Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.625
Peer-review started: March 24, 2021
First decision: October 16, 2021
Revised: November 4, 2021
Accepted: December 7, 2021
Article in press: December 7, 2021
Published online: January 14, 2022
Processing time: 293 Days and 15.6 Hours
We report a case of lorazepam-induced agitated delirium treated with hal
A 65-year-old man with a history of bipolar disorder presented to the emergency department with severe abdominal discomfort after binge eating. During his hospital stay, he received intravenous lorazepam for insomnia. On the next day, he became delirious and was thus treated with seven doses (5 mg each) of haloperidol over a 48 h period. Signs of NMS (hyperthermia, rigidity, myoclonus of upper limbs, impaired consciousness, tachypnea, and dark urine) became apparent and haloperidol was immediately suspended and brisk diuresis was initiated. On intensive care unit admission, he was confused, disoriented, and markedly agitated. Dexmedetomidine infusion was started with the goal of achieving a Richmond Agitation-Sedation Scale score of -1 or 0. NMS was resolved gradually and the patient stabilized, permitting discontinuation of dexmedetomidine after 3 d.
Dexmedetomidine may be clinically helpful for the management of NMS, most likely because of its sympatholytic activity.
Core Tip: Neuroleptic malignant syndrome (NMS), a life-threatening condition characterized by hyperthermia, rigidity, altered mental status, and dysautonomia, can occur in 0.02%–3% of patients who receive haloperidol. Herein, we report a case of lorazepam-induced agitated delirium treated with haloperidol, which further triggered NMS. The NMS was effectively managed with medical therapy and dexmedetomidine, a versatile and highly selective short-acting alpha-2 adrenergic agonist with sedative-hypnotic and anxiolytic effects. Dexmedetomidine may be clinically helpful for the management of NMS because of its sympatholytic activity. In the presence of rapid shallow breathing, dexmedetomidine may provide better patient comfort with less respiratory depression than does propofol.
- Citation: Yang CJ, Chiu CT, Yeh YC, Chao A. Successful management of delirium with dexmedetomidine in a patient with haloperidol-induced neuroleptic malignant syndrome: A case report. World J Clin Cases 2022; 10(2): 625-630
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/625.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.625
Delirium, a transient, reversible organic mental syndrome characterized by dis
Herein, we report a case of lorazepam-induced agitated delirium treated with haloperidol, which in turn triggered the onset of NMS. The latter condition, a medical emergency, was effectively managed with medical treatment and dexmedetomidine, a versatile and highly selective short-acting alpha-2 adrenergic agonist with sedative-hypnotic and anxiolytic effects[6]. Because extreme sympathetic nervous system activation is involved in the pathophysiology of NMS[7], we hypothesized that dexmedetomidine may be clinically beneficial for managing this medical emergency because of its sympatholytic activity[8].
A 65-year-old man presented to the emergency department with severe abdominal discomfort after binge eating.
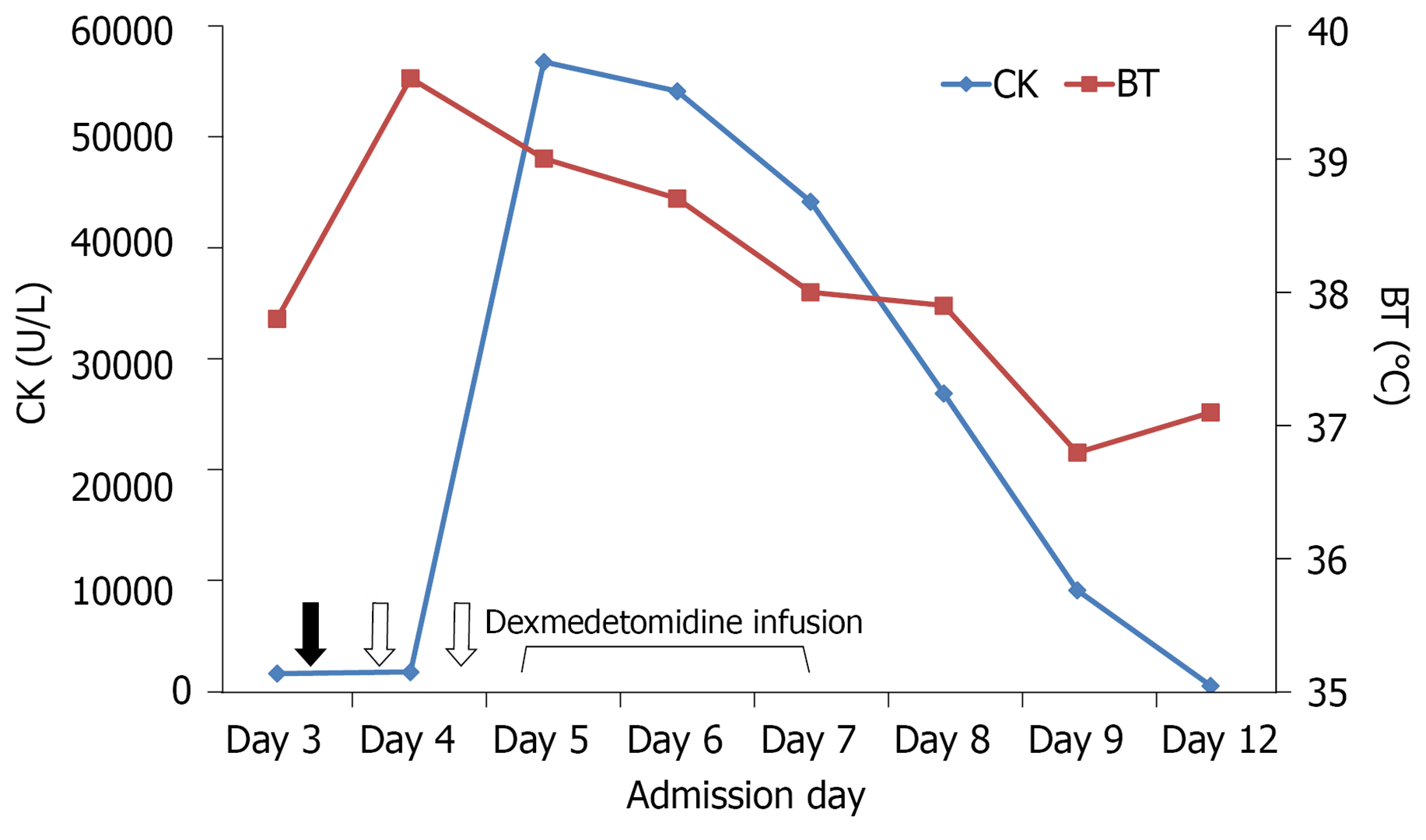
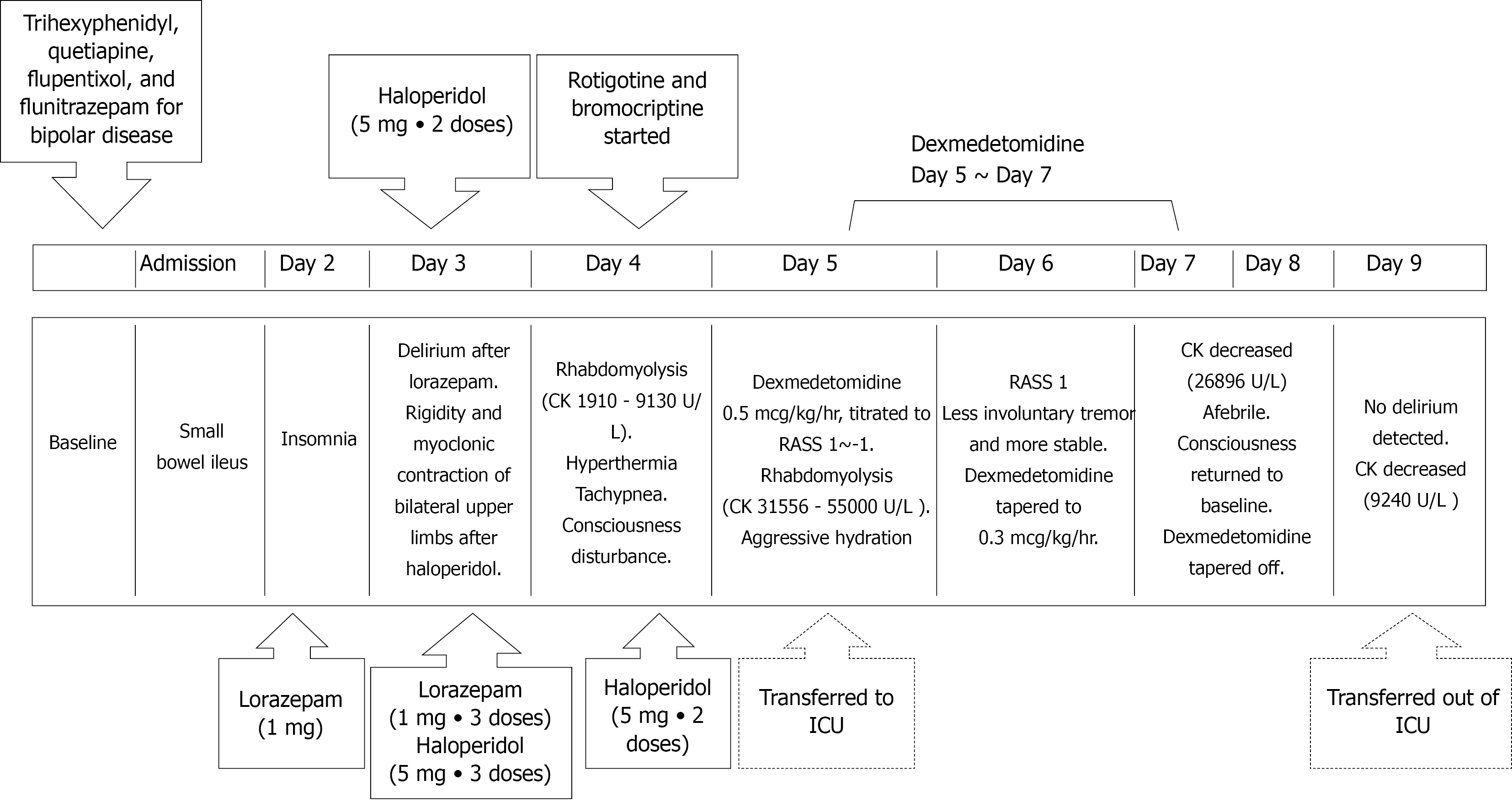
Because of food impaction, oral intake and medications were discontinued. However, lorazepam was administered intravenously to treat insomnia. The next day, the patient became delirious and was thus treated with seven doses (5 mg each) of haloperidol over a 48 h period. Unfortunately, he developed hyperthermia (body temperature: 40.6 °C) accompanied by tachypnea (respiratory rate: 40 breaths per min), tachycardia (heart rate: 128 beats per min), impaired consciousness, muscle rigidity, and dark urine. The serum creatine kinase (CK) levels were markedly increased (1910 U/L) (Figure 1), indicating rhabdomyolysis. NMS was diagnosed, and haloperidol was immediately stopped. Adequate hydration and body cooling were implemented; additionally, bromocriptine (2.5 mg/3 times daily) and transdermal patches containing rotigotine (2 mg/24 h) were applied to overcome the hypodopaminergic state. The patient was subsequently transferred to the ICU for intensive monitoring and treatment of NMS.
The patient’s medical history was notable for bipolar disorder, and his medications included trihexyphenidyl, quetiapine, flupentixol, and flunitrazepam.
Bipolar disorder.
Upon initial evaluation, the patient’s temperature was 36 °C, the heart rate 102 beats per min, and the respiratory rate 20 breaths per min. A physical examination revealed abdominal tension.
The laboratory findings upon ICU admission were as follows: CK, 21000 U/L; blood urea nitrogen, 29 mg/dL; creatinine, 1.4 mg/dL; potassium, 4.5 mEq/L; blood pH, 7.4; PCO2, 31 mmHg; PO2, 70 mmHg; HCO3, 23 mEq/L; and base excess, -1.5 mmol/L.
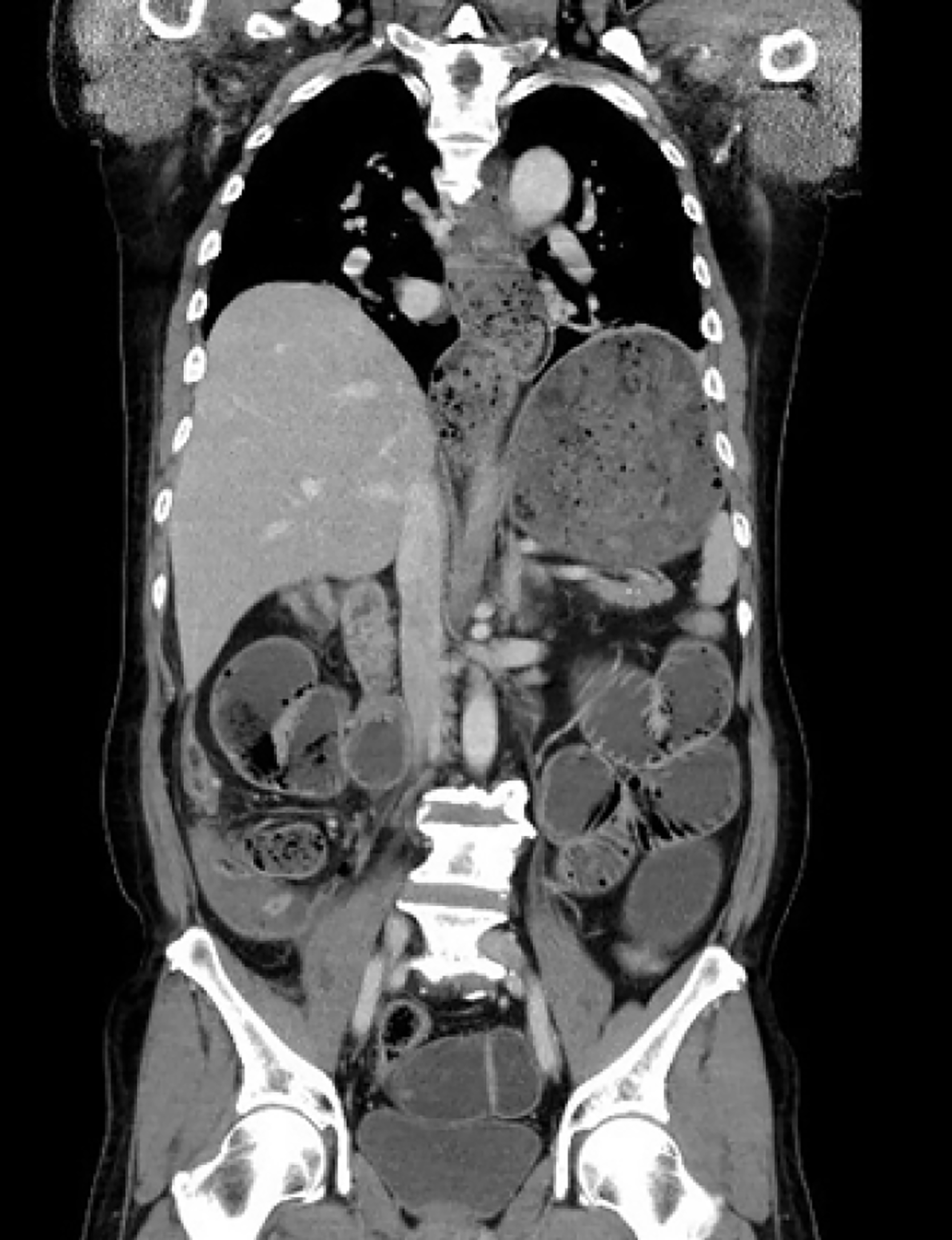
Computed tomography revealed food impaction accompanied by bowel distension from the esophagus to the small intestine (Figure 2).
Lorazepam-induced delirium treated with haloperidol, which in turn triggered NMS.
On ICU admission, the patient was confused and disoriented to time, space, situation, and persons. His Glasgow coma score (GCS) was 13 (Eye 4, Motor 5, and Verbal 4) and papillary light reflexes were bilaterally positive. Severe rigidity and myoclonus (especially in the upper extremities) were evident. Additionally, the patient was severely agitated (Richmond Agitation-Sedation Scale (RASS)[9] score = 3), and made attempts at removing his intravenous catheter and nasogastric tube. In addition to continuation of medical management, dexmedetomidine infusion was started (initial rate: 0.2 µg/kg/h) with the goal of achieving a RASS score of -1 or 0. Owing to persistent agitation (accompanied by shouting and attempts to remove restraints), dexmedetomidine dosing was further increased to 0.5 µg/kg/h. Gradual resolution of muscle rigidity, myoclonus, and agitation was observed over the next day (GCS score: 14). Thus, the dosage of dexmedetomidine was lowered to 0.3 µg/kg/h. The family’s presence at the patient’s bed side was encouraged. Containment measures were removed and the dexmedetomidine dosing was further reduced. Full cooperation was achieved 72 h after the initial infusion of dexmedetomidine, which was thus discontinued. After full regression of rhabdomyolysis and rigidity, the patient was transferred to the general ward for further care. Figure 3 depicts the timeline of the main clinical events.
The patient did not present additional episodes of delirium and was successfully discharged 18 d after admission. He is currently undergoing regular follow-up at our psychiatric clinic.
We describe a complex case of lorazepam-induced delirium occurring in an elderly hospitalized patient who was admitted for food impaction. Treatment of delirium with haloperidol precipitated the onset of NMS, an uncommon, yet life-threatening, complication of antipsychotics. Early diagnosis and removal of precipitating agents are very important in the management of NMS. NMS was managed with dexme
Benzodiazepines are a common cause of drug-induced delirium. In the elderly, because of the decreased renal clearance and other age-related pharmacodynamic and pharmacokinetic changes, these drugs can accumulate and cause toxicity and delirium[10]. Our patient became delirious after in-hospital administration of lorazepam for insomnia. Although the mechanisms of benzodiazepine-induced delirium are not well defined, neurotransmitter imbalances with excess brain dopaminergic activity are common in delirious patients[10,11]. Importantly, while medications can induce delirium, they may also be used to manage its symptoms[10]. Owing to their antidopaminergic effects, typical antipsychotics like haloperidol are commonly used in managing delirium[3]. As in our patient, haloperidol can be given at multiple doses (2–5 mg every 15–30 min) until clinical improvement is achieved[12]. However, the use of haloperidol for managing delirium in our case precipitated the onset of NMS, possibly as a result of excess dopamine D2 receptor blockade in the hypothalamic, nigrostriatal, mesolimbic, and mesocortical pathways[13].
Once NMS is diagnosed, ICU drugs like dexmedetomidine or propofol are potentially effective management options[14,15]. Because extreme sympathetic nervous system activation is involved in the pathophysiology of NMS[7], we reasoned that dexmedetomidine may be helpful in the management of this medical emergency because of its sympatholytic activity[8]. In the presence of rapid shallow breathing as in our case, dexmedetomidine may also provide better patient comfort with less respiratory depression than does propofol[16]. Importantly, dexmedetomidine also suppresses the spontaneous firing rate of locus coeruleus neurons and decreases heat generation by alpha-2 adrenergic receptor blockade in the hypothalamus[17]. Hence, it can successfully correct the profound thermogenesis and hypermetabolism typical of NMS as it did in our patient. While recommendations for specific treatments in NMS continue to be based on small-sized studies and clinical experience, this case serves as a useful reminder to clinicians to consider dexmedetomidine as part of the current therapeutic armamentarium.
Dexmedetomidine may be clinically beneficial for managing NMS because of its sympatholytic activity and its capacity to reduce heat generation. In the presence of rapid shallow breathing, dexmedetomidine may also provide better patient comfort with less respiratory depression than does propofol.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan M, Song J S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Wang JL
| 1. | Rieck KM, Pagali S, Miller DM. Delirium in hospitalized older adults. Hosp Pract (1995). 2020;48:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Collier R. Hospital-induced delirium hits hard. CMAJ. 2012;184:23-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Shen YZ, Peng K, Zhang J, Meng XW, Ji FH. Effects of Haloperidol on Delirium in Adult Patients: A Systematic Review and Meta-Analysis. Med Princ Pract. 2018;27:250-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Pileggi DJ, Cook AM. Neuroleptic Malignant Syndrome. Ann Pharmacother. 2016;50:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Ware MR, Feller DB, Hall KL. Neuroleptic Malignant Syndrome: Diagnosis and Management. Prim Care Companion CNS Disord. 2018;20:0-0. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet. 2017;56:893-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 708] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 7. | Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent). 2001;14:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2147] [Cited by in RCA: 2407] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 10. | Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 199] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Ali S, Patel M, Jabeen S, Bailey RK, Patel T, Shahid M, Riley WJ, Arain A. Insight into delirium. Innov Clin Neurosci. 2011;8:25-34. [PubMed] |
| 12. | Wang EH, Mabasa VH, Loh GW, Ensom MH. Haloperidol dosing strategies in the treatment of delirium in the critically ill. Neurocrit Care. 2012;16:170-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Berman BD. Neuroleptic malignant syndrome: a review for neurohospitalists. Neurohospitalist. 2011;1:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Yu C, Kaul R, Ostwani W. Dexmedetomidine infusion as a novel supportive therapy for fluphenazine-induced neuroleptic malignant syndrome in a 10-year-old boy: a case report and review of literature. J Pediatr Neurol. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Sakamoto A, Hoshino T, Suzuki H, Kimura M, Ogawa R. Repeated propofol anesthesia for a patient with a history of neuroleptic malignant syndrome. Nihon Ika Daigaku Zasshi. 1999;66:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Tomar GS, Singh F, Ganguly S, Gaur N. Is dexmedetomidine better than propofol and fentanyl combination in minor day care procedures? Indian J Anaesth. 2015;59:359-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar S. Reduction in the incidence of shivering with perioperative dexmedetomidine: A randomized prospective study. J Anaesthesiol Clin Pharmacol. 2012;28:86-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |