Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.618
Peer-review started: March 23, 2021
First decision: September 28, 2021
Revised: October 10, 2021
Accepted: December 8, 2021
Article in press: December 8, 2021
Published online: January 14, 2022
Processing time: 294 Days and 22 Hours
The hereditary antithrombin (AT) deficiency caused by SERPINC1 gene mutation is an autosomal dominant thrombotic disorder. An increasing number of studies have shown that mutations in the SERPINC1 rs2227589 polymorphic site are correlated with a risk of venous thromboembolism (VTE) at common sites, such as lower extremity deep venous thrombosis and pulmonary thromboembolism. Currently, there are no reports of cerebral venous sinus thrombosis (CVST), a VTE site with a low incidence rate and rs2227589 polymorphism.
Here, we report a Chinese CVST case with a mutation of the SERPINC1 rs2227589 polymorphic site, which did not cause significant AT deficiency. In a 50-year-old male patient presenting with multiple cerebral venous sinus thromboses no predisposing factors were detected, although a relative had a history of lower extremity deep venous thrombosis. We performed sequencing of the SERPINC1 gene for the patient and his daughter, which revealed the same heterozygous mutation at the rs2227589 polymorphic site: c.41+141G>A.
The results showed that more studies should be conducted to assess the correlation between rs2227589 polymorphism and CVST.
Core Tip: The hereditary antithrombin (AT) deficiency caused by SERPINC1 gene mutation is an autosomal dominant thrombotic disorder. Currently, there are no reports on SERPINC1 rs2227589 polymorphism and cerebral venous sinus thrombosis (CVST). Here, we report for the first time a Chinese CVST case with a mutation of the SERPINC1 rs2227589 polymorphic site, which did not cause significant AT defi
- Citation: Liao F, Zeng JL, Pan JG, Ma J, Zhang ZJ, Lin ZJ, Lin LF, Chen YS, Ma XT. Patients with SERPINC1 rs2227589 polymorphism found to have multiple cerebral venous sinus thromboses despite a normal antithrombin level: A case report. World J Clin Cases 2022; 10(2): 618-624
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/618.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.618
Thrombophilia refers to a tendency for pathological venous or arterial thrombosis caused by interactions between multiple genetic and/or acquired susceptibility factors[1]. Venous thromboembolism (VTE) is a typical hereditary thromboembolism, which is common in lower extremity deep venous thrombosis (DVT) and pulmonary thromboembolism (PTE)[2]. Cerebral venous sinus thrombosis (CVST) is a rare occurrence of VTE, with an incidence rate at least 25-50 times lower than that observed in common sites of VTE[3]. Common genetic risk factors for venous thrombosis include coagulation factor V and coagulation factor II, protein C and protein S, as well as mutations in the antithrombin (AT) gene[4]. The polymorphism of antithrombin gene SERPINC1 rs2227589 has been studied mostly in Caucasian people, where its association with the risk of DVT and recurrent pregnancy loss (RPL) has been demonstrated[5-7]. Studies on Chinese people have also reported an association with familial DVT, PTE, and coronary heart disease. However, no reports on rs2227589 polymorphism and CVST currently exist, due to the rarity of the symptom.
As a member of the serine protease inhibitor superfamily, AT is the most important anticoagulant molecule in the body and is involved in regulating thrombin, factor Xa, and other clotting factors[8]. Hereditary AT deficiency is caused by various SERPINC1 gene mutations and is a thrombotic disease of autosomal dominant inheritance[9]. AT deficiency increases the risk of first-onset VTE by about 16-fold and recurrent VTE by about four-fold. Additionally, an increasing number of studies have found that even AT levels near the lower limit of its normal range may increase the risk of VTE significantly[10,11].
Here, on the other hand, we report a CVST case with a mutation of the SERPINC1 rs2227589 polymorphic site, which did not result in significant AT deficiency.
A 50-year-old male patient was admitted to the emergency room after experiencing headache pain for 10 d.
Headache for 10 d.
The patient did not have a history of hypertension, diabetes, hyperlipidemia, surgery, infection, liver or kidney dysfunction, smoking or drinking. He was married.
His grandfather had a history of venous thrombosis in the lower extremities.
The physical examination yielded the following results: body mass index = 21 kg/m2, with stable vital signs, and clear consciousness, The patient's ophthalmoscopy indicated the presence of optic papilledema, normal results for the rest of the cranial nerves and for muscle tension of the extremities, limb muscle strength was rated as grade 5 based on the Medical Research Council scale, normal depth of feeling, normal tendon reflex, and negative Babinski, Chaddock, and meningeal irritation signs. The patient’s Montreal Cognitive Assessment was 29.
The blood test results were as follows: D-dimer: 1430 g/L, prothrombin time: 16.3 s (reference range: 10.6-14.3 s), plasma partial thromboplastin time: 45.4 s (26.0-40.0 s), without obvious abnormalities on routine examinations of blood, urine, and feces, serum homocysteine concentration, anticardiolipin antibodies, rheumatic antineutrophil cytoplasmic antibodies, negative antinuclear antibodies, rheumatoid factors, thyroid function, erythrocyte sedimentation rate, and creatine kinase level.
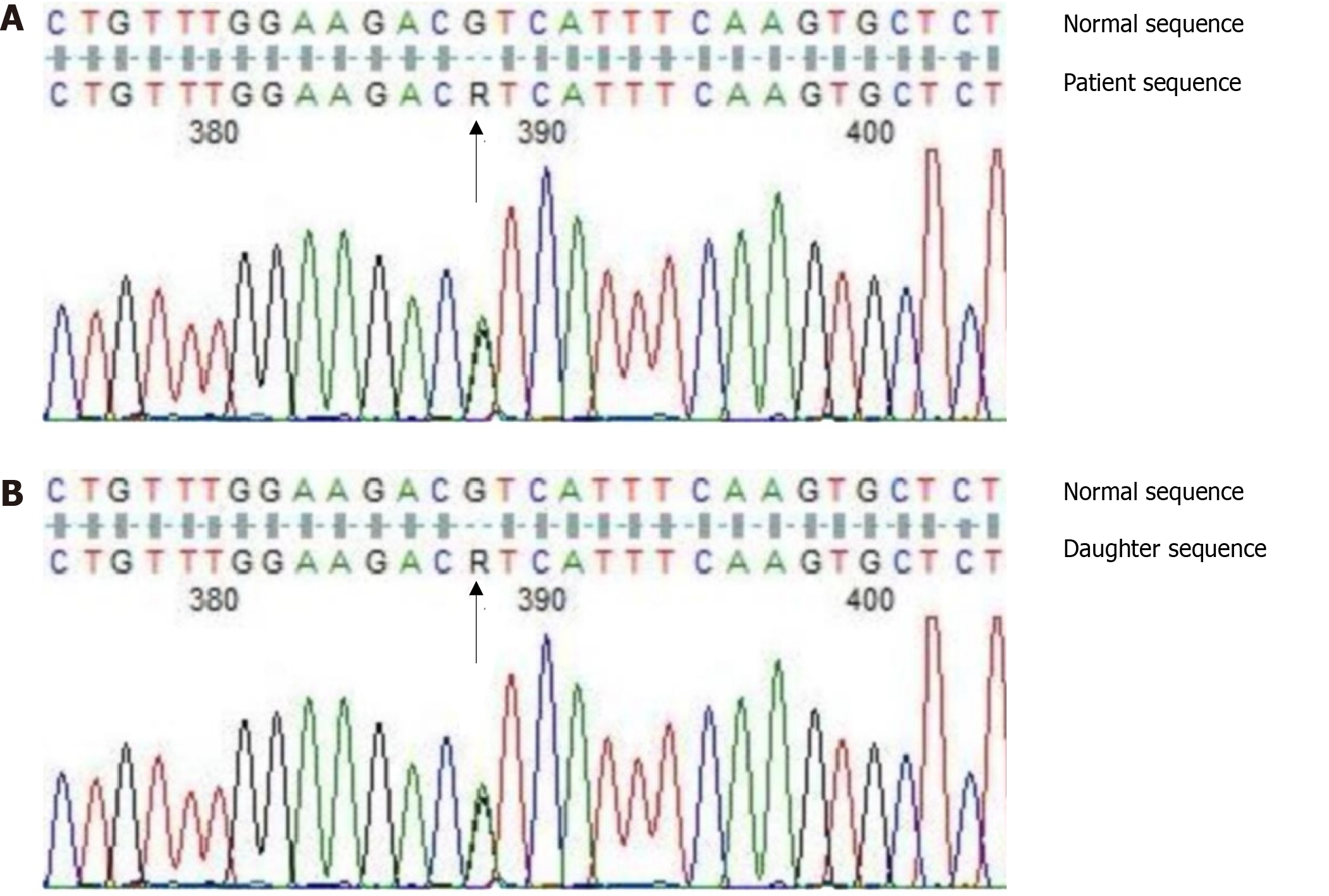
To determine the reason for CVST, coagulation tests were performed. The results were as follows: AT activity: 81.8% (reference range: 75%-125%), protein C: 52.8%, protein S: 31.9% (reference range: 70%-140%), and LA1/LA2 for preliminary screening/diagnosis of lupus: 1.16 (reference range: 0.8-1.2) (Table 1). Because the patient had taken warfarin for one week for anticoagulation the anticoagulant protein test was performed. Warfarin is known to reduce the content and activity of plasma protein C and protein S antigens[12]; hence, the drug was considered to be the reason for the observed decrease in activity of protein C and protein S. As the patient had no acquired risk factors for thrombophilia, such as surgery, immobilization, trauma, or infection, the possibility of hereditary thrombophilia was considered. Coagulation factor II, V, and SERPINC1 gene detection showed that the patient had no gene mutation of coagulation factor II and V, but had heterozygous mutations in the introns around Exon 1 (rs2227589 site) of SERPINC1 that encoded the AT gene: c.41+141G>A (Figure 1A).
| Subjects | Age | Clinical phenotype | AT activity, % | Protein C, % | Protein S, % | LA1/LA2 |
| Patient | 50 | 1 episode | 81.8 | 52.8 | 31.9 | 1.16 |
| Daughter | 27 | No thrombotic events | 90.1 | 104.6 | 56.4 | - |
Considering that the patient’s daughter was of childbearing age, we also conducted blood coagulation tests and SERPINC1 gene test for the patient's daughter, to determine whether thromboprophylaxis needed to be given during perinatal and contraceptive periods when the risk of VTE is increased. The results showed that her AT-III, protein C, and protein S were all normal (Table 1). SERPINC1 gene detection showed the same heterozygous mutations in the introns around Exon 1 (rs2227589 site) as her father: c.41+141G>A (Figure 1B).
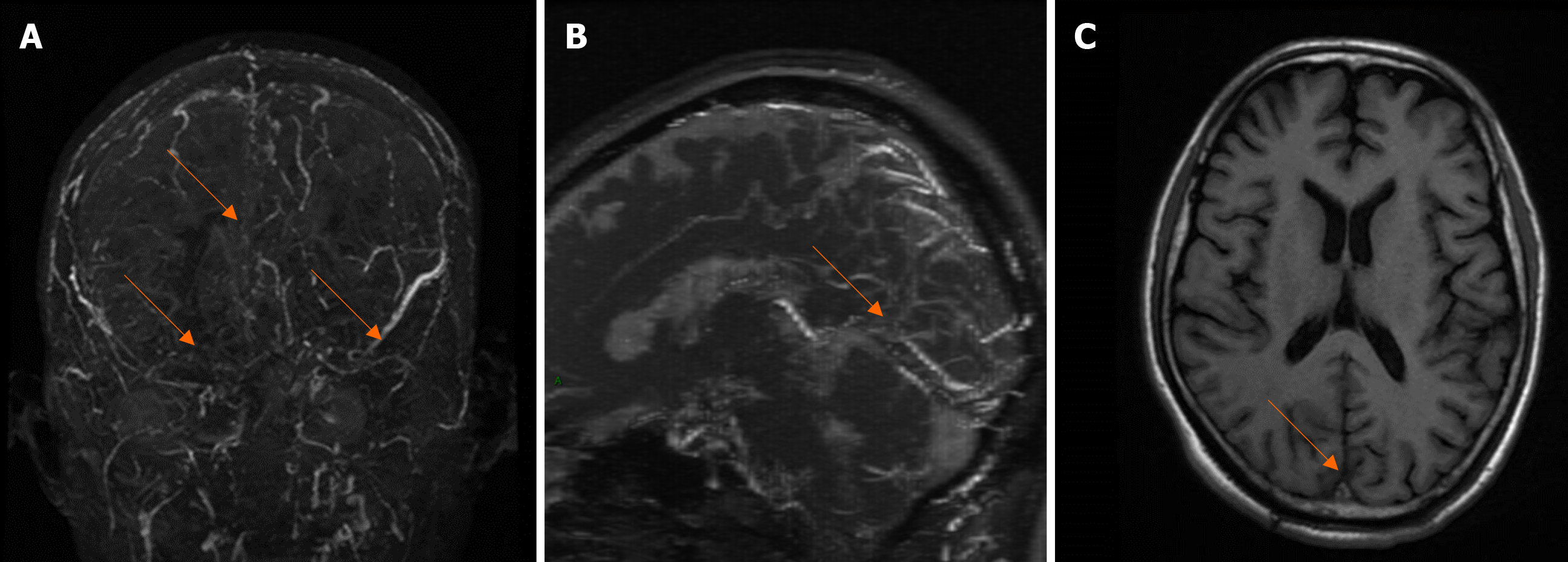
The patient had an unexplained acute headache, with nausea, vomiting and intracranial hypertension with fundus optic papilledema. He had a family history of venous thrombosis and was highly vigilant against CVST. Subsequently, a brain magnetic resonance imaging (MRI) scan was performed. As expected, cranial magnetic resonance venography (MRV) and MRI revealed several abnormal findings: filling defects were observed in the superior sagittal sinus, inferior sagittal sinus, straight sinus, torcular herophili, bilateral sigmoid sinus, and transverse sinus (Figure 2), which enabled a diagnosis of multiple CVST.
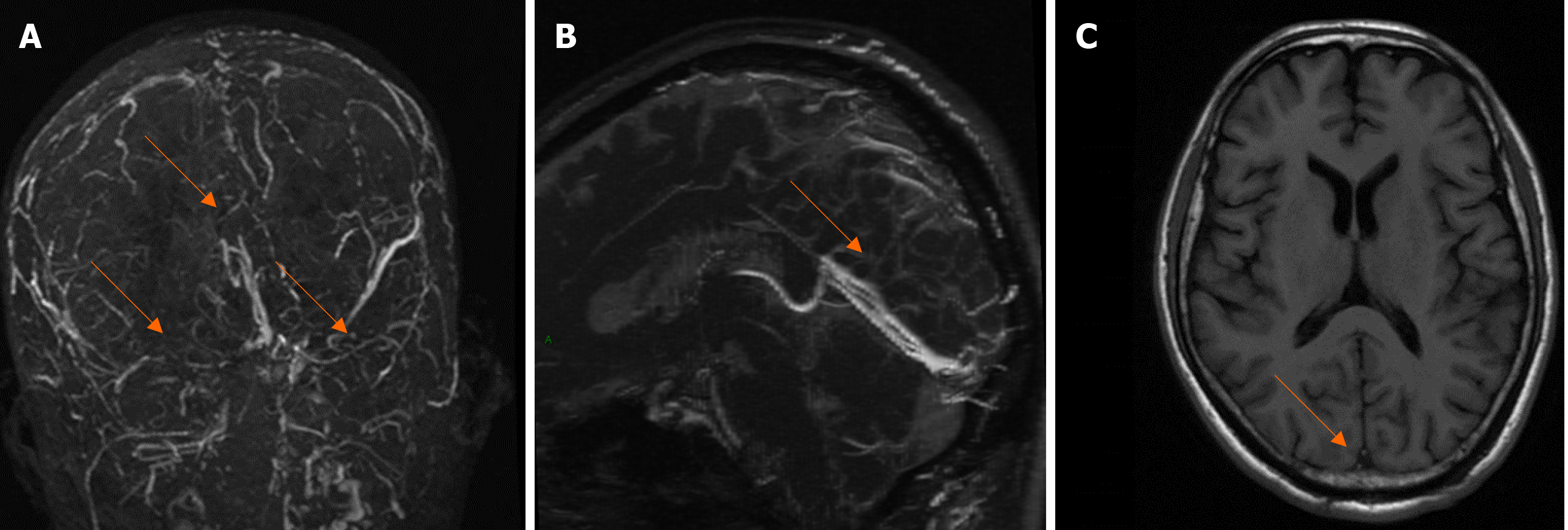
MRV and MRI examination 2 wk later showed that the inferior sagittal sinus and straight sinus were significantly clearer than that before treatment, while the superior sagittal sinus, bilateral sigmoid sinus, and transverse sinus were slightly fuller than that before treatment (Figure 3).
Cerebral venous sinus thromboses.
The treatment regimen included a subcutaneous injection of 4100U nadroparin calcium, q12h. The headache was relieved and relevant conditions were stable. After 3 d, warfarin was administered orally for anticoagulation at a dose of 2.5 mg, q.d., with the international normalized ratio (INR) maintained between 2.0 and 3.0.
MRV and MRI examination 2 wk later showed that the inferior sagittal sinus and straight sinus were significantly clearer than before treatment, while the superior sagittal sinus, bilateral sigmoid sinus, and transverse sinus were slightly fuller than before treatment.
Single nucleotide polymorphisms (SNPs) are the least complex mutations and are caused by the variation of a single nucleotide at the DNA level[13]. Although they usually have no effect on human health, they may affect the expression or stability of mRNA, resulting in medical impairment in 24% of cases when located at the transcription factor binding site or the non-translational region of the mRNA[7]. rs2227589 is an SNP site on introns near the AT gene SERPINC1 in Exon1. After evaluation of 19,682 SNP sites on 10887 genes in three controlled studies, Bezemer et al[7] first proposed that the rs2227589 polymorphism in the SERPINC1 gene was associated with DVT formation (OR: 1.29, 95%CI: 1.10-1.49). In a study of a normal Spanish cohort, Anton et al[14] confirmed that in SERPINC1 rs2227589 mutation carriers, AT activity (94.6 ± 8.4%) and levels (94.8 ± 5.6%) were also slightly reduced, possibly explaining the functional effect of the rs2227589 polymorphism. The correlation between the rs2227589 polymorphism and VTE risk has been shown to vary between ethnic groups. On the other hand, a systematic analysis of multiple groups by Yue et al[15] showed that rs2227589 and VTE were significantly correlated in the additive (OR: 1.09, 95%CI: 1.08-1.18) and dominant (OR:1.10, 95%CI: 1.01-1.20) genetic models. Therefore, we conclude that a mutation at the SERPINC1 rs2227589 site is a predisposing factor for CVST. The AT level was 81.8% (normal range: 75%-125%) in this patient. Although the AT level fell within the normal range, a controlled study on a large number of VTE cases stratified by AT level showed that an AT level around the lower limit of its normal range (76%-85%) increases the risk of VTE by two-fold[11]. We think that the increase in risk contributed to the patient’s CVST.
The latest European Academy of Neurology - European Stroke Organization guide for treatment of adult patients with CVST suggested that relevant patients without contraindications should be given anticoagulation treatment as soon as possible, with low molecular weight heparin given in the acute phase, and then warfarin administered orally for further anticoagulation according to an INR controlled within 2.0-3.0. The duration of treatment depends on thrombophilia and the risk of its recurrence[16]. Based on the medical history and relevant examination results of this patient, we first adopted 3 d of treatment with Nadroparin calcium during hospitalization, and then changed to oral warfarin for further anticoagulation. The dose was gradually increased, with the INR monitored and controlled between 2.0 and 3.0. A head MRI reexamination 2 wk later indicated partial thrombolysis and significant relief of headache symptoms. Therefore, the treatment was effective.
For the patient’s daughter, the AT level was 90.1%. Relevant studies have confirmed that the AT level is negatively correlated with age[17], and the risk for first-onset venous thrombosis may increase with a decrease in AT levels[10]. We speculate, therefore, that the asymptomatic condition of the patient’s daughter may be due to a relatively high AT level and low risk of VTE, and relatively old age expected for her first-onset VTE.
Prevention of VTE during pregnancy among women with genetic risk factors for thromboembolism is a challenge. The American College of Obstetricians and Gynecologists (ACOG) guide (2018) indicates the likelihood of synergistic effects between homozygous FVL, compound heterozygous FVL and PT20210A, AT deficiency, and high estrogen status, which are high genetic risk factors for thrombophilia[18]. The patient’s daughter was a rs2227589 polymorphic site mutation carrier without serious AT defects at this stage. Considering that existing data on rs2227589 research are limited, we consider similar conditions to be low risk for hereditary thrombophilia. For these patients, with first-degree relatives who have a family history of VTE, the ACOG recommends only prenatal monitoring without anticoagulant therapy or prophylactic use of heparin and, after delivery, the use of anticoagulant therapy or moderate heparin dosage to prevent thrombosis. Estrogen-containing drugs may increase the risk of VTE[19]. We recommend the use of a condom for contraception for the patient’s daughter if necessary.
To sum up, most studies of rs2227589 polymorphisms have investigated the risk of VTE at common sites of VTE, but have not studied the risk of rs2227589 polymorphism for CVST due to the rarity of CVST at the more common VTE sites. We report a case of CVST case with a mutation of SERPINC1 at the rs2227589 polymorphic site, which did not exhibit significant AT deficiency. Therefore, further studies are needed to confirm the correlation between rs2227589 polymorphism and CVST, as well as ongoing exploration to identify new genetic risk factors related to CVST. In addition, the mutation of the rs2227589 special site only led to a slight decrease in AT level in this patient, suggesting that serious CVST can still occur even when the AT level is in the normal range. This report illustrates that when severe CVST occurs and common reasons for thrombosis are not identified, the possibility of rs2227589 polymorphism site variation should be considered if the AT level is slightly lower but still in the normal range.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Watanabe A S-Editor: Wang LL L-Editor: Webster JR P-Editor: Wang LL
| 1. | Stevens SM, Woller SC, Bauer KA, Kasthuri R, Cushman M, Streiff M, Lim W, Douketis JD. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 2. | Colucci G, Tsakiris DA. Thrombophilia Screening: Universal, Selected, or Neither? Clin Appl Thromb Hemost. 2017;23:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Riva N, Ageno W. Cerebral and Splanchnic Vein Thrombosis: Advances, Challenges, and Unanswered Questions. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Rosendaal FR. Causes of venous thrombosis. Thromb J. 2016;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Bhakuni T, Sharma A, Rashid Q, Kapil C, Saxena R, Mahapatra M, Jairajpuri MA. Antithrombin III deficiency in Indian patients with deep vein thrombosis: identification of first India based AT variants including a novel point mutation (T280A) that leads to aggregation. PLoS One. 2015;10:e0121889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Guerra-Shinohara EM, Bertinato JF, Tosin Bueno C, Cordeiro da Silva K, Burlacchini de Carvalho MH, Pulcineli Vieira Francisco R, Zugaib M, Cerda A, Morelli VM. Polymorphisms in antithrombin and in tissue factor pathway inhibitor genes are associated with recurrent pregnancy loss. Thromb Haemost. 2012;108:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Bezemer ID, Bare LA, Doggen CJ, Arellano AR, Tong C, Rowland CM, Catanese J, Young BA, Reitsma PH, Devlin JJ, Rosendaal FR. Gene variants associated with deep vein thrombosis. JAMA. 2008;299:1306-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Quinsey NS, Greedy AL, Bottomley SP, Whisstock JC, Pike RN. Antithrombin: in control of coagulation. Int J Biochem Cell Biol. 2004;36:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Sekiya A, Taniguchi F, Yamaguchi D, Kamijima S, Kaneko S, Katsu S, Hanamura M, Takata M, Nakano H, Asakura H, Ohtake S, Morishita E. Causative genetic mutations for antithrombin deficiency and their clinical background among Japanese patients. Int J Hematol. 2017;105:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Di Minno MN, Dentali F, Veglia F, Russolillo A, Tremoli E, Ageno W. Antithrombin levels and the risk of a first episode of venous thromboembolism: a case-control study. Thromb Haemost. 2013;109:167-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Bucciarelli P, Passamonti SM, Biguzzi E, Gianniello F, Franchi F, Mannucci PM, Martinelli I. Low borderline plasma levels of antithrombin, protein C and protein S are risk factors for venous thromboembolism. J Thromb Haemost. 2012;10:1783-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | MacCallum P, Bowles L, Keeling D. Diagnosis and management of heritable thrombophilias. BMJ. 2014;349:g4387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Shastry BS. SNPs in disease gene mapping, medicinal drug development and evolution. J Hum Genet. 2007;52:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Antón AI, Teruel R, Corral J, Miñano A, Martínez-Martínez I, Ordóñez A, Vicente V, Sánchez-Vega B. Functional consequences of the prothrombotic SERPINC1 rs2227589 polymorphism on antithrombin levels. Haematologica. 2009;94:589-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Yue YJ, Sun Q, Xiao L, Liu SG, Huang QJ, Wang ML, Huo M, Yang M, Fu YY. Association of SERPINC1 Gene Polymorphism (rs2227589) With Pulmonary Embolism Risk in a Chinese Population. Front Genet. 2019;10:844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F, di Minno M, Maino A, Martinelli I, Masuhr F, Aguiar de Sousa D, Stam J. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the European Academy of Neurology. Eur Stroke J. 2017;24:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 396] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 17. | Amin H, Mohsin S, Aslam M, Hussain S, Saeed T, Ullah MI, Sami W. Coagulation factors and antithrombin levels in young and elderly subjects in Pakistani population. Blood Coagul Fibrinolysis. 2012;23:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | American College of Obstetricians and Gynecologists' Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 197: Inherited Thrombophilias in Pregnancy. Obstet Gynecol. 2018;132:e18-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 19. | Gialeraki A, Valsami S, Pittaras T, Panayiotakopoulos G, Politou M. Oral Contraceptives and HRT Risk of Thrombosis. Clin Appl Thromb Hemost. 2018;24:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |