVC, RA tumor thrombus | NR | NR |
| 5 | Saboo et al[16] | 26 | F | L | Large | IVC tumor thrombus | No surgery | NR |
| 6 | Present case | 20 | F | R | 22 | Liver, IVC, RA tumor thrombus | Liver + Adr + IVCt + RAt | Alive |
The anesthetic management for adrenal Ewing’s sarcoma with IVC thrombus has perioperative challenges. One of the challenges during surgery is tumor infiltration of the liver, which may not be resected completely, leading to a poor prognosis. The other challenge relates to anesthetic management, including long surgery time, massive hemorrhage, hemodynamic instability, tumor thrombus spread and pulmonary embolism, etc. Here, we summarize the anesthesia treatment strategy and perioperative management of our patient and provide a basis for treating similar cases in the future.
The patient presented with intermittent pain under the right costal arch for four months.
A 20-year old female patient presented with intermittent pain under the right costal arch for four months, and was diagnosed with an adrenal tumor with IVC and right atrium tumor thrombus. Before admission, the patient had visited a number of different hospitals. However, due to the position and size of the tumor, and complications such as pulmonary embolism, no surgery was performed. The Department of Urology in our hospital has treated more than 100 cases of adrenal or renal tumors with Mayo Clinic stage I-IV tumor thrombus[17,18]. Thus, we have experience in tumor excision and emergency treatment for these circumstances. Considering the rapid development of the tumor and risks of a stuck valve that could occur at any time, the patient was admitted to hospital. Prior to admission, the patient and her family members were fully informed that the operation was a form of palliative treatment.
The patient had no remarkable past medical history.
The patient had no remarkable personal and family history.
Physical examination showed a temperature of 37 ºC, heart rate (HR) of 101 bpm, blood pressure (BP) of 92/60 mmHg, respiratory rate of 17 breaths/min, and oxygen saturation (SpO2) of 91% in room air.
Laboratory examinations showed an elevation of renin 1.51 ng/mL/h (normal range: 0.05-0.79 ng/mL/h) and angiotensin II 191.01 pg/L (normal range: 55.3-115.3 pg/L). The high renin-angiotensin secretion was possibly attributed to decreased renal perfusion. The 24 h urine catecholamine levels were normal and pheochromocytoma was excluded. Although endocrine changes were not typical, adrenal cortical carcinoma was highly suspected. Reduced platelets (PLT, 68 × 109/L) and an abnormal coagulation test (prothrombin time (PT): 15.5 s and international normalized ratio (INR): 1.45) were present. The hematologist recommended maintaining perioperative PLT > 80 × 109 in order to reduce intraoperative blood loss.
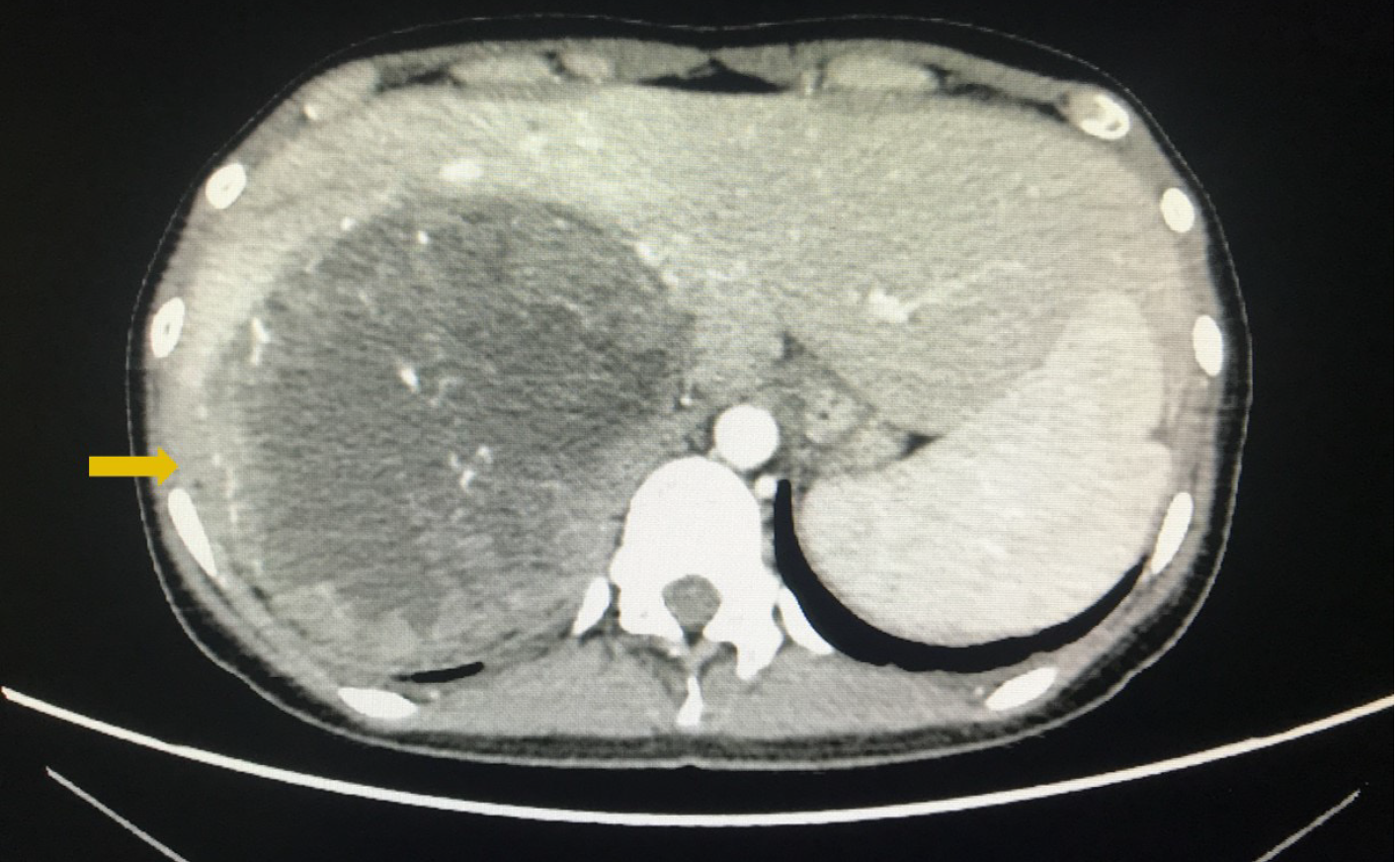
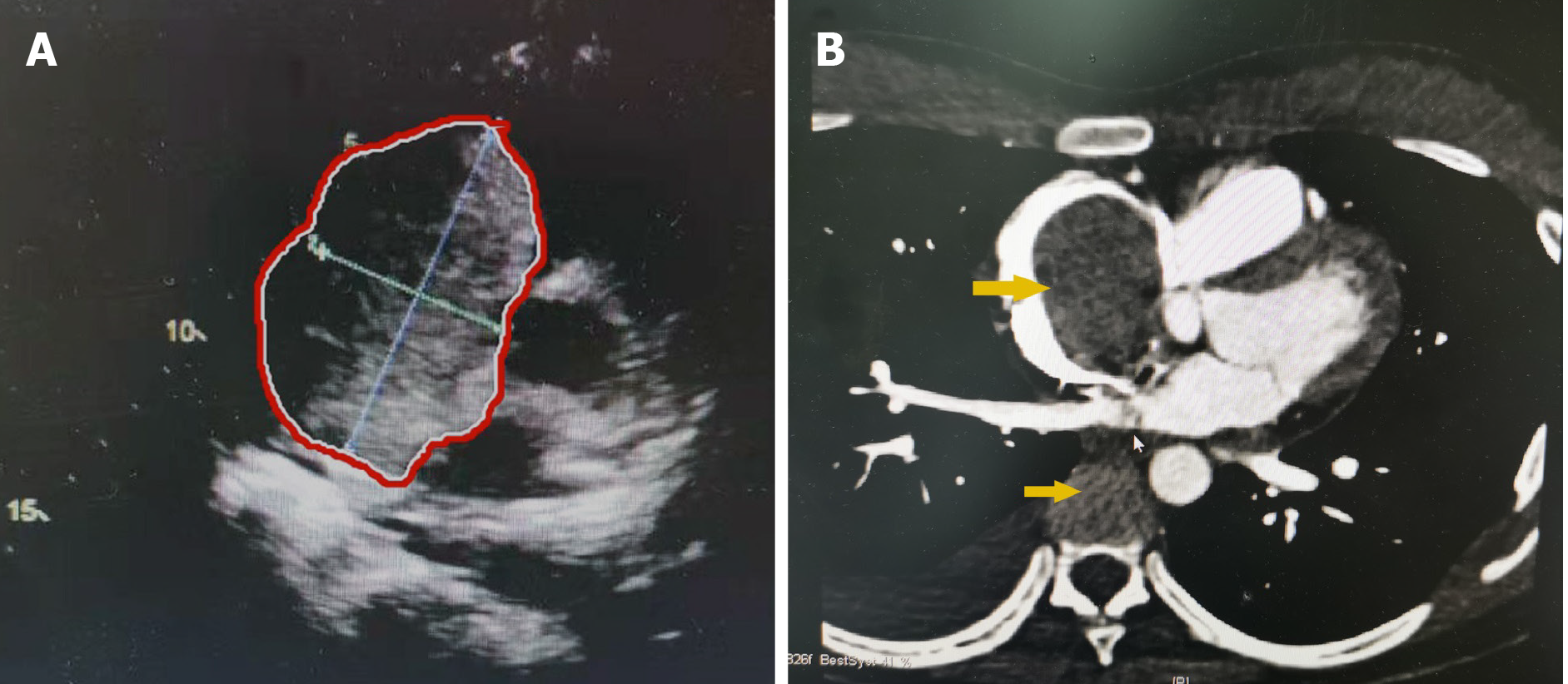
Enhanced abdominal computed tomography (CT) showed a retroperitoneal mass with a diameter of 22 cm, which most likely originated from the right adrenal gland and was closely related to the liver (Figure 1). Positron emission tomography-CT (PET-CT) revealed a huge heterogeneous mass with elevated glucose metabolism in the right hepatic lobe and the space between the liver and the right kidney. Transthoracic echocardiography (TTE) showed that the proximal IVC was 25.7 mm wide with a strong echogenic filling extending into the right atrium and ventricle and the distant tumor thrombus was 70 mm approximately. Preoperative left ventricular ejection fraction (LVEF) was 64% (Figure 2A). Cardiac enhanced magnetic resonance imaging (MRI) confirmed the filling in the right atrium and ventricle (Figure 2B).
Five days before surgery, the patient developed a sudden shortness of breath at rest, accompanied by a sense of pressure behind the sternum and in the scapular area for one hour. During this time, her BP and HR were 105/79 mmHg and 116 bpm, respectively. Immediate arterial blood gas analysis showed: pH 7.49, PaCO2 22.5 mmHg, PaO2 58 mmHg, lactate 3 mmol/L, SpO2 94%. Electrocardiogram showed sinus tachycardia (118 bpm), inverted T waves were observed in chest leads, and visible Q waves in II, III, aVF leads, but there were no significant changes in myocardial injury markers. Cardiac MRI and pulmonary artery Computed Tomography Angiography (CTA) showed no pulmonary embolism. Her symptoms were relieved after 4 L/min oxygen therapy (nasal cannula). The cardiologist examined the patient and speculated that the tumor thrombus in the right atrium and ventricle affected tricuspid valve motion, as well as the occurrence of pulmonary embolism.
Several days later, the multidisciplinary team composed of urological surgeons, cardiac surgeons, general surgeons, anesthesiologists and ICU physicians evaluated the tumor status and cardiac function and decided to perform surgery to prevent a severe stuck valve and right heart failure. The most significant indication for a stuck valve is the “roller coaster”-like BP, accompanied by clinical symptoms such as dyspnea at rest, chest pain, low output, shock, embolization, and even cardiac arrest[19,20]. Considering that a severe stuck valve and right heart failure could occur at any time, the multidisciplinary team decided to perform tumor and thrombus resection directly, but not after preoperative biopsy.
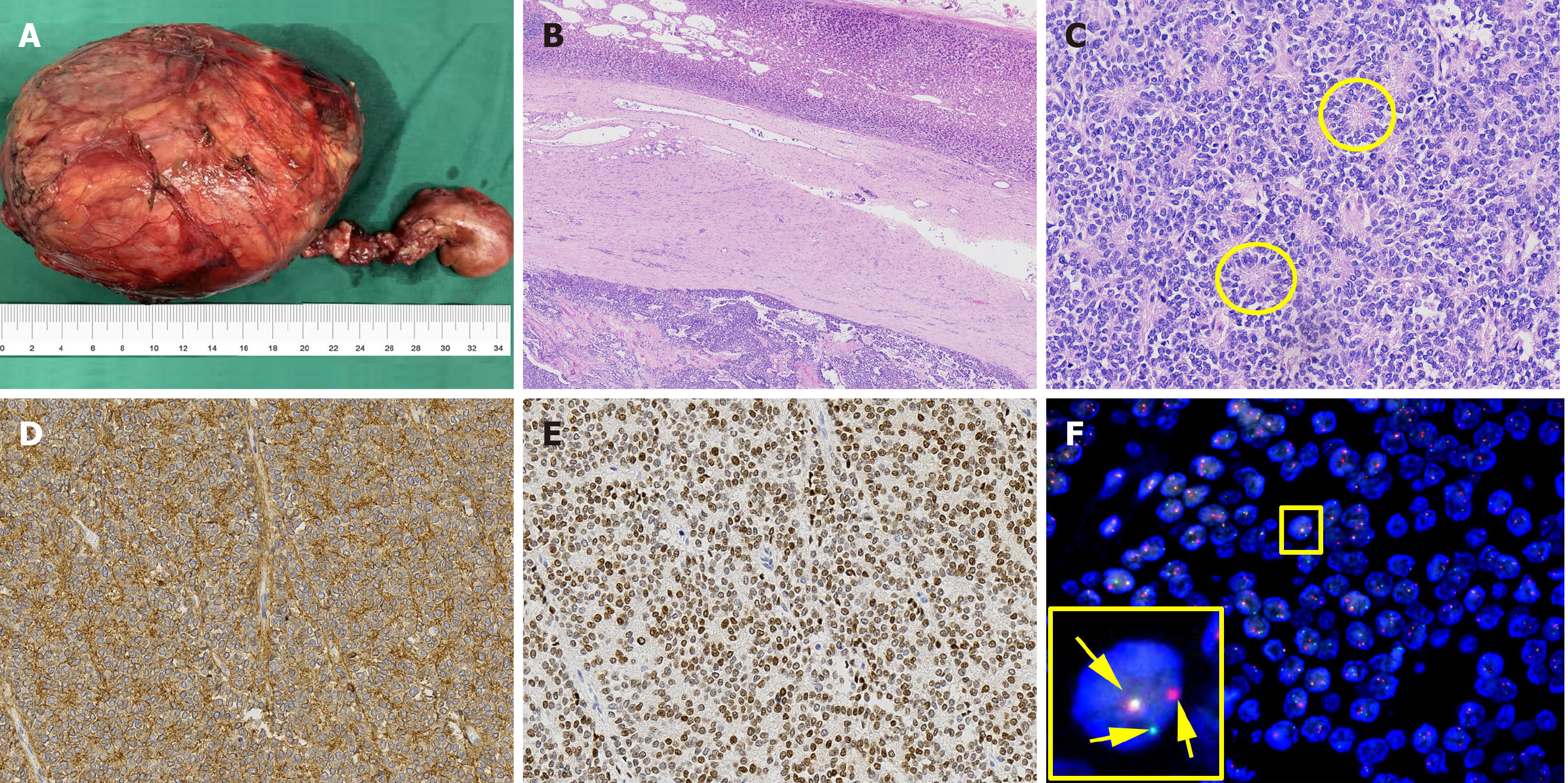
Histopathologic examination of the resected tissue showed that the tumor was surrounded by normal adrenal tissue, which suggested that the tumor originated in the adrenal gland (Figure 3A and B). Figure 3C shows tumor cells with round nuclei and pale cytoplasm, as well as their rosette structures, which confirmed the diagnosis of Ewing’s sarcoma. Immunohistochemistry revealed that the tumor cells were positive for CD99 and Nkx2.2, well-known markers for Ewing’s sarcoma (Figure 3D and E). Furthermore, fluorescence in situ hybridization studies showed rearrangement of the EWSR1 gene in the nuclei of tumor cells (Figure 3F). These results confirmed that the tumor was Ewing’s sarcoma with an adrenal origin.
The patient underwent surgery. In the pre-operation room, 10 mg diazepam was given intravenously to relieve the patient's anxiety, and she was then transferred to the operating room. The initial BP, HR and SpO2 were 93/65 mmHg, 110 bpm and 90% (in room air), respectively. Two peripheral venous access points (16 G) were established, and the left radial artery was cannulated for continuous arterial blood pressure monitoring. Following the above preparation, a 10-min preoxygenation (FiO2: 50%) was performed prior to induction. For anesthesia induction, 20 μg sufentanil, 10 mg etomidate and 6 mg cisatracurium were injected in sequence. After 2 min of positive mask ventilation, a reinforced tracheal tube was intubated. Anesthesia induction was relatively stable without obvious variation in both BP and HR. Sufentanil at 25 μg/h, 1-2 MAC of sevoflurane and intermittent cisatracurium were used for anesthesia maintenance. The bispectral index (BIS) was maintained within 40 and 60. Right internal jugular vein puncture was performed to place the central venous catheter for central venous pressure (CVP) monitoring. The TEE probe was inserted to assist surgeons and anesthesiologists to determine the clearance of thrombus, the changes in heart function and the occurrence of pulmonary embolism.
After a series of preparations, the surgery was performed on schedule. As the tumor was closely related to the liver, laparotomy was chosen to reduce blood loss. At the beginning of surgery, an incision was made 2 cm below the right costal margin from the xiphoid to the posterior line of the axilla and was extended about 10 cm below the left costal margin. It was found that the liver was compressed to the left by the tumor. Firstly, the liver and duodenum were dissociated to expose the right kidney and IVC. Then, the dissociation between the lower pole of the tumor and the kidney was extended, and other parts of the tumor were dissociated to expose the right renal vein, right renal artery, right ureter, and to retain the right kidney. During tumor dissoci
| Time point | Preoperative | After intubation | During dissociation | After CPB | After Surgery | After CPR |
| HR (bpm) | 110 | 112 | 120 | 120 | 80 | 100 |
| ABP (mmHg) | 92/60 | 102/70 | 70/40 | 90/60 | 105/70 | 140/100 |
| SpO2 (%) | 90 | 98 | 100 | 100 | 100 | 100 |
| CVP (cmH2O) | 13 | 13 | 9 | 18 | 20 | 11 |
| BIS | 90 | 50 | 43 | 45 | 40 | / |
The cardiac surgeon continued with a median sternotomy, and after systemic heparinization, the IVC, superior vena cava and the ascending aorta were cannulated to establish cardiopulmonary bypass. Blood gas analysis was performed hourly and parameters were modulated. Before the ascending aorta was cross-clamped, the patient was gradually cooled to 32 oC. After the infusion of cold cardioplegia into the aortic root, cardiac arrest was achieved. The right atrium was opened to reveal part of the tumor thrombus. The remaining tumor thrombus was pushed into the IVC, which was removed after blocking the proximal and distal ends of the IVC. Intraoperative TEE examination confirmed the removal of tumor thrombus in the right atrium and ventricle. However, tough cord-like thrombus remained in the IVC closely related to the liver. Blind removal could damage the IVC and tumor fragmentation may cause pulmonary embolism[22], and satisfactory complete resection can only be achieved via liver transplantation[23,24]. Furthermore, the previous reports of renal cell carcinoma indicated that resection of the residual thrombus did not improve the prognosis[10]. After repairing the right atrium and vena cava, the patient was rewarmed and weaned off cardiopulmonary bypass. During cardiopulmonary bypass, her BP remained relatively stable. Vital signs and ventilation parameters during surgery are shown in Table 2, and arterial gas results during surgery are shown in Table 3. The resected tumor is shown in Figure 3A.
| Time point | After intubation | Before CPB | After CPB | Postoperative | After CPR |
| pH | 7.29 | 7.20 | 7.32 | 7.46 | 7.35 |
| PaO2 (mmHg) | 198 | 385 | 372 | 419 | 370 |
| PaCO2 (mmHg) | 44 | 36 | 32 | 30 | 44 |
| BE (mEq/L) | -5.9 | -12.9 | -4.5 | -2.5 | -1.9 |
| Hb (g/L) | 120 | 82 | 54 | 55 | 70 |
| K+ (mmol/L) | 4.3 | 5.6 | 4.7 | 3.8 | 4.4 |
| HCO3- (mmol/L) | 20.5 | 14 | 21.1 | 21.0 | 23.6 |
| Glu (mmol/L) | 4.7 | 8.4 | 7.8 | 7.2 | 8.7 |
In summary, the surgical time was 587 min, intraoperative blood loss and urine were 4,500 mL and 600 mL, respectively. 14 U of red blood cells, 2200 mL of plasma, 1000 mL of colloid solution and 7200 mL of crystal solution were infused. Intraoperative complications were limited to blood loss of 500 mL from the lower margin of the liver during the dissociation and incomplete tumor and thrombus resection. Based on previous studies, blind removal may not improve the prognosis obviously in some patients[10], and the main focus of intraoperative anesthesia management was circulation maintenance and monitoring.
When the patient was transferred to the ICU, the normal ECG waveform and pulse oxygen waveform suddenly disappeared, along with the carotid pulse. Cardiopulmonary resuscitation (CPR) was performed immediately, 1 mg adrenaline was injected intravenously, and the defibrillator was prepared. One minute later, the heartbeat was restored. After 5 min, the BP, HR and SpO2 were 140/100 mmHg, 120 bpm and 100%, respectively. Emergency TTE examination indicated that the chamber of ventricles was small with hypokinesis, and LVEF was 34%, but no obvious tumor thrombus was detected in the right atrium or pulmonary arteries.
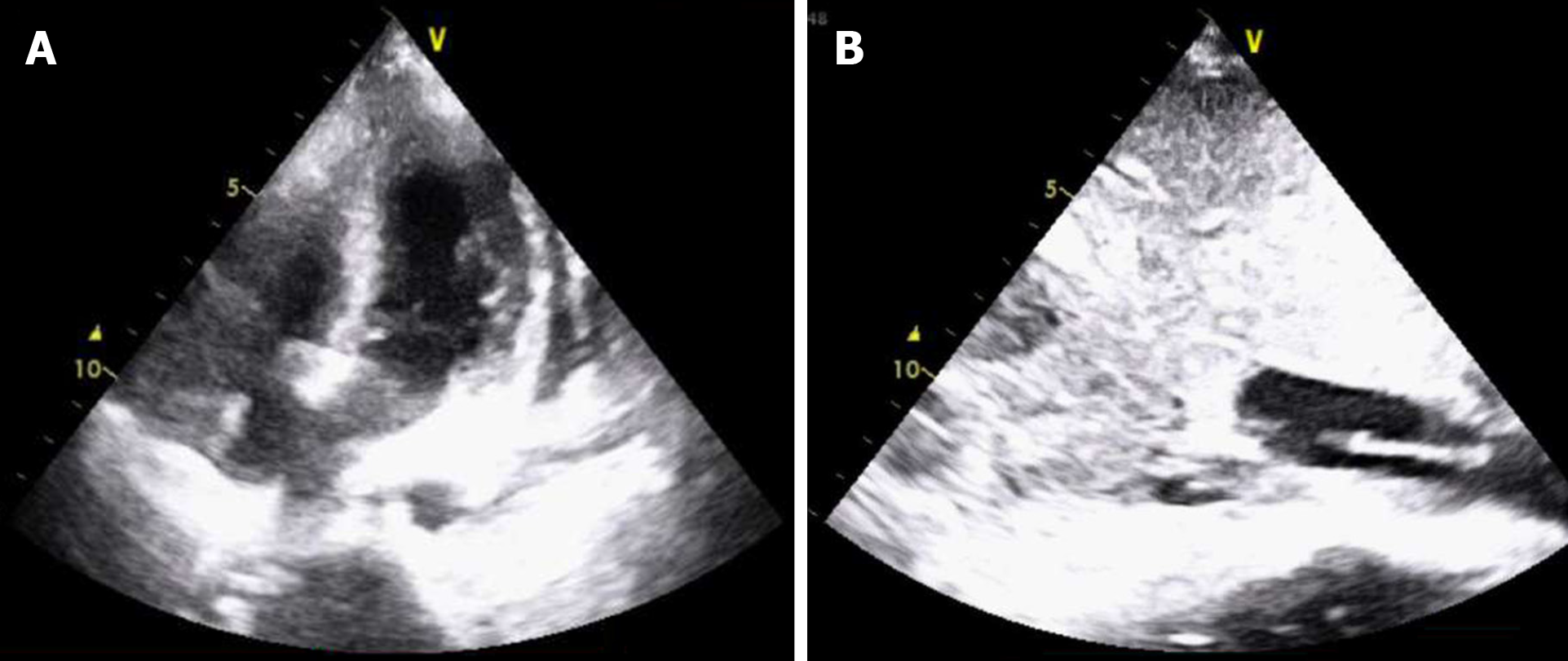
After the vital signs of the patient had stabilized for 30 min, she was transferred to the ICU. On the first day, the patient developed acute liver failure (AST 7,448 U/L, ALT 3,458 U/L, ALB 24.2 g/L, TBIL 72.2 μmol/L) and renal failure (no urine with Cr 136 μmol/L). CRRT was performed, hepatoprotective agents including magnesium isoglycyrrhizinate and glutathione were used, and coagulation factors were supplemented to improve coagulation function. Cardiogenic shock and acidosis occurred after cardiac resuscitation and could not be corrected by medication. TTE indicated abnormal motion in the wall of the right ventricle and the middle and apex of the left ventricle (LVEF 38%). Therefore, ECMO was given on day two. The principle of treatment in the ICU was mainly to improve liver, kidney and heart function. During CRRT and ECMO treatment, anticoagulation was provided, which caused bleeding in the surgical area. On day twelve in the ICU, TTE showed a cord-like mass in the IVC (Figure 4), which was consistent with the intraoperative TEE result, without obvious progress. ECMO was successfully withdrawn on day twelve when the LVEF recovered (LVEF 46%) and BP was relatively stable. A total of 12 U of red blood cells, 3600 mL of plasma and 6 U of platelets were infused. On day thirteen in the ICU, the patient was conscious. The BP and HR were 99-120/50-60 mmHg and 85 bpm, respectively. TTE indicated that LVEF was 58%. The endotracheal tube was removed, and the patient was transferred to the urology ward.
In the urology ward, treatment was focused on postoperative pleural effusion and rehabilitation. Closed thoracic drainage was used for pleural effusion, and opioids and flurbiprofen were used for pain management. Eventually, the patient was discharged with improved physical status and sleep quality, and without chest pain. Cardiac function was restored to the preoperative level (LVEF 63%). Perioperative blood tests for red and white cells, hemoglobin, coagulation and inflammation (procalcitonin, PCT, a parameter to estimate the severity, prognosis, and time course of the inflammatory response) are summarized in Table 4.
| Time point | Preoperative | Postoperative | Discharged |
| Red blood cell ( × 109) | 5.02 | 3.15 | 3.09 |
| Hemoglobin (g/L) | 113 | 96 | 94 |
| White cell count ( × 109) | 5.27 | 8.02 | 2.86 |
| Neutrophils (%) | 57.7 | 84.7 | 70 |
| Lymphocytes (%) | 22.7 | 11.1 | 8.5 |
| Procalcitonin (ng/mL) | --- | 1.82 | 0.126 |
| Platelets ( × 109) | 113 | 63 | 156 |
| APTT (s) | 32 | 32.5 | 29.5 |
| PT (s) | 15.5 | 14.5 | 12.1 |
| INR | 1.45 | 1.36 | 1.21 |
| Coagulation factor VIII (%) | --- | 94.2 | 150 |
| D-dimer | --- | 8.31 | 2.78 |
| Fibrinogen (g/L) | 3.8 | 1.08 | 2.9 |
The patient received chemotherapy after surgery. Firstly, she received three courses of Ifosfamide and Etoposide combination chemotherapy every 21 d. Considering the local recurrence of liver metastasis, she received another six courses of Gemcitabine and Taxotere combination chemotherapy every 21 d. So far, liver metastasis has reduced and the IVC tumor thrombus has not changed since surgery. The timeline of perioperative therapies and outcome are summarized in Figure 5. There was no obvious progression of the residual IVC tumor thrombus. Since discharge, the patient has survived for 20 mo, and we will continue to monitor her for some time.
Extraosseous Ewing’s sarcoma is rare, and its overall incidence is 1% of all sarcomas[25]. To date, 39 cases of Ewing’s sarcoma arising from the adrenal gland have been reported[26-29], five of which were initially diagnosed with IVC thrombus (Table 1). The follow-up time ranged from 1 to 36 mo and the survival time was correlated with the clinical stage at diagnosis. There are two prognostic factors to predict the recurrence of Ewing's sarcoma: disease-free interval (DFI) between diagnosis and first relapse and the site of recurrence. The patients with DFI > 2 years have an estimated 5-year overall survival of approximately 30%, but those with DFI < 2 years have an estimated 5-year overall survival of only 7%. In addition, patients with combined local and distant relapse have the worst outcomes, while those with isolated local recurrences appear to fare better[30].
Laparotomy combined with thoracotomy is the primary treatment and has become a major significant challenge for anesthesia and perioperative management. Furthermore, the present case was unconventional in anesthetic practice according to the adrenal Ewing’s sarcoma and its combination with IVC and right atrium thrombus, which resulted in vascular obstruction and hemodynamic disturbance. Besides, the primary tumor also directly induced abnormal hormone levels. During transfer to the ICU, the patient developed sudden cardiac arrest. Although the heartbeat was soon restored, cardiac function was still poor, requiring ECMO support. Liver and kidney dysfunction were also a problem. Appropriate treatment has led to improved prognosis, but the underlying mechanisms and therapeutic improvements are still worth exploring.
To date, published cases with tumor thrombus have focused on adrenal pheo
In this case, the tumor was approximately 22 cm in diameter, and the thrombus extended from the IVC into the right atrium and ventricle. According to the Mayo Clinic classification, the thrombus level was grade IV[33]. The key point of anesthetic management is to maintain perioperative hemodynamic stability, and multiple monitoring including EEG, IABP, CVP, temperature, respiratory parameters and blood gas analysis was employed during the perioperative period. Diazepam was given to relieve preoperative anxiety, and anesthetics with little effect on hemodynamics including etomidate, sevoflurane, sufentanil and cisatracurium were used for anesthetic induction and maintenance. Fluid management and vasoactive drugs are also important components for perioperative hemodynamic management. It has been reported that the perioperative outcomes favored goal directed therapy rather than liberal fluid therapy[37]. Considering infiltration of the tumor in the liver and possible bleeding, we performed volume preloading, and used norepinephrine and dopamine to prevent hypotension during the process of dissociation. During and after cardiopulmonary bypass, her BP remained relatively stable, and transfusion was performed according to blood loss. The total intraoperative transfusion included 14 U of red blood cells, 3200 mL of plasma and colloid solution, and 7200 mL of crystal solution. TEE can be used for these procedures, as close monitoring is pivotal for hemodynamic maintenance[38]. Furthermore, TTE was used in the ICU to monitor heart function and residual thrombus, as well as the occurrence of pulmonary embolism.
During surgery, although the circulation fluctuated due to excessive bleeding, the circulation was controlled by a series of therapies. However, the patient experienced a sudden cardiac arrest after surgery. The multidisciplinary team considered that this was due to the following reasons: (1) According to the liver infiltration, the tumor was not completely resected, thus, pulmonary embolism caused by residual tumor was considered as the primary cause. Although TTE did not provide definite evidence of the embolus, the chamber of the right ventricle was small with hypokinesis, which could be the result of acute pulmonary embolism; (2) During surgery, the patient lost 4,500 mL of blood and received 13,550 mL fluid and blood transfusions. The bleeding and rehydration, as well as cardiopulmonary bypass, could be an unusual burden on the heart and result in acute coronary syndrome, even cardiac arrest; and (3) Pathological examination indicated that the tumor was Ewing’s sarcoma, which can secrete a range of pro-inflammatory cytokines, and lead to perioperative septic shock[33,39,40]. In addition, the tumor excision process promoted the release of these mediators and could invoke a systemic inflammatory response (SIRS) and shock. Perioperative vital laboratory data are shown in Table 4. The level of PCT (1.82 ng/mL) and the percentage of neutrophils (84.7%) increased after surgery. PCT has been reported to be a better parameter for estimating the severity, prognosis, and time course of SIRS, than CRP[41]. These results indicated possible postoperative SIRS in the present case.
In conclusion, giant adrenal Ewing’s sarcoma with IVC and right atrium thrombus is a rare and challenging scenario. In the present case, a comprehensive evaluation of tumor thrombus extension was performed preoperatively. For tumor resection with excessive bleeding, adequate blood product preparation, proper selection of anesthetics and vasoactive agents, as well as intraoperative monitoring including TEE, BP, CVP, airway pressure and blood gas analysis are equally important. Perioperative compromised cardiac function and circulation fluctuations, as well as pulmonary embolism and acute cardiovascular event, are the major difficulties in anesthetic management.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Watanabe A, Xie Y S-Editor: Wang LL L-Editor: Webster JR P-Editor: Wang LL
| 1. | Teicher BA, Bagley RG, Rouleau C, Kruger A, Ren Y, Kurtzberg L. Characteristics of human Ewing/PNET sarcoma models. Ann Saudi Med. 2011;31:174-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Khosla D, Verma S, Punia RS, Dass A, Dimri K, Kaur G, Pandey AK. Extraosseous Ewing's Sarcoma of the Parapharyngeal Space - A Rare Entity - with Review of Literature. Iran J Otorhinolaryngol. 2019;31:51-54. [PubMed] |
| 3. | Smith R, Owen LA, Trem DJ, Wong JS, Whangbo JS, Golub TR, Lessnick SL. Expression profiling of EWS/FLI identifies NKX2.2 as a critical target gene in Ewing's sarcoma. Cancer Cell. 2006;9:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Abi-Raad R, Manetti GJ, Colberg JW, Hornick JL, Shah JG, Prasad ML. Ewing sarcoma/primitive neuroectodermal tumor arising in the adrenal gland. Pathol Int. 2013;63:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 456] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 6. | Xie CF, Liu MZ, Xi M. Extraskeletal Ewing's sarcoma: a report of 18 cases and literature review. Chin J Cancer. 2010;29:420-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Alkhouli M, Morad M, Narins CR, Raza F, Bashir R. Inferior Vena Cava Thrombosis. JACC Cardiovasc Interv. 2016;9:629-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Alkhouli M, Shafi I, Bashir R. Inferior vena cava filter thrombosis and suprarenal caval stenosis: a double whammy. JACC Cardiovasc Interv. 2015;8:e23-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | McAree BJ, O'Donnell ME, Fitzmaurice GJ, Reid JA, Spence RA, Lee B. Inferior vena cava thrombosis: a review of current practice. Vasc Med. 2013;18:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Moinzadeh A, Libertino JA. Prognostic significance of tumor thrombus level in patients with renal cell carcinoma and venous tumor thrombus extension. Is all T3b the same? J Urol. 2004;171:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Bissada NK, Yakout HH, Babanouri A, Elsalamony T, Fahmy W, Gunham M, Hull GW, Chaudhary UB. Long-term experience with management of renal cell carcinoma involving the inferior vena cava. Urology. 2003;61:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Topaktaş R, Ürkmez A, Tokuç E, Kayar R, Kanberoğlu H, Öztürk Mİ. Surgical management of renal cell carcinoma with associated tumor thrombus extending into the inferior vena cava: A 10-year single-center experience. Turk J Urol. 2019;45:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Laan DV, Thiels CA, Glasgow A, Wise KB, Thompson GB, Richards ML, Farley DR, Truty MJ, McKenzie TJ. Adrenocortical carcinoma with inferior vena cava tumor thrombus. Surgery. 2017;161:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Li H. Primitive neuroectodermal tumors of adrenal gland. Jpn J Clin Oncol. 2010;40:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Kim MS, Kim B, Park CS, Song SY, Lee EJ, Park NH, Kim HS, Kim SH, Cho KS. Radiologic findings of peripheral primitive neuroectodermal tumor arising in the retroperitoneum. AJR Am J Roentgenol. 2006;186:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Saboo SS, Krajewski KM, Jagannathan JP, Ramaiya N. IVC tumor thrombus: an advanced case of rare extraosseous Ewing sarcoma of the adrenal gland. Urology. 2012;79:e77-e78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Liu Z, Ma LL, Tian XJ, Wang GL, Hou XF, Zhang SD, Deng SH. [Radical nephrectomy and thrombectomy for Mayo clinic stage III tumor thrombus: a surgical technique and clinical experience]. Beijing Da Xue Xue Bao Yi Xue Ban. 2017;49:597-604. [PubMed] |
| 18. | Ye JF, Ma LL, Zhao L, Wang GL. [Segmental vena cava resection for the treatment of renal tumor with invading tumor thrombus]. Beijing Da Xue Xue Bao Yi Xue Ban. 2018;50:183-187. [PubMed] |
| 19. | Luo W, Wang X, Li J, Mu Y, Ni Y. Intermittent stuck valve after aortic valve replacement with a mechanical valve: A case report. Medicine (Baltimore). 2017;96:e6214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Deviri E, Sareli P, Wisenbaugh T, Cronje SL. Obstruction of mechanical heart valve prostheses: clinical aspects and surgical management. J Am Coll Cardiol. 1991;17:646-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 209] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Dowd NP, Karski JM, Cheng DC, Carroll JA, Lin Y, James RL, Butterworth J. Pharmacokinetics of tranexamic acid during cardiopulmonary bypass. Anesthesiology. 2002;97:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Kwon TW, Kim H, Moon KM, Cho YP, Song C, Kim CS, Ahn H. Surgical treatment of inferior vena cava tumor thrombus in patients with renal cell carcinoma. J Korean Med Sci. 2010;25:104-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Kaplan S, Ekici S, Doğan R, Demircin M, Ozen H, Paşaoğlu I. Surgical management of renal cell carcinoma with inferior vena cava tumor thrombus. Am J Surg. 2002;183:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 24. | Vaidya A, Ciancio G, Soloway M. Surgical techniques for treating a renal neoplasm invading the inferior vena cava. J Urol. 2003;169:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 25. | Maccioni F, Della Rocca C, Salvi PF, Manicone AM, Ascarelli A, Longo F, Rossi P. Malignant peripheral neuroectodermal tumor (MPNET) of the kidney. Abdom Imaging. 2000;25:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Koufopoulos N, Kokkali S, Manatakis D, Balalis D, Nasi D, Ardavanis A, Korkolis D, Khaldi L. Primary peripheral neuroectodermal tumor (PNET) of the adrenal gland: a rare entity. J BUON. 2019;24:770-778. [PubMed] |
| 27. | Guo H, Chen S, Liu S, Wang K, Liu E, Li F, Hou Y. Rare adrenal gland incidentaloma: an unusual Ewing's sarcoma family of tumor presentation and literature review. BMC Urol. 2017;17:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Toda K, Ishii S, Yasuoka H, Nishioka M, Kobayashi T, Horiguchi K, Tomaru T, Ozawa A, Shibusawa N, Satoh T, Koshi H, Segawa A, Shimizu SI, Oyama T, Yamada M. Adrenal Ewing's Sarcoma in an Elderly Man. Intern Med. 2018;57:551-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Ibabao C, Tsetse C, Sheth Y, Maitland C, Mohammed M. Primary Ewing sarcoma of the adrenal gland: A rare cause of abdominal mass. Radiol Case Rep. 2020;15:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Leavey PJ, Mascarenhas L, Marina N, Chen Z, Krailo M, Miser J, Brown K, Tarbell N, Bernstein ML, Granowetter L, Gebhardt M, Grier HE; Children's Oncology Group. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2008;51:334-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Chen J, Liu C, Fu Q, Pei D, Ren L, Yan H. Anesthetic management of gigantic pheochromocytoma resection with inferior vena cava and right atrium tumor thrombosis: a case report. BMC Anesthesiol. 2019;19:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Ku CF, Lo CY, Chan WF, Chiu SW, Fan ST, Lam KS. Resection of phaeochromocytoma extending into the right atrium in a patient with multiple endocrine neoplasia type 2A. Hong Kong Med J. 2005;11:59-62. [PubMed] |
| 33. | Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol. 1987;59:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 368] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Castro EC, Parwani AV. Ewing sarcoma/primitive neuroectodermal tumor of the kidney: two unusual presentations of a rare tumor. Case Rep Med. 2012;2012:190581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Jundt G. [Updates to the WHO classification of bone tumours]. Pathologe. 2018;39:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Biermann JS. Updates in the treatment of bone cancer. J Natl Compr Canc Netw. 2013;11:681-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg. 2012;114:640-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 38. | Blute ML, Leibovich BC, Lohse CM, Cheville JC, Zincke H. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int. 2004;94:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 450] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 39. | Yera H, Poulain D, Lefebvre A, Camus D, Sendid B. Polymicrobial candidaemia revealed by peripheral blood smear and chromogenic medium. J Clin Pathol. 2004;57:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Flavin K, Gunning P. Peri‐operative systemic inflammation and intractable shock in a patient with Ewing's sarcoma. Anaesthesia Cases. 2016;4: 22-26. [DOI] [Full Text] |
| 41. | Castelli GP, Pognani C, Meisner M, Stuani A, Bellomi D, Sgarbi L. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care. 2004;8:R234-R242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 278] [Article Influence: 13.2] [Reference Citation Analysis (0)] |