Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.585
Peer-review started: June 18, 2021
First decision: September 28, 2021
Revised: October 14, 2021
Accepted: December 2, 2021
Article in press: December 2, 2021
Published online: January 14, 2022
Processing time: 207 Days and 18.7 Hours
Argatroban is a novel direct thrombin inhibitor that has been used for treatment of acute ischemic stroke (AIS). To our knowledge, no systematic analysis has assessed the efficacy and safety of argatroban for treatment of AIS.
To evaluate the efficacy and safety of argatroban for treatment of AIS.
Cochrane Library, Medline, PubMed, and Web of Science were searched to retrieve all studies associated with argatroban and AIS. Effective rate, adverse events rate, and 95% confidence intervals were calculated and pooled using meta-analysis methodology.
We only found four randomized controlled studies, comprising 354 cases with 213 in the argatroban group and 141 in the control group. Great heterogeneity was found in the four studies (c2 = 11.44, I2 = 74%, P = 0.01). Subgroup analysis could not be performed because of the absence of detailed data. The two most recent studies showed acceptable heterogeneity (c2 = 1.56, I2 = 36%, P = 0.21). Our analysis showed that argatroban was not more effective than the control therapy in the acute phase of ischemic stroke (Z = 0.01, P = 0.99). Argatroban did not increase the risk of bleeding compared with the control group (c2 = 0.37, I2 = 0%, P = 0.54, Z = 0.80, P = 0.42).
Patients with AIS might not benefit from argatroban and combination therapy with argatroban does not increase bleeding tendency.
Core Tip: This study is the first meta-analysis that systematically assessed the efficacy and safety of argatroban as a cure for acute ischemic stroke (AIS). The results showed that argatroban might not benefit for AIS. Also, this meta-analysis further suggested that argatroban does not increase the risk of bleeding for AIS.
- Citation: Lv B, Guo FF, Lin JC, Jing F. Efficacy and safety of argatroban in treatment of acute ischemic stroke: A meta-analysis. World J Clin Cases 2022; 10(2): 585-593
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/585.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.585
Acute ischemic stroke (AIS) is the most common type of cerebrovascular disease. Ischemic stroke (IS) is the leading cause of adult disability and has the second-highest fatality rate in the world[1]. Still, morbidity and mortality have shown a growing trend in recent years[2]. Evidence suggests that only aspirin and recombinant tissue-type plasminogen activator (r-tPA) have a definite curative effect on the acute phase of IS (Class A evidence, level I recommendation). The efficacy of other drugs is still lacking evidence-based support. Anticoagulant therapy has always been a focus in the field of AIS, but the results were controversial. Although anticoagulant therapy can reduce the recurrence of IS and the incidence of pulmonary embolism and deep vein thrombosis, its effect on the mortality and disability rate of IS is still unknown[3]. Also, anticoagulation increases the incidence of intracranial hemorrhage (ICH)[4]. So, traditionally used anticoagulant drugs, such as heparin, low molecular weight heparin, and warfarin are not recommended for AIS treatment. Argatroban is a novel, small-molecule, direct thrombin inhibitor. It exerts its anticoagulant function by binding with thrombin, not only in the state of dissolution but also in blood clotting[5]. It has been mainly proved for the treatment of thrombosis caused by heparin-induced thrombocytopenia. There is a growing body of evidence on the safety and efficiency of argatroban therapy for AIS[6-8]. In Japan and South Korea, argatroban therapy is also used in ischemic diseases including myocardial and cerebral ischemia[9]. However, there is still a lack of evidence for its efficacy and safety. To provide more reliable evidence for clinical practice, we conducted a Cochrane Collaboration systematic review that included the randomized controlled studies on AIS treatment using argatroban.
A search of PubMed, Embase, Science Citation Index, Medline, and Cochrane Library was performed up to October 2020. The search was conducted using medical subject headings and keywords including “argatroban”, “4-methyl-1-(N(2)-(3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl)-L-arginyl)-2-piperidinecarboxylic acid”, “cerebral infarction”, “ischemic stroke”, “cerebrovascular disorder”, and “cerebrovascular accident”. Meanwhile, we retrieved references listed in studies and reviews researched from the online databases to obtain relevant data.
We only enrolled randomized controlled studies that assessed the efficacy and safety of argatroban in treating AIS. All the studies were in English and published as full articles. Case reports, reviews, commentaries, editorials, and studies written in abstract form or published repeatedly were excluded to prevent homogeneity. The methodological quality of the included studies was assessed using the risk assessment tool for RCT bias in the Cochrane Systematic Reviewers’ Handbook.
The outcome and adverse effects were calculated from the data provided by the researchers. Validity and adverse effect assessment were based on the information synthesized from the studies. Validity mainly referred to therapeutic effect, assessed by neurological function scores. Adverse effects mainly referred to bleeding.
Relative risk ratio (RR) and 95% confidence interval (CI) were used as effect analysis statistics for categorical data. Efficiency and safety were calculated for all of the studies that were identified for the meta-analysis, and the results were combined using fixed- or random-effects modeling. Statistical heterogeneity was assessed using χ2 tests (P < 0.05 indicated statistical significance) and I2 tests (P <0.05, I2 > 50% indicated significant heterogeneity; P > 0.05, I2 < 50% indicated insignificant heterogeneity). The fixed-effects model was used if there was no statistical heterogeneity, otherwise, the random-effects model was used. Subgroup analyses were conducted for further investigation. Meta-analysis was conducted using RevMan version 5.4 (Cochrane collaboration), and P < 0.05 was defined as statistically significant.
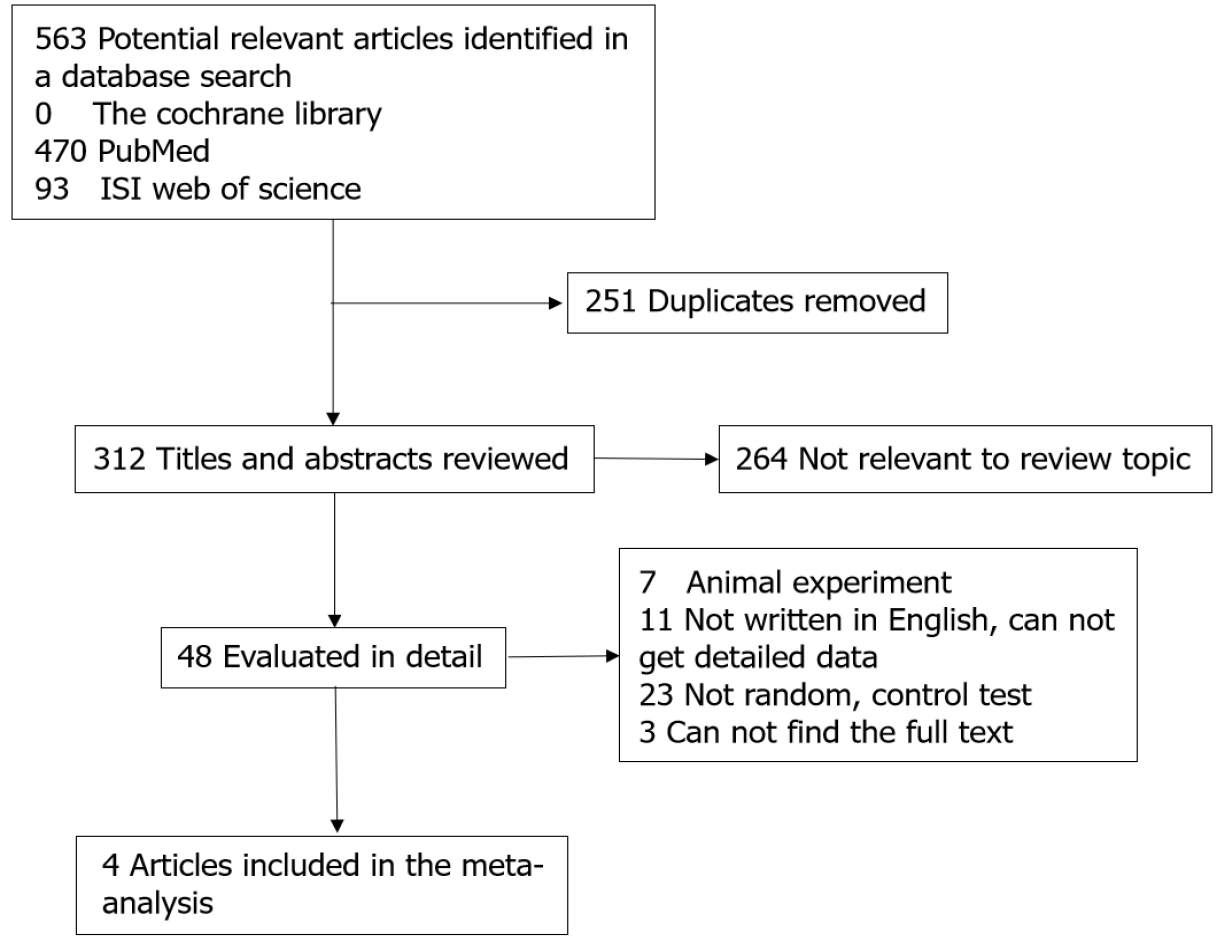
A total of 412 relevant studies were retrieved, and 408 were excluded because of duplication or failure to meet the inclusion criteria. Finally, four trials were included in our study[6,10-12]. The studies included 354 cases with 213 in the argatroban group and 141 in the control group. The literature screening process and results are showed in Figure 1. Two of the studies were conducted in North America and two in Japan. Three studies were multicenter and one was single center. The main characteristics of the included studies are presented in Table 1.
| Title | Country | Multiple/single center | Date | Argatroban/control |
| Thrombin inhibition in the acute phase of IS using argatroban | Japan | Single center | 1995 | 7/6 |
| Effect of the argatroban in acute cerebral thrombosis | Japan | Multicenter | 1997 | 59/59 |
| Argatroban in patients with acuteischemic stroke | United States | Multicenter | 2004 | 86/47 |
| Randomized, multicenter trial of ARTSS-2 (Argatroban with Recombinant Tissue Plasminogen Activator for Acute Stroke) | United States | Multicenter | 2017 | 61/29 |
All four studies used improvement of neurological deficits to assess the efficiency of argatroban. The National Institutes of Health Stroke Scale, Modified Rankin Scale, Barthel Index, and activity in daily living were used in three studies. The evaluation method was not described in the other study[10]. Although there was no uniform standard, all the enrolled studies reported the effective rate of nerve function improvement, which was used to assess the efficacy of argatroban in the treatment of AIS. Three studies[6,11,12] ICH or major bleeding as an adverse reaction, which was not found in the fourth study[10].
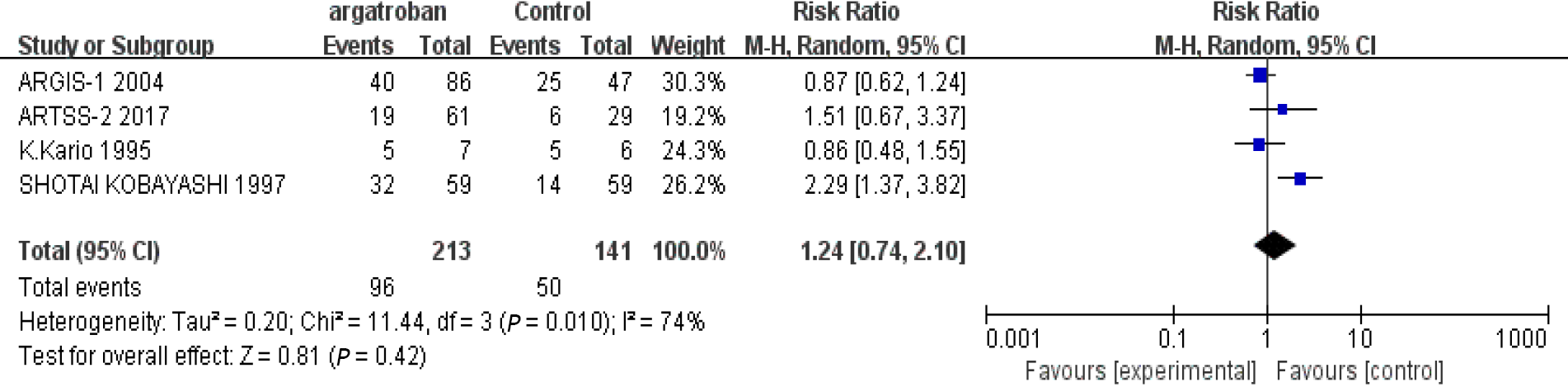
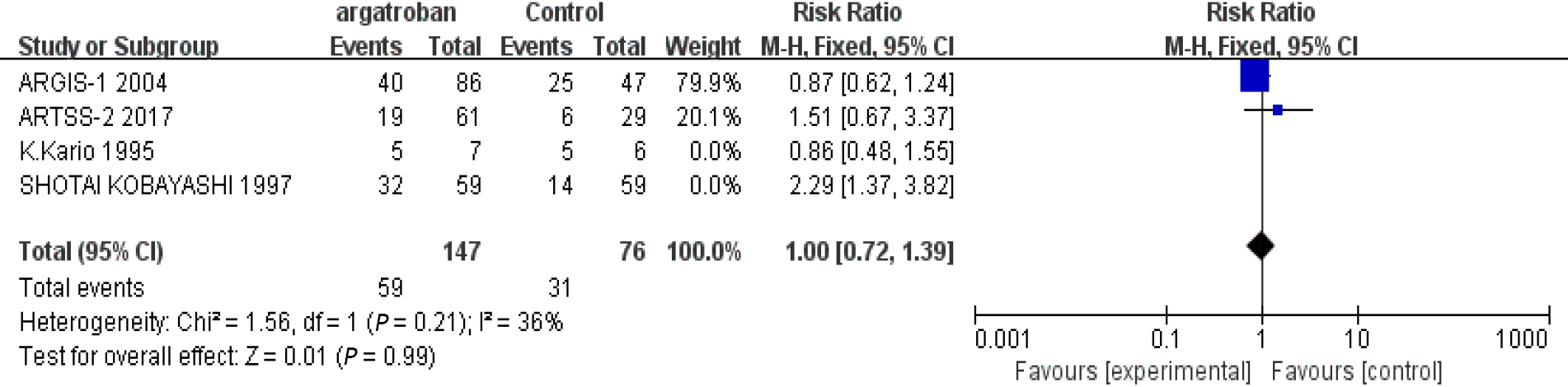
We performed a meta-analysis of the four studies mentioned above. The efficacy of argatroban was controversial. Two studies reported superior improvements in the argatroban group than in the control group[6,11]. However, the other two studies did not find definitive effectiveness of argatroban in the treatment of AIS compared with the control groups[10,12]. The P value of heterogeneity among the studies was significant (P < 0.05, I2 = 74%), so the random-effects model was used for the analysis. The result showed that the overall effect was not significant (RR = 1.24; 95%CI: 0.74–2.10; P = 0.42) (Figure 2). Since there was considerable heterogeneity among the four studies, the result was not reliable. The nonconformity of enrollment criteria and result evaluation might have been the cause of the heterogeneity. We found that the inclusion and assessment criteria of two studies[6,12] performed in recent years were in good coincidence. Therefore, we only analyzed the results of these two studies. The results showed that the heterogeneity was insignificant (P = 0.21, I2 = 36%). And the fixed-effects model was used for the analysis. The overall effect was also not significant (RR = 1.0; 95%CI: 0.72–1.39; P = 0.99) (Figure 3). The existing research results do not support the efficacy of argatroban in treating AIS.
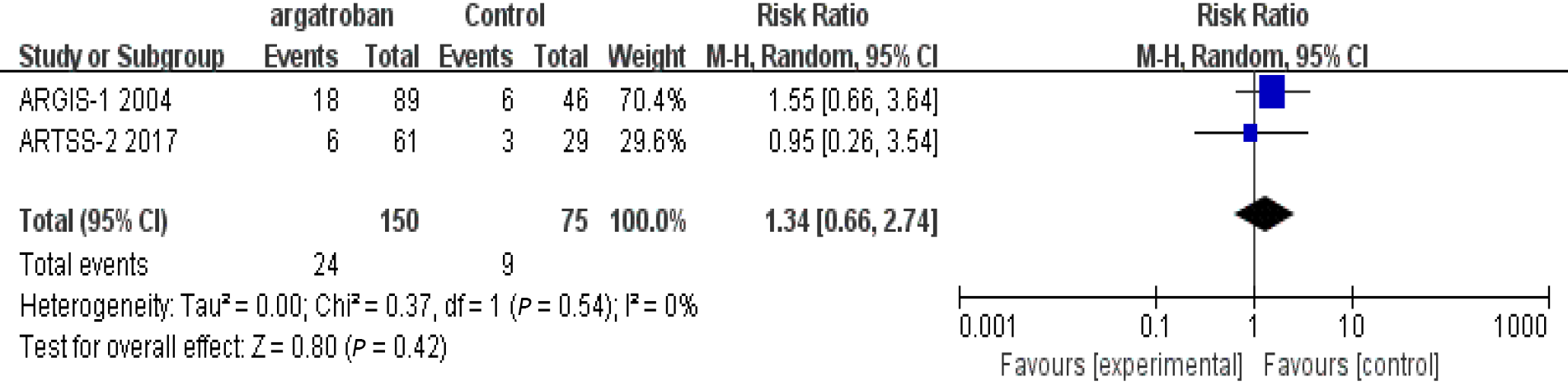
In the three studies that assessed adverse reactions, none of them found that argatroban increased the risk of bleeding. Detailed data were not provided by Kobayashi et al[11], so only the studies of Barreto et al[6] and LaMonte et al[12] were included in our analysis. The heterogeneity of the two studies was insignificant (P = 0.54, I2 = 0%) and the fixed-effects model was used. The overall analysis showed that there was no significant difference between the argatroban and control groups (RR = 1.34; 95%CI: 0.66–2.74; P = 0.42) (Figure 4). The results indicated that argatroban does not increase the risk of bleeding in AIS. In all the four studies, there was no gender difference between the argatroban and control groups (P > 0.05). The safety and efficacy of argatroban were not assessed according to gender. Therefore, the impact of gender on the safety and efficacy of argatroban cannot be evaluated. We analyzed the impact of patient age. In the studies of Barreto et al[6], Kari et al[10], and Kobayashi et al[11], the mean age of different groups was described but comparisons were not made. In LaMonte et al[12]’s study, there was an age difference between the arga
Although the results of this meta-analysis suggested that argatroban did not increase the risk of ICH in the acute phase of cerebral infarction, it failed to show the advantage of argatroban over other drugs in the treatment of AIS. Anticoagulants have been used to treat AIS for > 70 years[13]. The use of anticoagulants for prevention and treatment of IS is still controversial. Anticoagulants are effective in preventing recurrence of cerebral infarction but can also cause bleeding. So far, there is no evidence to support short or long-term benefit of anticoagulants for patients with AIS[14], and more evidence-based data are needed.
Argatroban is a small-molecule thrombin inhibitor that was first synthesized by Japanese scientists[15]. It can inhibit coagulation by interacting with the catalytic site of thrombin reversibly[16]. Compared with other anticoagulant drugs, argatroban has some advantages. First, argatroban can penetrate and inhibit thrombin effectively despite the fibrin barrier, benefiting from its small molecular size. That means that argatroban has a therapeutic effect on more organized thrombi[5]. Second, argatroban acts quickly. Normally, it can reach steady-state plasma levels in 1–3 h after intravenous administration. Besides, the dose–response curve of argatroban is steady and predictable, which means that it has a wide margin of safety of dose titration[17,18]. Third, argatroban is metabolized rapidly in the liver. The elimination half-life is 39–51 min and is mostly affected by hepatic function, despite age, gender, and renal function[19]. Although there is no specific antidote, the coagulation parameters generally return to normal within 2–4 h after withdrawal of argatroban, as long as liver function is normal[20]. Also, its pharmacological mechanism is selective and it hardly influences other serine proteases.
At present, argatroban is mainly used to treat heparin-induced thrombocytopenia[21]. In Japan and Korea, it has also been used to treat AIS[19]. Several reports have shown that argatroban is effective in treating AIS. Compared with high-dose aspirin (300 mg daily), argatroban plus standard-dose aspirin (100 mg daily) was as effective and safe for the treatment of moderate AIS[22]. Several single-center, nonrandomized, controlled studies have found that argatroban is effective for treating AIS[23-25]. However, the number of patients enrolled in the published studies was small and the studies were all carried out in Asia. In addition to the anticoagulant effect, some studies have shown that argatroban can improve ischemic symptoms by ameliorating cerebral blood flow in patients with AIS[26,27]. However, the number of studies is small and evidence-based medicine is insufficient. Therefore, these findings cannot be extrapolated to clinical application.
The efficiency and safety of argatroban in treating cardiogenic and non-cardioem
As far as we know, this study is the first systematic review of the safety and efficacy of argatroban for treatment of AIS. We only analyzed four studies. We found considerable heterogeneity among the studies. Clinical heterogeneity might occur for many reasons, such as geographic region, racial difference in severity of initial symptoms, and interference with other treatment. The four studies were not designed to the same standard, which may have caused heterogeneity. Also, subgroup analysis was not possible because of the absence of detailed data. Although we did not find evidence supporting the efficacy of argatroban for treatment of AIS, there were some short
Patients with AIS might not benefit from argatroban and combination therapy with argatroban does not increase bleeding tendency.
Acute ischemic stroke (AIS) has been a global health challenge. And new treatments have been explored. Argatroban as a novel direct thrombin inhibitor has been used in treating AIS. However, the exact efficiency and safety remain unclear.
The drug safety of argatroban has been proved by many studies. However, the results of present studies on evaluating curative effect of argatroban on AIS were quite controversial, which has puzzled us in confirming the role of argatroban in AIS treatment. Therefore, it is necessary to do such an analysis to further evaluate the efficiency and safety of argatroban in treating AIS.
The objective of this study was to evaluate the efficiency and safety of argatroban in treating AIS by extracting available data from existing studies.
We have searched database PubMed, Embase, Science, Medline, and Cochrane Library to retrieve all of the studies associated with argatroban and AIS. Only randomized controlled clinical studies were screened for this review. Meta-analysis methodology was used and the standard mean difference values and 95% confidence intervals were estimated to get final results.
Only four studies that met the criteria were included in our review, which contained a total of 354 cases with 213 cases in the argatroban group and 141 in the control group. The overall analysis showed that patients with AIS did not improve more with argatroban treatment. And argatroban did not increase the bleeding risk in AIS patients.
Our study that integrated the existing data suggested that patients with AIS might not benefit more from argatroban and combination therapy with argatroban will not increase bleeding tendency.
More high-quality studies are needed for further evaluation of the efficacy and safety of argatroban in treating AIS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciarambino T S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Thrift AG, Howard G, Cadilhac DA, Howard VJ, Rothwell PM, Thayabaranathan T, Feigin VL, Norrving B, Donnan GA. Global stroke statistics: An update of mortality data from countries using a broad code of "cerebrovascular diseases". Int J Stroke. 2017;12:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Thrift AG, Thayabaranathan T, Howard G, Howard VJ, Rothwell PM, Feigin VL, Norrving B, Donnan GA, Cadilhac DA. Global stroke statistics. Int J Stroke. 2017;12:13-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 293] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 3. | Geeganage CM, Sprigg N, Bath MW, Bath PM. Balance of symptomatic pulmonary embolism and symptomatic intracerebral hemorrhage with low-dose anticoagulation in recent ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. J Stroke Cerebrovasc Dis. 2013;22:1018-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Kelly AG, Holloway RG. Guideline: The AHA/ASA made 217 recommendations for early management of acute ischemic stroke in adults. Ann Intern Med. 2018;168:JC63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Jeske WP, Fareed J, Hoppensteadt DA, Lewis B, Walenga JM. Pharmacology of argatroban. Expert Rev Hematol. 2010;3:527-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Barreto AD, Ford GA, Shen L, Pedroza C, Tyson J, Cai C, Rahbar MH, Grotta JC; ARTSS-2 Investigators. Randomized, Multicenter Trial of ARTSS-2 (Argatroban With Recombinant Tissue Plasminogen Activator for Acute Stroke). Stroke. 2017;48:1608-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Berekashvili K, Soomro J, Shen L, Misra V, Chen PR, Blackburn S, Dannenbaum M, Grotta JC, Barreto AD. Safety and Feasibility of Argatroban, Recombinant Tissue Plasminogen Activator, and Intra-Arterial Therapy in Stroke (ARTSS-IA Study). J Stroke Cerebrovasc Dis. 2018;27:3647-3651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 8. | Yang Y, Zhou Z, Pan Y, Chen H, Wang Y; ARAIS Protocol Steering Group. Randomized trial of argatroban plus recombinant tissue-type plasminogen activator for acute ischemic stroke (ARAIS): Rationale and design. Am Heart J. 2020;225:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Sanchez R, Picard N, Mouly-Bandini A, Chalvignac V, Lacarelle B, Sampol-Manos E. Severe decrease of cyclosporine levels in a heart transplant recipient receiving the direct thrombin inhibitor argatroban. Ther Drug Monit. 2014;36:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Kario K, Kodama K, Koide M, Matsuo T. Thrombin inhibition in the acute phase of ischaemic stroke using argatroban. Blood Coagul Fibrinolysis. 1995;6:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 11. | Kobayashi S, Tazaki Y. Effect of the thrombin inhibitor argatroban in acute cerebral thrombosis. Semin Thromb Hemost. 1997;23:531-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 12. | LaMonte MP, Nash ML, Wang DZ, Woolfenden AR, Schultz J, Hursting MJ, Brown PM; ARGIS-1 Investigators. Argatroban anticoagulation in patients with acute ischemic stroke (ARGIS-1): a randomized, placebo-controlled safety study. Stroke. 2004;35:1677-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 13. | Gubitz G, Counsell C, Sandercock P, Signorini D. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2000;CD000024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Wang X, Ouyang M, Yang J, Song L, Yang M, Anderson CS. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2021;10:CD000024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Okamoto S, Hijikata-Okunomiya A. Synthetic selective inhibitors of thrombin. Methods Enzymol. 1993;222:328-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Yeh RW, Jang IK. Argatroban: update. Am Heart J. 2006;151:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Swan SK, Hursting MJ. The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy. 2000;20:318-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 269] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Escolar G, Bozzo J, Maragall S. Argatroban: a direct thrombin inhibitor with reliable and predictable anticoagulant actions. Drugs Today (Barc). 2006;42:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Walenga JM. An overview of the direct thrombin inhibitor argatroban. Pathophysiol Haemost Thromb. 2002;32 Suppl 3:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Swan SK, St Peter JV, Lambrecht LJ, Hursting MJ. Comparison of anticoagulant effects and safety of argatroban and heparin in healthy subjects. Pharmacotherapy. 2000;20:756-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Prince M, Wenham T. Heparin-induced thrombocytopaenia. Postgrad Med J. 2018;94:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Chen L, Cao S, Yang J. Argatroban plus aspirin versus aspirin in acute ischemic stroke. Neurol Res. 2018;40:862-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Urabe T, Tanaka R, Noda K, Mizuno Y. Anticoagulant therapy with a selective thrombin inhibitor for acute cerebral infarction: usefulness of coagulation markers for evaluation of efficacy. J Thromb Thrombolysis. 2002;13:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Park JS, Park SS, Koh EJ, Eun JP, Choi HY. Treatment for Patients with Acute Ischemic Stroke Presenting beyond Six Hours of Ischemic Symptom Onset : Effectiveness of Intravenous Direct Thrombin Inhibitor, Argatroban. J Korean Neurosurg Soc. 2010;47:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Liu S, Liu P, Wang P, Zhang F, Wang L, Wang Y, Lu H, Ma X. Argatroban Increased the Basal Vein Drainage and Improved Outcomes in Acute Paraventricular Ischemic Stroke Patients. Med Sci Monit. 2020;26:e924593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Yamashita T, Hayashida O, Nagamitsu T, Nagatsuna T, Wakuta Y, Fudaba H. The regional cerebral blood flow amelioration of argatroban in the acute stage of cerebral thrombosis. Keio J Med. 2000;49 Suppl 1:A141-A144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Maruki Y, Onoda A, Matsuzaki M, Narabayashi Y, Sawada M, Shimazu K. A specific thrombin inhibitor (argatroban) ameliorated cerebral blood flow in the patients with acute cerebral infarction. Keio J Med. 2000;49 Suppl 1:A138-A140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 28. | Hosomi N, Naya T, Kohno M, Kobayashi S, Koziol JA; Japan Standard Stroke Registry Study Group. Efficacy of anti-coagulant treatment with argatroban on cardioembolic stroke. J Neurol. 2007;254:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Wada T, Yasunaga H, Horiguchi H, Matsubara T, Fushimi K, Nakajima S, Yahagi N. Outcomes of Argatroban Treatment in Patients With Atherothrombotic Stroke: Observational Nationwide Study in Japan. Stroke. 2016;47:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Oguro H, Mitaki S, Takayoshi H, Abe S, Onoda K, Yamaguchi S. Retrospective Analysis of Argatroban in 353 Patients with Acute Noncardioembolic Stroke. J Stroke Cerebrovasc Dis. 2018;27:2175-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Chen S, Cai D, Huang P, Liu J, Lai Y, He J, Zhou L, Sun H. Early and long-term outcomes of argatroban use in patients with acute noncardioembolic stroke. Clin Neurol Neurosurg. 2020;198:106233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Kern R, Nagayama M, Toyoda K, Steiner T, Hennerici MG, Shinohara Y. Comparison of the European and Japanese guidelines for the management of ischemic stroke. Cerebrovasc Dis. 2013;35:402-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |