Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.631
Peer-review started: May 3, 2021
First decision: October 16, 2021
Revised: November 1, 2021
Accepted: December 7, 2021
Article in press: December 7, 2021
Published online: January 14, 2022
Processing time: 253 Days and 23.4 Hours
Solitary fibrous tumor (SFT) of the central nervous system is rare. It is predominantly benign and rarely malignant. There is no established standardized treatment regimen for malignant intracranial SFTs.
We present a rare case of SFT in a 9-year-old girl with a space-occupying effect in the frontal-parietal lobes. She underwent craniotomy, and the mass was resected. Immunohistochemistry examination of the specimen showed that Ki-67 proliferation index staining was highly positive in 80% of tumor cells. Whole exome sequencing of the surgical tissue showed 38 somatic gene mutations and 1 gene amplification such as fibroblast growth factor receptor 4 or TP53. At 1.5 mo after surgery, head magnetic resonance imaging revealed that the tumor had recurred. The patient received 60 Gy and 30 fractions of intensity modulated radiotherapy. The patient then received anlotinib 8 mg po qd for 1-14 d of a 21 d cycle. Foll
This is the first reported case of SFT of the central nervous system treated with surgery, radiotherapy and anlotinib. This regimen may be an effective treatment option for malignant intracranial SFT patients.
Core Tip: Solitary fibrous tumor (SFT) of the central nervous system is rare. There is no established standardized treatment regimen for malignant intracranial SFTs. This is the first reported case of SFT in the central nervous system treated with surgery, radi
- Citation: Zhang DY, Su L, Wang YW. Malignant solitary fibrous tumor in the central nervous system treated with surgery, radiotherapy and anlotinib: A case report. World J Clin Cases 2022; 10(2): 631-642
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/631.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.631
Solitary fibrous tumor (SFT) is a rare soft tissue tumor of mesenchymal cell origin. An SFT mainly develops in the pleural cavity, but it can arise in a variety of non-pleural soft tissue sites throughout the body. The incidence of SFT in the central nervous system (CNS) is very low and usually originates from the cranial meninges. The NGFI-A-binding protein (NAB2) and signal transducer and activator of transcription (STAT6) gene fusion was identified as a driver mutation of SFT. Based on SFT and hemangiopericytoma (HPC) containing identical genetic abnormalities, they were considered a new combined entity in the 2016 CNS classification[1]. This classification described three grades of SFT/HPC, grade I, grade II and grade III. The first case of intracranial SFT was described by Carneiro[2] in 1966. This tumor most commonly affects adults and is generally benign in the CNS[3]. Malignant SFT of the CNS is exceedingly rare.
No standardized treatment guideline is available for malignant intracranial SFTs. The main treatments are surgical resection and postoperative radiotherapy. Despite the combination of surgery and adjuvant radiotherapy, the control rates of malignant SFT have been disappointing. Targeted therapy for the treatment of the soft tissue tumors has been developed recently. Anlotinib (Chia-tai Tianqing Pharmaceutical Co., Ltd, China) is a newly multitargeted tyrosine kinase inhibitor with anti-neoplastic and anti-angiogenic activities. It inhibits tumor angiogenesis and proliferation. There are ongoing phase I/II/III clinical trials of anlotinib for different carcinomas and sarcomas in China and other countries. To our knowledge, there are no studies on anlotinib for the treatment of intracranial SFT. In this report, we present a girl with intracranial SFT who was effectively treated by surgery, radiotherapy and anlotinib. We discuss the histopathological features, next-generation sequencing results and anlotinib treatment of cancer, together with a brief review of the literature on SFT treatment by monotherapy.
A 9-year-old girl presented to our hospital emergency department with a 3-wk history of ineffective right limb movement.
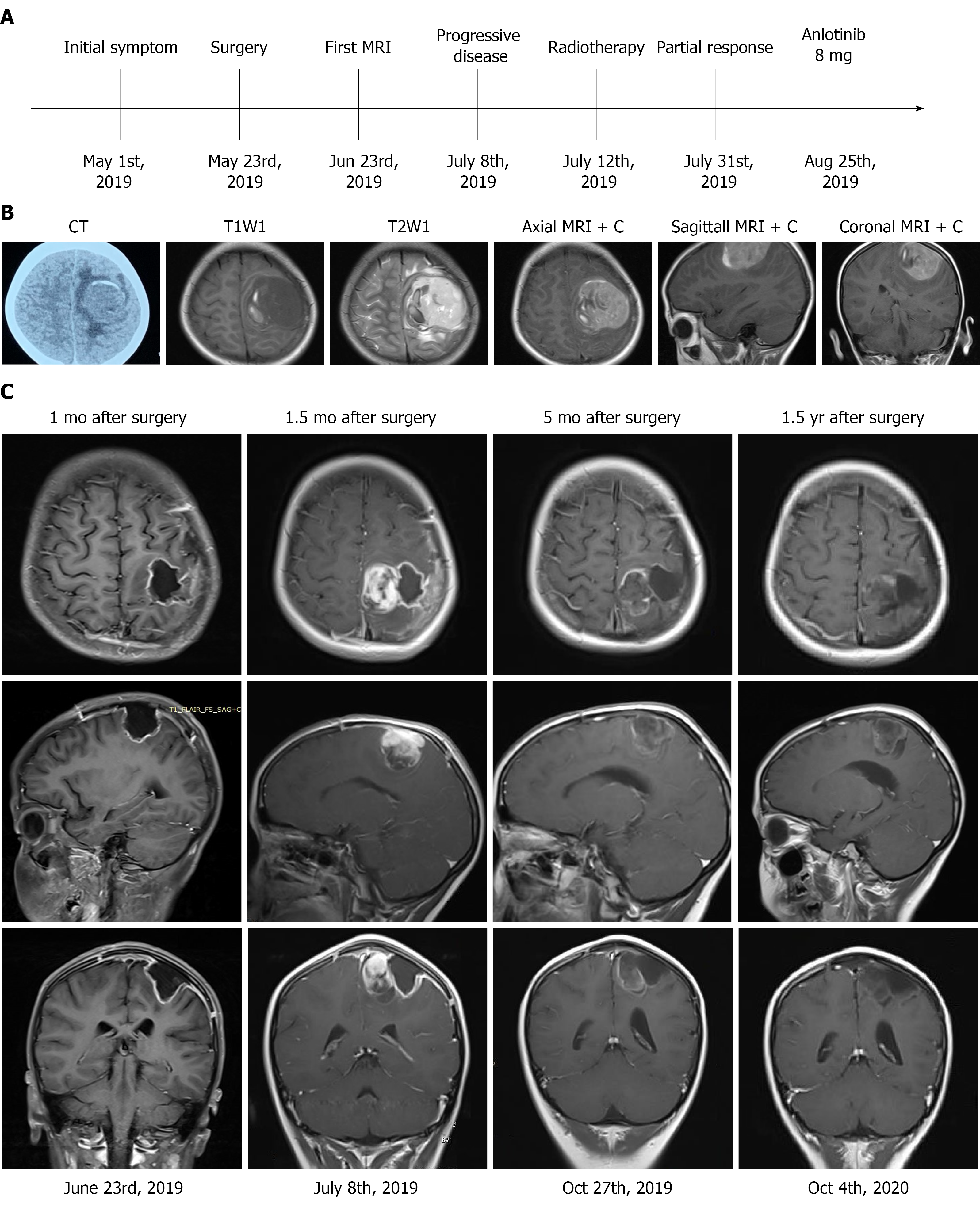
The treatment timeline is shown in Figure 1A. A head computed tomography (CT) scan revealed a quasi-circular mass in the left frontal-parietal region with high-density and associated hemorrhage (Figure 1B). Brain magnetic resonance imaging (MRI) revealed low signals on T1 weighted imaging with high surrounding signals. High signals on T2 weighted imaging with low surrounding signals were observed, with marked enhancement on contrast measuring 4.8 cm × 5.0 cm × 4.5 cm in the left motor area of the frontal-parietal lobes (Figure 1B). The imaging characteristics were similar to meningioma. An unenhanced chest CT scan revealed no nodules in the chest.
Laboratory tests including complete blood cell counts, bleeding time, activated partial thromboplastin time, prothrombin time, liver and renal function and blood glucose level were within normal ranges.
Her temperature was 36.6 ℃, resting respiratory rate was 16 breaths/min, heart rate was 90 bpm and blood pressure was 120/75 mmHg. Neurological examination showed that her Glasgow Coma Scale score was 15 (E4V5M6), and muscle strength was grade 2 in the right limbs. She did not have any other neurological deficits.
She had no personal or family history of benign or malignant tumors.
The patient had no history of prior illness.
The patient had ineffective right limb movement for 3 wk. She also had headaches, accompanied by nausea and vomiting and excess sleep. A head CT scan revealed a quasi-circular mass in the left frontal-parietal region with high-density and associated hemorrhage.
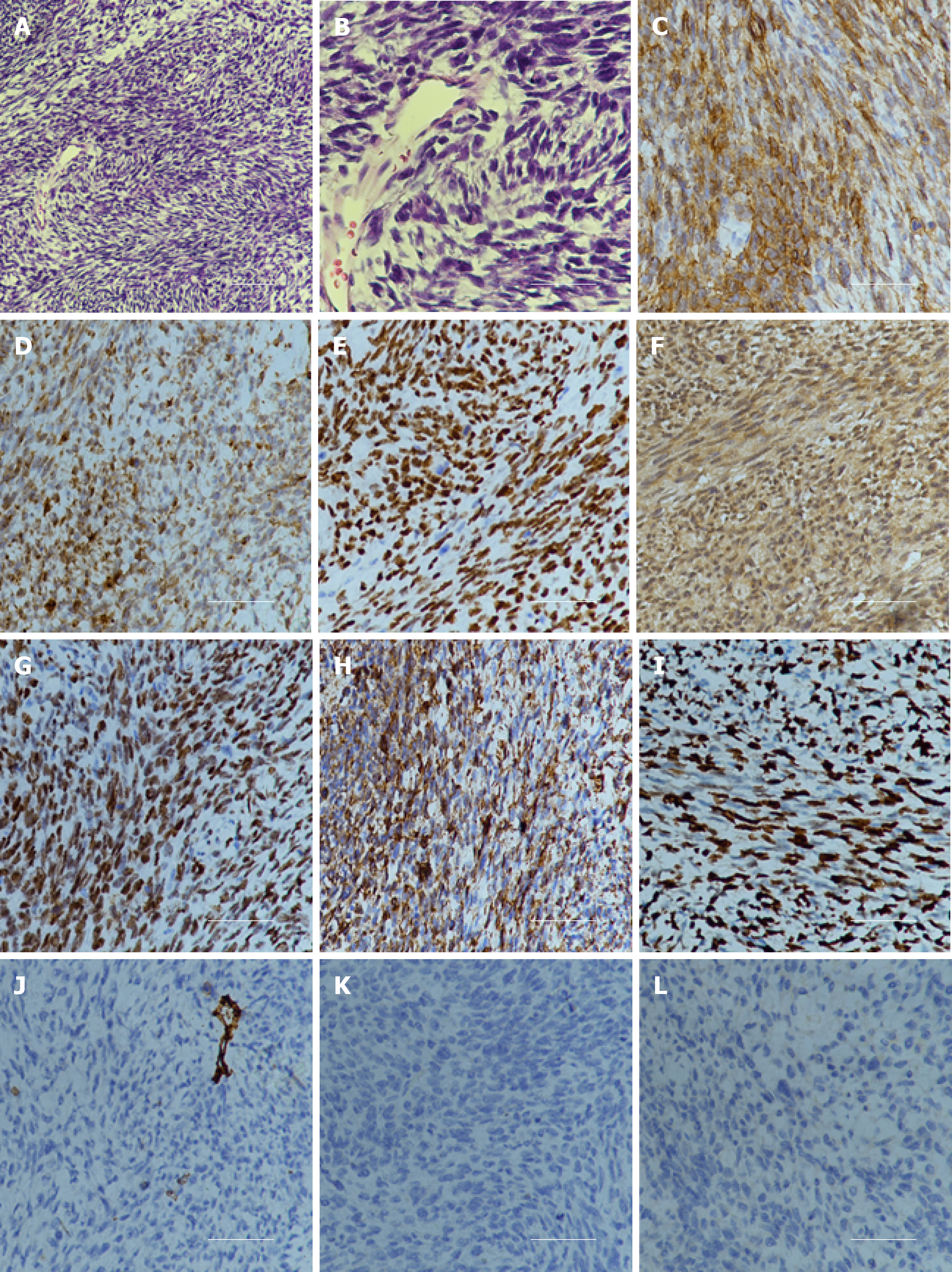
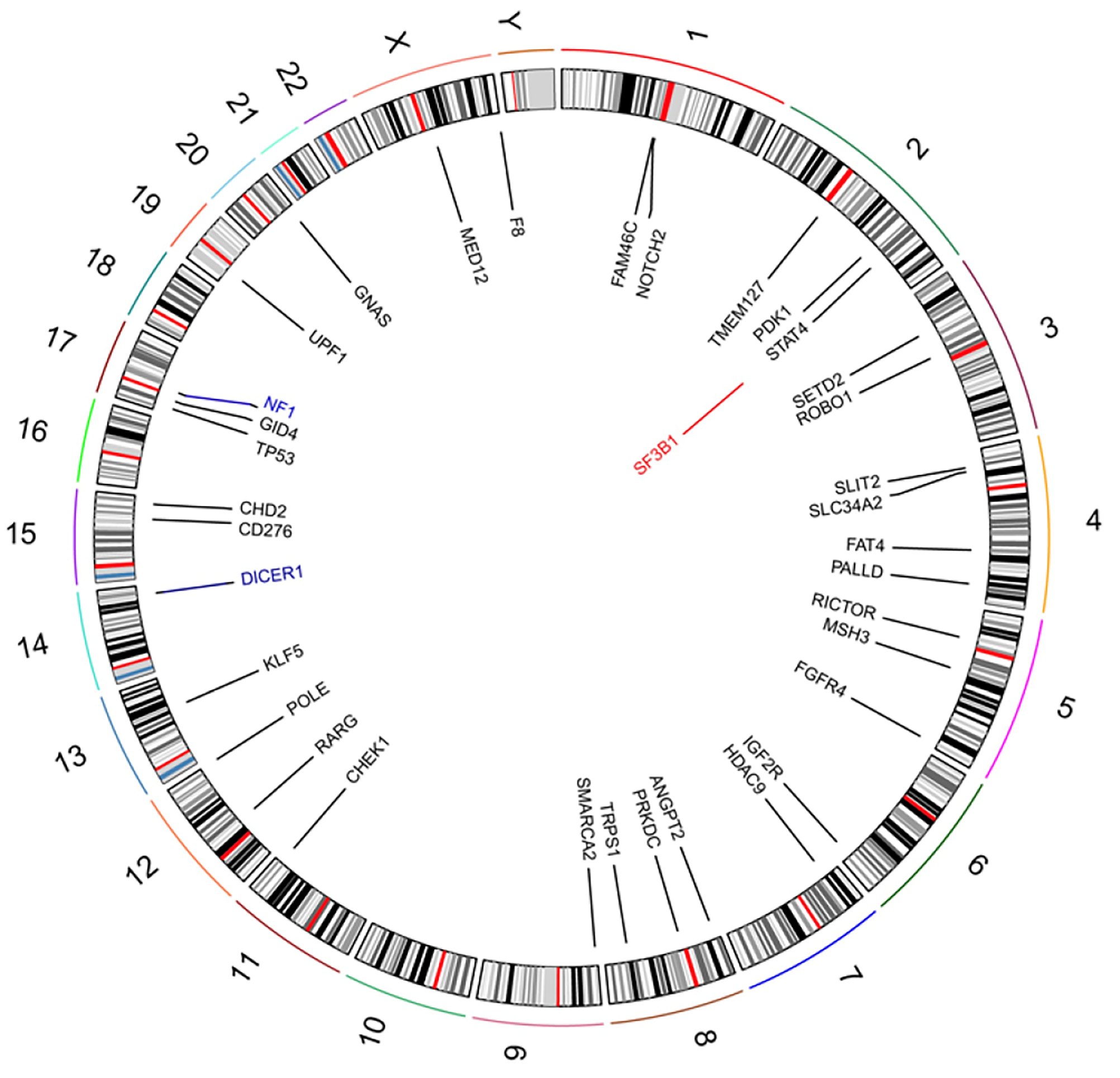
Examination of a frozen section of the biopsy revealed features of malignant tumor. Hematoxylin and eosin staining showed that a large number of spindle or oval cells were diffusely distributed, with deep staining of a null, “staghorn” vascular pattern, hypercellularity and increased mitotic activity were observed in the tumor (> 4 mitosis/10 high-power fields) (Figures 2A and B). Immunohistochemistry examination of the specimen showed positivity for CD99, Bcl-2, TP53, IDH1, TLE-1 and vimentin (Figures 2C-2H) and negativity for CD34, STAT6, CK, EMA, Olig2, PR, SSTR2, CD68, S-100 and GFAP (Figures 2J-2L). Ki-67 proliferation index staining was highly positive in 80% of tumor cells (Figure 2I). Based on the above findings, the pathological diagnosis of malignant SFT was confirmed. The patient’s parents sent the specimen to the Department of Neuropathology, Beijing Neurosurgical Institute to confirm the diagnosis. The pathological diagnosis concurred with that at our hospital. The primary surgical tissue was subjected to whole-exome sequencing by next-generation sequencing (Genetron Health Co., Ltd, Beijing, China). A global landscape of gene mutations was generated from the whole exome sequencing data (Figure 3). A total of 38 somatic gene mutations, including 36 missense mutations, 2 frameshift mutations and 1 gene amplification were detected. These gene alterations were divided into: genes related to chemotherapy and targeted drug-related genes. In targeted drug-related genes, fibroblast growth factor receptor (FGFR) 4 (c.1463G > A, p.Gly488Asp) and TP53 (c.751A > T, p.Ile251Phe) were detected (Table 1). However, this test did not reveal any gene fusion, especially NAB2-STAT6 gene fusion.
| Gene | Type | Nucleotide | Protein | Frequency, % | Chromosome | Exon |
| ANGPT2 | Missense | c.52G > A | p.Ala18Thr | 29.6 | 8 | 1/9 |
| CD276 | Missense | c.91G > T | p.Val31Phe | 59.4 | 15 | 3/10 |
| CHD2 | Missense | c.1972T > G | p.Ser658 Ala | 59.2 | 15 | 16/39 |
| CHEK1 | Missense | c.497A > T | p.Lys166Met | 32.4 | 11 | 6/13 |
| DICER1 | Missense | c.3923A > G | p.Asp1308Gly | 37.3 | 14 | 21/27 |
| DICER1 | Missense | c.5439G > T | p.Glu1813Asp | 32.4 | 14 | 25/27 |
| DICER1 | frameshift | c.2031dup | p.Ser678L eufsTer14 | 55.6 | 14 | 12/27 |
| F8 | missense | c.3347T > A | p.Phe1116Tyr | 36.4 | X | 14/26 |
| FAM46C | missense | c.476A >G | p.Asn159Ser | 56.4 | 1 | 2/2 |
| FAT4 | missense | c.7269A > C | p.Leu2423Phe | 70.3 | 4 | 8/17 |
| FAT4 | missense | c.12512G >A | p.Cys4171Tyr | 21.5 | 4 | 14/17 |
| FGFR4 | missense | c.1463G > A | p.Gly488Asp | 55.6 | 5 | 10/16 |
| GID4 | missense | c.785T > G | p.Phe262Cys | 92.9 | 17 | 5/6 |
| GNAS | missense | c.981G > T | p.Glu327Asp | 22.3 | 20 | 12/13 |
| HDAC9 | missense | c.2704G > C | p.Ala902Pro | 58.7 | 7 | 21/25 |
| IGF2R | missense | c.2842A > T | p.Asn948 | 32.2 | 6 | 21/48 |
| KLF5 | missense | c.757A > C | p.Asn253His | 41.3 | 13 | 2/4 |
| MED12 | missense | c.4809A > T | p.Gln1603His | 36.5 | X | 35/45 |
| MSH3 | missense | c.2404G > T | p.Asp802Tyr | 19.7 | 5 | 17/24 |
| NF1 | frameshift | c.3053_306 7delinsCAGT | p.Leu1018 SerfsTer7 | 89.3 | 17 | 23/58 |
| NOTCH2 | missense | c.5854G > C | p.Ala1952Pro | 35.8 | 1 | 32/34 |
| PALLD | missense | c.207T > G | p.Ser69Arg | 70.5 | 4 | 2/21 |
| PDK1 | missense | c.374A > T | p.Lys125Met | 97.2 | 2 | 3/12 |
| POLE | missense | c.3218G > A | p.Gly1073Glu | 56.4 | 12 | 26/49 |
| PRKDC | missense | c.1413A > T | p.Lys471Asn | 66.1 | 8 | 13/86 |
| RARG | missense | c.744G > T | p.Leu248Phe | 24.7 | 12 | 7/10 |
| RICTOR | missense | c.3786A > T | p.Lys1262Asn | 36.3 | 5 | 31/38 |
| ROBO1 | missense | c.3240G > T | p.Gln1080His | 28.2 | 3 | 23/31 |
| ROBO1 | missense | c.3736G > T | p.Ala1246Ser | 31.8 | 3 | 26/31 |
| SETD2 | missense | c.4407G > C | p.Met1469Ile | 28.3 | 3 | 3/21 |
| SLC34A2 | missense | c.225G > C | p.Gln75His | 22.8 | 4 | 3/13 |
| SLIT2 | missense | c.2425A > T | p.Ser809Cys | 66.1 | 4 | 24/37 |
| SMARCA2 | missense | c.394G > A | p.Glu132Lys | 22.9 | 9 | 4/34 |
| STAT4 | missense | c.1450A > T | p.Asn484Tyr | 98.4 | 2 | 17/24 |
| TMEM127 | missense | c.380G > T | p.Arg127Leu | 36.5 | 2 | 3/4 |
| TP53 | missense | c.751A > T | p.Ile251Phe | 90.3 | 17 | 7/11 |
| TRPS1 | missense | c.2206A > G | p.Thr736Ala | 31.4 | 8 | 5/7 |
| UPF1 | missense | c.1880A > T | p.Lys627Met | 45.1 | 19 | 14/24 |
The patient was diagnosed with malignant SFT.
The patient underwent left craniotomy under general anesthesia, and the mass was resected. After surgery, her motor examination showed improvement in the right limb, and she could walk unaided. Head MRI was performed 1 mo after surgery, and the results showed no residual tumor (Figure 1C). The patient was advised to undergo adjuvant radiotherapy due to high Ki-67, and MRI (1.5 mo after surgery) before radiotherapy planning showed a solid mass, suggesting a progressive tumor (Figure 1C). The patient was unable to walk by herself, and physical examination showed muscle strength was grade 3 in the right limbs. The patient received 60 Gy and 30 fractions of intensity modulated radiotherapy. Mannitol was administered to relieve her symptoms by reducing intracranial pressure. After 19 d of radiotherapy, the patient could walk unaided. Considering her pathological diagnosis, FGFR4 and TP53 mutation and progression of the disease, the oncologist at Beijing Cancer Hospital advised the patient to undergo anlotinib treatment. She received anlotinib 8 mg po qd for 1-14 d of a 21 d cycle at home 3 mo after surgery. During anlotinib treatment, the patient occasionally suffered from loss of appetite, and her laboratory results were within the normal range. MRI performed 5 mo after surgery showed that the tumor had not progressed (Figure 1C). The patient took anlotinib orally for 17 mo.
Follow-up MRI showed that the tumor had not progressed 1.5 years after surgery (Figure 1C). An unenhanced chest CT scan revealed no nodules in the chest.
We present the first known case of malignant intracranial SFT treated with surgery, adjuvant radiotherapy and anlotinib, leading to a good prognosis. SFT is a rare soft tissue tumor in the CNS. The first case of SFT in the CNS was described by Carneiro[2] in 1966. SFT most commonly affects adults and is generally benign. A few cases have been described in children. An MRI scan 1 mo after surgery showed that the tumor had been successfully resected in our case. However, 2 wk after the first MRI examination, the tumor rapidly progressed as shown by head MRI in this 9-year-old patient. The immunohistochemical features of intracranial SFT and HPC overlap. They share an inversion at 12q13 fused with NAB2 and STAT6 genes, and the combined term is “SFT/HPC”[1]. This classification described three grades of SFT/HPC, grade I-III. Grade I was previously diagnosed as SFT. Grade II was previously diagnosed as CNS HPC. Grade III was previously termed anaplastic HPC and malignant SFT. Our patient had grade III CNS SFT and had a poor prognosis. However, disease prog
The nuclear expression of STAT6 on immunohistochemistry is very important in the pathological diagnosis of SFT[4,5]. Although NAB2-STAT6 fusion seems to be important in the diagnosis of SFT, its detection may be difficult unless high-throughput sequencing is performed to detect breakpoints. Most SFTs have shown NAB2-STAT6 fusion, and the remaining 11% of cases lacked an identifiable NAB2-STAT6 fusion[5]. Our case did not show NAB2-STAT6 fusion following high-throughput sequencing, and no nuclear STAT6 expression was observed on immunohistochemical analysis. Hematoxylin and eosin staining showed a high-grade component mimicking a spindle cell sarcoma, small-cell sarcoma and other entities that have no morphological resemblance to SFT. The dedifferentiation of SFT can induce the loss of STAT6 expression on immunohistochemistry[6-8]. We speculated that Ki-67 proliferation index was 80%, and the tumor was highly dedifferentiated, which suggest the reasons for negative STAT6 on immunohistochemistry. Although STAT6 is a sensitive marker in the diagnosis of SFT, other immunohistochemical indices, such as CD99, Bcl-2 and vimentin are also sensitive markers for the diagnosis of intracranial SFT[9-11]. Han et al[12] analyzed 53 cases using immunohistochemistry and found that CD99 was positive in 94.3% of cases and Bcl-2 was positive in 96.2% of cases.
The differential diagnosis of SFT in the CNS includes meningioma. MRI initially showed that the mass was broadly attached to the dura matter in our patient. However, the dural tail sign, which is the most important characteristic of meningioma on MRI, was absent. Secondly, EMA and SSTR2 were negative in our case by immunohistochemistry, as opposed to meningioma in which EMA and SSTR2 are both positive[13]. Thirdly, meningioma is usually a benign tumor, and SFT tends to be aggressive. In our case, Ki-67 proliferation index was 80% and showed that the tumor was very malignant. These features can be used to differentiate SFT from meningioma.
As our patient was young, a diagnosis of synovial sarcoma was considered. The diagnosis of synovial sarcoma depends on the cytogenetic change t (X; 18) (p11; q11), which is detected by fluorescence in situ hybridization assay[14]. In our case, fluorescence in situ hybridization assay showed that the t (X; 18) was negative. Thus, the diagnosis in our patient was not synovial sarcoma. Another differential diagnosis we considered was schwannoma. However, the tumor was located in the frontal-parietal lobe, not the area where the cranial nerves are located. Therefore, a schwannoma was ruled out.
A malignant SFT has been shown to be hypercellular, mitotically active (> 4 mitosis/10 high-power fields), with cytological atypia, tumor necrosis and /or infiltrative margins[15]. Although our case did not show NAB2-STAT6 fusion following high-throughput sequencing and STAT6 expression on immunohistochemistry, CT scanning, MRI imaging, morphologic examination, conventional immunohistochemistry (positive for CD99, Bcl-2 and vimentin), high Ki-67 proliferation index and the exclusion of other tumors resulted in the diagnosis of malignant SFT.
SFT is frequently benign and if gross total resection is performed, it will not recur. HPC tends to be an aggressive tumor with the potential for local recurrence and metastases[16]. Although gross total resection offers a potential cure, improvement or preservation of motor function is crucial for patients. During surgery, we found that the tumor invaded the arachnoid, and gross total resection could cause the damage to the underlying cortex. Thus, gross total resection was not performed in our patient. Her muscle strength after surgery was better than that before surgery, and she could walk unaided. However, the disadvantage of this surgical method is that the tumor can recur. MRI (1.5 mo after surgery) showed a solid mass on the cut margin. SFT after subtotal resection has a high risk of local recurrence[17], and adjuvant radiation may be beneficial in some cases[18]. Rana et al[19] analyzed 155 intracranial SFT patients and found that adjuvant radiation did not prolong overall survival time compared with the surgery group. However, Bishop et al[20] found that the treatment of SFT with combined surgery and radiotherapy led to excellent local control. Therefore, the role of radiotherapy in SFT is still unclear. Due to the low number of patients, it is difficult to conduct high-quality clinical trials on this treatment regimen.
The tumor location in our case was in the motor area, and gross total resection of the tumor while preserving neurological function was relatively difficult. Head MRI 1 mo after surgery showed that the tumor had been successfully resected. However, 2 wk after the first MRI examination, brain MRI revealed tumor recurrence. The patient was advised to undergo adjuvant radiotherapy due to a high Ki-67 index and rapid recurrence. The patient received 60 Gy and 30 fractions of intensity modulated radiotherapy. After 20 d of radiotherapy, her limb weakness improved. We speculated that there were three main reasons for rapid alleviation of the patient’s symptoms. The first is that the time for the tumor to press against the motor zone of the cortex was relatively short and did not cause necrosis of cortical cells. Radiotherapy induces tumor cell necrosis, and the functions of these cells are then restored. The second is that we administered mannitol to relieve symptoms by reducing intracranial pressure. The third is that the tumor was more sensitive to radiotherapy, and it was effectively controlled. Our patient showed that radiotherapy resulted in excellent local control of intracranial malignant SFT.
Activation of oncogenes is a key mechanism of tumorigenesis and generally arises from a genetic mutation or amplification. As the therapeutic value of the genetic mutation depends heavily on the clinical prognosis, proving their direct involvement in personal targeted therapy has become a task for doctors. However, the extent of genetic intratumoral heterogeneity in SFTs is largely unknown. In our study, next-generation sequencing analysis showed significant germline aberrations and somatic point mutations were identified in hotspot cancer-related genes in this patient, such as FGFR4 and TP53.
Anlotinib, a newly designed oral small-molecule receptor tyrosine kinase inhibitor, was developed independently by Chia-Tai Tianqing Pharmaceutical Co., Ltd. in China. This drug was approved by the China Food and Drug Administration for the treatment of non-small cell lung cancer, soft tissue sarcoma, metastatic renal cell carcinoma and medullary thyroid cancer[21]. Anlotinib inhibits tumor angiogenesis and proliferation, targeting vascular endothelial growth factor, FGFR, platelet-derived growth factor α/β and c-Kit[21]. Several prospective studies suggest the potential efficacy of antiangiogenic treatments in SFT[22-27] (Table 2). Our patient received anlotinib 8 mg po qd for 1-14 d of a 21 d cycle after adjuvant radiotherapy for 17 mo. The follow-up MRI showed that the tumor had not progressed.
| Ref. | Yr | Number of participants | CNS | Drug | RECIST | Choi | Median PFS in mo | |||
| PR | SD | PR | SD | RECIST | Choi | |||||
| Park et al[22] | 2011 | 14 | 6 | Temozolomide + bevacizumab | 2 | 12 | 11 | 2 | 10.8 | 9.67 |
| Stacchiotti et al[23] | 2012 | 35 | 6 | Sunitinib | 2 | 17 | 14 | 5 | 6 | 7 |
| Valentin et al[24] | 2013 | 5 | 1 | Sorafenib | NA | 2 | NA | NA | NA | NA |
| Maruzzo et al[25] | 2015 | 13 | NA | Pazopanib | 1 | 8 | 5 | 4 | 4.7 | NA |
| Ebata et al[26] | 2018 | 9 | 2 | Pazopanib | 0 | 8 | 4 | 2 | 6.2 | NA |
| Martin-Broto et al[27] | 2019 | 36 | 5 | Pazopanib | 2 | 21 | 18 | 9 | 5.57 | 5.57 |
Oncogene alterations are present in all FGFR family members in human cancers. FGFR is a receptor tyrosine kinase consisting of an intracellular tyrosine-kinase domain and an extracellular ligand-binding domain. FGFR 1-3 often occur in amplifications or fusions in some cancers. However, FGFR 4 is infrequently mutated in cancers[28]. FGFR 4 mutations are present in 6% of melanomas[29]. One recent report found that 7% of cancers had FGFR aberrations, and FGFR 4 mutation was found in 0.5% of 4853 tumors[30]. Y367C mutation of the FGFR 4 gene in the breast cancer cell line MDA-MB453 promoted tumor growth[31]. Futami et al[32] identified FGFR 4 mutation in one of 83 gastric tumor specimens, and cells expressing this mutation showed a malignant phenotype. Multi-targeted tyrosine kinase inhibitors can be used to inactivate FGFR 4 by disrupting ATP binding in its tyrosine kinase domains[33]. Potential mechanisms of FGFR 4 activation include FGFR 4 overexpression and somatic mutations[34]. Therefore, we speculated that FGFR 4 mutation was likely to be the “driver” mutation and resulted in increased FGFR signaling in this patient. FGFR 4 mutation might be a key anticancer target for anlotinib in the treatment of malignant intracranial SFT.
TP53 is a tumor suppressor gene and plays a crucial role in malignant tumor progression. There is mounting basic and clinical evidence to show that tumors with TP53 gene mutations have a better response to antiangiogenic drugs than TP53 wild-type tumors[35]. Recent research found that anlotinib induced apoptosis in TP53 D259Y and R248G mutants, which were able to induce apoptosis through their transcription-independent function[36]. Fang et al[37] identified three cases with TP53 mutations (p.S183X on exon 5, p.S241F on exon 7, p.R175H on exon 5, K320fs on exon 9) that might represent biomarkers for predicting the effects of anlotinib in non-small cell lung cancer. Wu et al[38] reported a patient with pulmonary artery sarcoma harboring a TP53 mutation (p.R110P in exon 4) who had a favorable response to anlotinib. Kurisaki-Arakawa et al[39] found dedifferentiated SFTs in the pelvis with a TP53 mutation (p.A158H in exon 5). Morimitsu et al[40] found the TP53 mutation p.A116T in 1 of 17 cases with solitary extrapleural fibrous tumors. These findings suggest that various mutations of TP53 in SFTs are common, and tumors with TP53 mutations are more likely to respond to anlotinib. Based on the next-generation sequencing analysis, we speculated that TP53 mutation also plays a very important role in malignant SFT of the CNS treated with anlotinib monotherapy.
There is currently no standard treatment regimen for malignant SFT of the CNS. There is no effective targeted drug that can improve the prognosis of malignant intracranial SFT. This is the first report in the world of a patient with malignant intracranial SFT treated with surgery, radiotherapy and anlotinib monotherapy. Based on preliminary data, we speculated that FGFR 4 and TP53 mutations might be beneficial in the treatment of malignant intracranial SFT with anlotinib. Basic research and larger, randomized controlled trial are needed to confirm the results of the present study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Maeda O, Sato T S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10993] [Cited by in RCA: 10858] [Article Influence: 1206.4] [Reference Citation Analysis (0)] |
| 2. | Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH. Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol. 1996;106:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 277] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Giordan E, Marton E, Wennberg AM, Guerriero A, Canova G. A review of solitary fibrous tumor/hemangiopericytoma tumor and a comparison of risk factors for recurrence, metastases, and death among patients with spinal and intracranial tumors. Neurosurg Rev. 2021;44:1299-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Tani E, Wejde J, Åström K, Wingmo IL, Larsson O, Haglund F. FNA cytology of solitary fibrous tumors and the diagnostic value of STAT6 immunocytochemistry. Cancer Cytopathol. 2018;126:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Fritchie K, Jensch K, Moskalev EA, Caron A, Jenkins S, Link M, Brown PD, Rodriguez FJ, Guajardo A, Brat D, Velázquez Vega JE, Perry A, Wu A, Raleigh DR, Santagata S, Louis DN, Brastianos PK, Kaplan A, Alexander BM, Rossi S, Ferrarese F, Haller F, Giannini C. The impact of histopathology and NAB2-STAT6 fusion subtype in classification and grading of meningeal solitary fibrous tumor/hemangiopericytoma. Acta Neuropathol. 2019;137:307-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Zhang P, Xiong K, Lv P, Zhang H, Wang Y, Yang Z, Tao Z, Zhang P, Song W. Malignant solitary fibrous tumor occurring in the mediastinal pleura showing NAB2ex4-STAT6ex2 fusion and negative STAT6 immunohistochemistry: A case report. Thorac Cancer. 2020;11:1344-1349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Schneider N, Hallin M, Thway K. STAT6 Loss in Dedifferentiated Solitary Fibrous Tumor. Int J Surg Pathol. 2017;25:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Dagrada GP, Spagnuolo RD, Mauro V, Tamborini E, Cesana L, Gronchi A, Stacchiotti S, Pierotti MA, Negri T, Pilotti S. Solitary fibrous tumors: loss of chimeric protein expression and genomic instability mark dedifferentiation. Mod Pathol. 2015;28:1074-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Bisceglia M, Galliani C, Giannatempo G, Lauriola W, Bianco M, D'angelo V, Pizzolitto S, Vita G, Pasquinelli G, Magro G, Dor DB. Solitary fibrous tumor of the central nervous system: a 15-year literature survey of 220 cases (August 1996-July 2011). Adv Anat Pathol. 2011;18:356-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Chen H, Zeng XW, Wu JS, Dou YF, Wang Y, Zhong P, Xu R, Jiang CC, Wang XQ. Solitary fibrous tumor of the central nervous system: a clinicopathologic study of 24 cases. Acta Neurochir (Wien). 2012;154:237-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Wu Z, Yang H, Weng D, Ding Y. Rapid recurrence and bilateral lungs, multiple bone metastasis of malignant solitary fibrous tumor of the right occipital lobe: report of a case and review. Diagn Pathol. 2015;10:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Han Y, Zhang Q, Yu X, Han X, Wang H, Xu Y, Qiu X, Jin F. Immunohistochemical detection of STAT6, CD34, CD99 and BCL-2 for diagnosing solitary fibrous tumors/hemangiopericytomas. Int J Clin Exp Pathol. 2015;8:13166-13175. [PubMed] |
| 13. | Boulagnon-Rombi C, Fleury C, Fichel C, Lefour S, Marchal Bressenot A, Gauchotte G. Immunohistochemical Approach to the Differential Diagnosis of Meningiomas and Their Mimics. J Neuropathol Exp Neurol. 2017;76:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Zhang G, Xiao B, Huang H, Zhang Y, Zhang X, Zhang J, Wang Y. Intracranial synovial sarcoma: A clinical, radiological and pathological study of 16 cases. Eur J Surg Oncol. 2019;45:2379-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Fletcher C, Bridge JA, Hogendoorn PCW, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone: WHO Classification of Tumours. World Health Organization. 2013;5. |
| 16. | Tihan T, Viglione M, Rosenblum MK, Olivi A, Burger PC. Solitary fibrous tumors in the central nervous system. A clinicopathologic review of 18 cases and comparison to meningeal hemangiopericytomas. Arch Pathol Lab Med. 2003;127:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Fargen KM, Opalach KJ, Wakefield D, Jacob RP, Yachnis AT, Lister JR. The central nervous system solitary fibrous tumor: a review of clinical, imaging and pathologic findings among all reported cases from 1996 to 2010. Clin Neurol Neurosurg. 2011;113:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Nakahara K, Yamada M, Shimizu S, Fujii K. Stereotactic radiosurgery as adjuvant treatment for residual solitary fibrous tumor. Case report. J Neurosurg. 2006;105:775-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Rana N, Kim E, Jaboin J, Attia A. The Role of Adjuvant Radiation in the Management of Solitary Fibrous Tumors of the Central Nervous System: A National Cancer Database Analysis of 155 Patients. Cureus. 2018;10:e2656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Bishop AJ, Zagars GK, Demicco EG, Wang WL, Feig BW, Guadagnolo BA. Soft Tissue Solitary Fibrous Tumor: Combined Surgery and Radiation Therapy Results in Excellent Local Control. Am J Clin Oncol. 2018;41:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 435] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 22. | Park MS, Patel SR, Ludwig JA, Trent JC, Conrad CA, Lazar AJ, Wang WL, Boonsirikamchai P, Choi H, Wang X, Benjamin RS, Araujo DM. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer. 2011;117:4939-4947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 23. | Stacchiotti S, Negri T, Libertini M, Palassini E, Marrari A, De Troia B, Gronchi A, Dei Tos AP, Morosi C, Messina A, Pilotti S, Casali PG. Sunitinib malate in solitary fibrous tumor (SFT). Ann Oncol. 2012;23:3171-3179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Valentin T, Fournier C, Penel N, Bompas E, Chaigneau L, Isambert N, Chevreau C. Sorafenib in patients with progressive malignant solitary fibrous tumors: a subgroup analysis from a phase II study of the French Sarcoma Group (GSF/GETO). Invest New Drugs. 2013;31:1626-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Maruzzo M, Martin-Liberal J, Messiou C, Miah A, Thway K, Alvarado R, Judson I, Benson C. Pazopanib as first line treatment for solitary fibrous tumours: the Royal Marsden Hospital experience. Clin Sarcoma Res. 2015;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Ebata T, Shimoi T, Bun S, Miyake M, Yoshida A, Shimomura A, Noguchi E, Yonemori K, Shimizu C, Fujiwara Y, Narita Y, Tamura K. Efficacy and Safety of Pazopanib for Recurrent or Metastatic Solitary Fibrous Tumor. Oncology. 2018;94:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Martin-Broto J, Stacchiotti S, Lopez-Pousa A, Redondo A, Bernabeu D, de Alava E, Casali PG, Italiano A, Gutierrez A, Moura DS, Peña-Chilet M, Diaz-Martin J, Biscuola M, Taron M, Collini P, Ranchere-Vince D, Garcia Del Muro X, Grignani G, Dumont S, Martinez-Trufero J, Palmerini E, Hindi N, Sebio A, Dopazo J, Dei Tos AP, LeCesne A, Blay JY, Cruz J. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019;20:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 28. | Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol. 2019;16:105-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 29. | Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9144] [Cited by in RCA: 12771] [Article Influence: 982.4] [Reference Citation Analysis (0)] |
| 30. | Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin Cancer Res. 2016;22:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 592] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 31. | Roidl A, Foo P, Wong W, Mann C, Bechtold S, Berger HJ, Streit S, Ruhe JE, Hart S, Ullrich A, Ho HK. The FGFR4 Y367C mutant is a dominant oncogene in MDA-MB453 breast cancer cells. Oncogene. 2010;29:1543-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Futami T, Kawase T, Mori K, Asaumi M, Kihara R, Shindoh N, Kuromitsu S. Identification of a novel oncogenic mutation of FGFR4 in gastric cancer. Sci Rep. 2019;9:14627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Lang L, Teng Y. Fibroblast Growth Factor Receptor 4 Targeting in Cancer: New Insights into Mechanisms and Therapeutic Strategies. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Levine KM, Ding K, Chen L, Oesterreich S. FGFR4: A promising therapeutic target for breast cancer and other solid tumors. Pharmacol Ther. 2020;214:107590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | Fu S, Hou MM, Naing A, Janku F, Hess K, Zinner R, Subbiah V, Hong D, Wheler J, Piha-Paul S, Tsimberidou A, Karp D, Araujo D, Kee B, Hwu P, Wolff R, Kurzrock R, Meric-Bernstam F. Phase I study of pazopanib and vorinostat: a therapeutic approach for inhibiting mutant p53-mediated angiogenesis and facilitating mutant p53 degradation. Ann Oncol. 2015;26:1012-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Ruan X, Shi X, Dong Q, Yu Y, Hou X, Song X, Wei X, Chen L, Gao M. Antitumor effects of anlotinib in thyroid cancer. Endocr Relat Cancer. 2019;26:153-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 37. | Fang S, Cheng W, Zhang M, Yang R. Association of TP53 Mutations with Response to Anlotinib Treatment in Advanced Non-Small Cell Lung Cancer. Onco Targets Ther. 2020;13:6645-6650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Wu Y, Huang J, Wang Q, Zhang M, Luo Y, Wang X, Zhu X, Liu H. Whole-exome sequencing insights into pulmonary artery sarcoma mimicking pulmonary embolism: a case report and review. Onco Targets Ther. 2019;12:6227-6235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Kurisaki-Arakawa A, Akaike K, Hara K, Arakawa A, Takahashi M, Mitani K, Yao T, Saito T. A case of dedifferentiated solitary fibrous tumor in the pelvis with TP53 mutation. Virchows Arch. 2014;465:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Morimitsu Y, Nakajima M, Hisaoka M, Hashimoto H. Extrapleural solitary fibrous tumor: clinicopathologic study of 17 cases and molecular analysis of the p53 pathway. APMIS. 2000;108:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |