Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.492
Peer-review started: September 16, 2021
First decision: October 18, 2021
Revised: October 19, 2021
Accepted: December 3, 2021
Article in press: December 3, 2021
Published online: January 14, 2022
Processing time: 117 Days and 16.9 Hours
Surgery for thyroid carcinoma offers a good prognosis; however, cervical lymph node metastasis may occur in the early stage. An effective diagnostic method can accurately guide clinical surgical planning and the scope of lymph node dissection, ultimately improving patient prognosis.
To explore the diagnostic value of fine-needle aspiration of thyroglobulin (FNA-Tg) combined with ultrasound (US)-guided fine-needle aspiration cytology for cervical lymph node metastasis in thyroid carcinoma.
We enrolled 209 pathologically confirmed thyroid carcinoma patients who visited our hospital between Jan 2017 and Dec 2020. Patients were tentatively diagnosed with cervical lymph node enlargement using preoperative US. They underwent US-guided fine-needle aspiration cytology and FNA-Tg. The value of single and combined application of the two methods for the diagnosis of cervical lymph node metastasis was calculated. The factors affecting FNA-Tg for diagnosis were analyzed using univariate and multivariate methods.
FNA-Tg values were significantly higher among patients with positive cervical lymph node metastasis. The sensitivity and specificity of US-guided fine-needle aspiration cytology, FNA-Tg, and US-guided fine-needle aspiration cytology + FNA-Tg were 85.48% and 90.59%, 83.06% and 87.06%, and 96.77% and 91.76%, respectively. The area under the receiver operating characteristic curve for US-guided fine-needle aspiration cytology, FNA-Tg, and the two combined, was 0.880, 0.851, and 0.943, respectively. A long diameter/short diameter ratio < 2, an insufficient number of acquired cells, a low serum thyroglobulin level, and an absence of typical metastatic US features increased the risk of cervical lymph node metastasis in thyroid carcinoma patients misdiagnosed using FNA-Tg.
The diagnostic value of FNA-Tg for detecting cervical lymph node metastasis is not high; however, combined with US-guided fine-needle aspiration cytology, it is significantly improved.
Core Tip: Fine-needle aspiration of thyroglobulin (FNA-Tg) has relatively high diagnostic value in lymph node metastasis and recurrence of differentiated thyroid carcinoma. FNA-Tg combined with ultrasonic-guided fine-needle aspiration cytology has a certain meaning in the thyroid carcinoma with lymph node metastasis.
- Citation: Zhang LY, Chen Y, Ao YZ. Value of thyroglobulin combined with ultrasound-guided fine-needle aspiration cytology for diagnosis of lymph node metastasis of thyroid carcinoma. World J Clin Cases 2022; 10(2): 492-501
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/492.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.492
Common clinical diagnostic methods for cervical lymph node metastasis in thyroid carcinoma patients include ultrasound (US), computed tomography, magnetic resonance imaging, radionuclide scanning, and other imaging methods, as well as US-guided fine-needle aspiration cytology (FNAC). However, all of these methods have limitations[1,2]. US is the most commonly used imaging method; however, comorbid inflammatory lymphadenopathy can lead to misdiagnosis; accurate differentiation between benign and malignant nodules requires extensive experience[3]. FNAC can offer further cytological diagnostic support for lymph nodes with suspicious US results. However, incorrect sampling sites, insufficient sample sizes, small metastases, and cystic alteration of the lesion can lead to false-negative results[4]. Fine-needle aspiration of thyroglobulin (FNA-Tg) reportedly has a relatively high diagnostic value in lymph node metastasis and recurrence of differentiated thyroid carcinoma[5]. In this study, we primarily aimed to explore and describe the value of FNA-Tg combined with US-guided FNAC to diagnose cervical lymph node metastasis in patients with thyroid carcinoma and explore factors influencing the diagnosis.
A total of 209 pathologically diagnosed thyroid carcinoma patients who visited the Thyroid Surgery Department of Affiliated Hospital of Chengde Medical University between Jan 2017 and Dec 2020 were selected. The inclusion criteria were as follows: (1) Patients who met the diagnostic criteria of thyroid cancer according to the National Comprehensive Cancer Network Guidelines for thyroid cancer criteria[6]; (2) Patients with confirmed pathological diagnosis; (3) Patients aged 20 to 67 years; (4) Patients who presented with suspicious lymph node enlargement on preoperative cervical lymph node US and then underwent US-guided FNAC and FNA-Tg; and (5) Patients with complete data. The exclusion criteria were as follows: (1) Patients with a history of radiation and chemotherapy; and (2) Patients with lung infections and heart failure.
Before the implementation of this study, the research plan was submitted to the Medical Ethics Committee of our hospital for approval and then implemented after the decision and document of the Medical Ethics Committee.
For FNAC, the patients were placed supine with a soft pillow under their neck to fully expose the puncture site. After routine disinfection of the puncture site, 1% lidocaine was applied under local anesthesia. A 22 G cell puncture needle (Yako, Japan) was selected, and the fine needle was inserted into the center of the lymph node under US guidance. The needle was rapidly retracted and inserted back and forth in different needle channels five times. Subsequently, the puncture needle was pulled out, the aspirated tissue was placed onto the slide, smeared, and fixed for pathological examination. Each lymph node was punctured at least three times. After HE staining, the smears were reviewed by two senior pathologists, and the cancer cells were either determined to be positive for lymph node metastases, or if no cancer cells were found, or if the number of cells was insufficient, the cells were determined to be negative.
For FNA-Tg measurement, 0.5 mL of 0.9% normal saline was absorbed with a 1-mL syringe, the needle was rinsed, and 1 mL of eluent was prepared. The supernatant was extracted after centrifugation at 3000 r/min for 5 min. Subsequently, the Tg content was detected using the COBAS E601 electrochemical analyzer (Roche, Basel, Switzerland) and an immunochemiluminescence method.
The judgment standards[7] were as follows: FNA-Tg > 1.0 ng/mL was diagnosed as positive thyroid cancer lymph node metastasis, and FNA-Tg ≤ 1.0 ng/mL was diagnosed as negative thyroid cancer lymph node metastasis.
In this study, age and other measurement indexes were tested for normal distribution, and all were in line with approximate normal distribution or normal distribution, which was expressed by mean ± SD. A t-test was performed using SPSS software (IBM Corp., Armonk, NY, USA). The measured data were analyzed using an χ2 test. For multivariate analysis, a logistic regression model was used to draw the ROC curve and obtain the area under the curve (AUC). The test level was α = 0.05.
On US, patients with positive cervical lymph node metastasis showed significantly higher rates of cortical centripetal thickening, hypoechogenicity of the cortex and the medulla, long diameter/short diameter ratio < 2, partial liquefaction or fusion of lymph nodes, abundant internal blood supply, and hilar absence than patients with negative lymph node metastasis (P < 0.05) (Table 1).
| Pathological results | n | Cortical centripetal thickening | Hypoechogenicity of cortex and medulla | Long diameter/short diameter < 2 | Partial liquefaction or fusion of the lymph nodes | Rich internal blood supply | Hilum deletion | ||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||
| Positive cervical lymph node metastasis | 124 | 69 (55.65) | 55 (44.35) | 55 (44.35) | 69 (55.65) | 67 (54.03) | 57 (45.97) | 39 (31.45) | 85 (68.55) | 63 (50.81) | 61 (49.19) | 32 (25.81) | 92 (74.19) |
| Negative cervical lymph node metastasis | 85 | 34 (40.00) | 51 (60.00) | 26 (30.59) | 59 (69.41) | 30 (35.29) | 55 (64.71) | 14 (16.47) | 71 (83.53) | 31(36.47) | 54 (63.53) | 11 (12.94) | 74 (87.06) |
| χ2 value | 4.939 | 4.027 | 7.120 | 5.980 | 4.188 | 5.108 | |||||||
| P value | 0.026 | 0.045 | 0.008 | 0.014 | 0.041 | 0.024 | |||||||
FNA-Tg values were significantly higher in patients with positive cervical lymph node metastasis than those with negative lymph node metastasis (P < 0.05) (Table 2).
| Pathological results | n | FNA-Tg (ng/mL) | t value | P value |
| Positive cervical lymph node metastasis | 124 | 1.56 ± 0.47 | 14.526 | 0.000 |
| Negative cervical lymph node metastasis | 85 | 0.77 ± 0.21 |
Considering pathological results as the gold standard, a four-grid table was prepared (Table 3). The sensitivity and specificity of FNAC in the diagnosing cervical lymph node metastasis of thyroid carcinoma were 85.48% and 90.59%, respectively. The sensitivity and specificity of FNA-Tg for diagnosing cervical lymph node metastasis of thyroid carcinoma were 83.06% and 87.06%, respectively. The sensitivity and specificity of FNAC + FNA-Tg for diagnosing cervical lymph node metastasis of thyroid carcinoma were 96.77% and 91.76%, respectively (Table 4).
| FNA | Pathological results | Total | FNA-Tg | Pathological results | Total | FNA + FNA-Tg | Pathological results | Total | |||
| Positive | Negative | Positive | Negative | Positive | Negative | ||||||
| Positive | 106 | 8 | 114 | Positive | 103 | 11 | 114 | Positive | 120 | 7 | 127 |
| Negative | 18 | 77 | 95 | Negative | 21 | 74 | 95 | Negative | 4 | 78 | 82 |
| Total | 124 | 85 | 209 | Total | 124 | 85 | 209 | Total | 124 | 85 | 209 |
| Diagnostic method | Sensitivity | Specificity | Rate of missed diagnosis | Misdiagnosis rate | Positive predictive value | Negative predictive value |
| FNA | 85.48 | 90.59 | 14.52 | 9.41 | 92.98 | 81.05 |
| FNA-Tg | 83.06 | 87.06 | 16.94 | 12.94 | 90.35 | 77.89 |
| FNA + FNA-Tg | 96.77 | 91.76 | 3.23 | 8.24 | 94.49 | 95.12 |
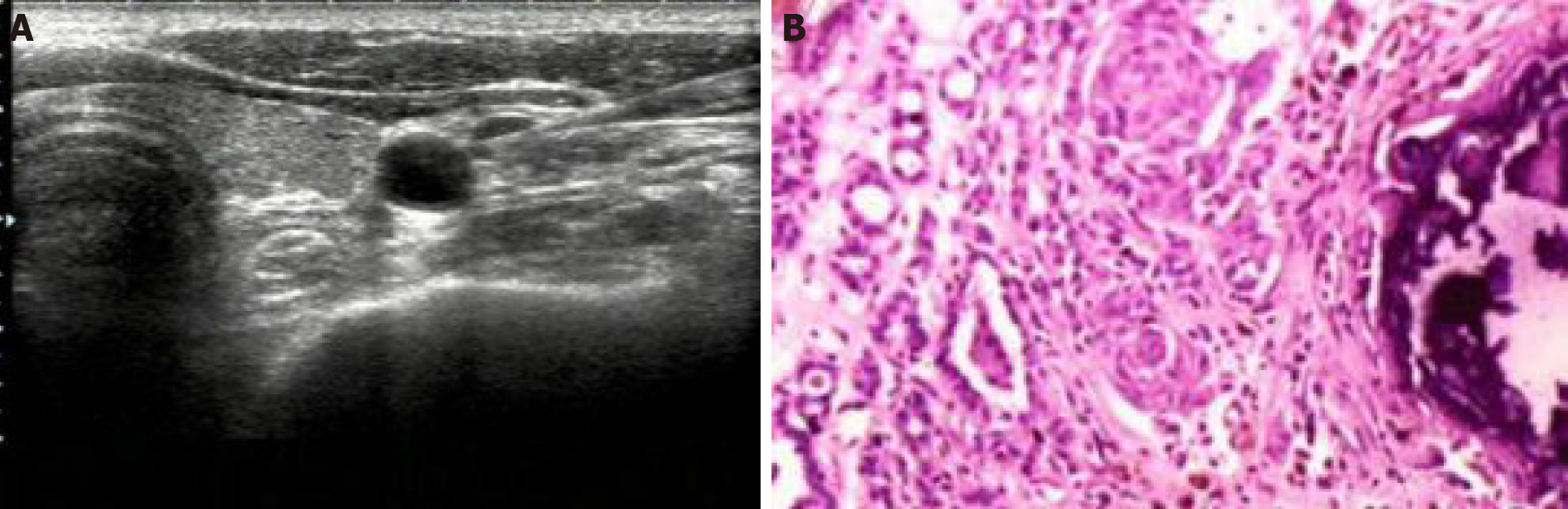
Figure 1 shows the results of US-guided FNA examination of cervical lymph nodes and postoperative pathological examination of lymph nodes in patients with papillary thyroid carcinoma with positive lymph node metastasis.
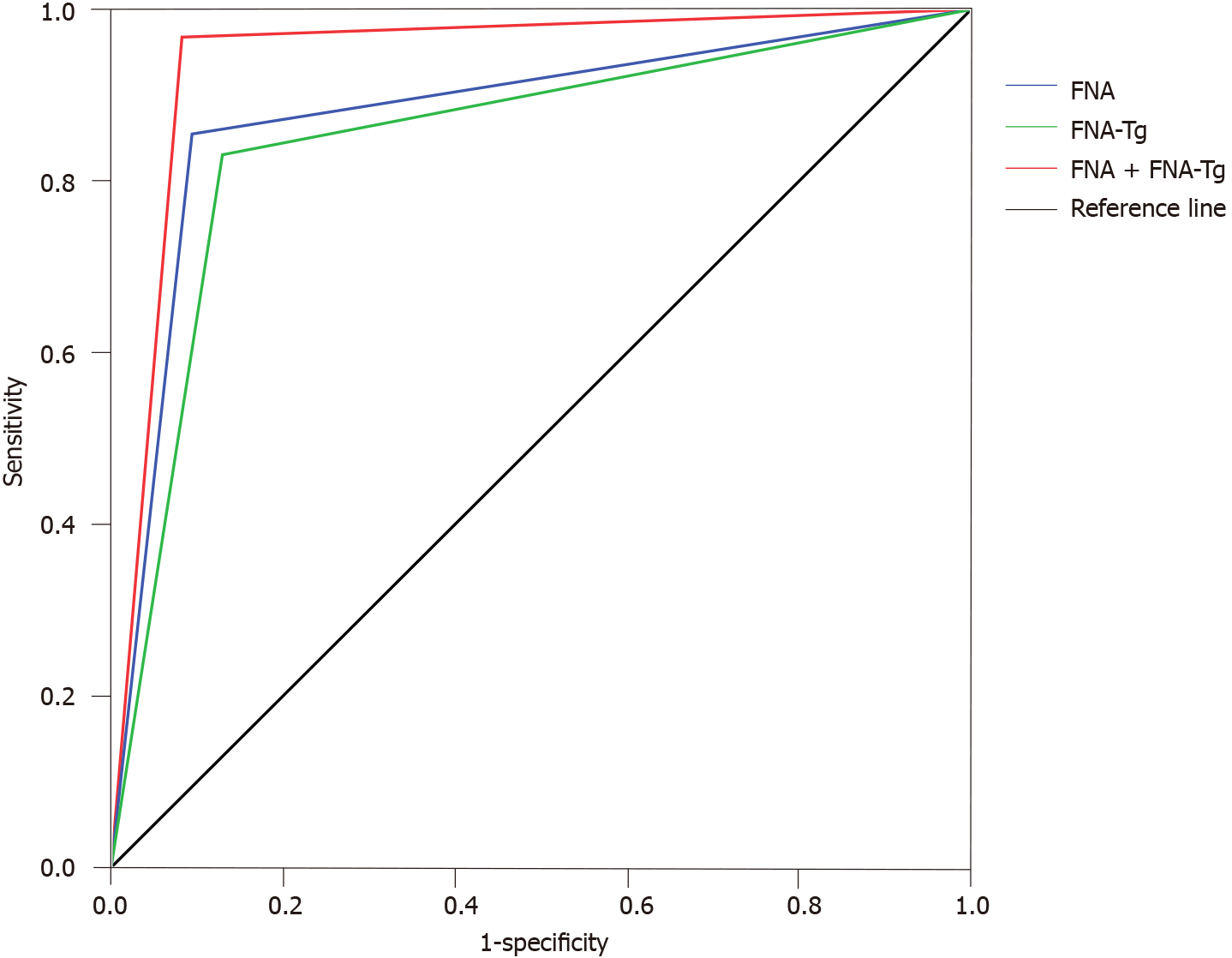
An ROC curve was drawn adopting the pathological results as the gold standard. Results showed that the AUC value for FNAC diagnosis of thyroid carcinoma with cervical lymph node metastasis was 0.880. The AUC value for FNA-Tg diagnosis of thyroid carcinoma with cervical lymph node metastasis was 0.851. The AUC value for FNAC + FNA-Tg for diagnosing thyroid carcinoma with cervical lymph node metastasis was 0.943 (Figure 2).
Patients were divided into groups based on FNA-Tg differential diagnosis. The univariate analysis showed that the differences between the two groups were statistically significant (P < 0.05), including the rate of long diameter, long diameter/short diameter lymph node ratio, the number of collected cells, serum thyroid stimulating hormone (TSH), serum Tg, and US characteristics (Table 5).
| Index | Correct diagnosis (n = 177) | Error diagnosis (n = 32) | t/χ2 value | P value |
| Age (yr) | 49.3 ± 5.8 | 48.2±6.6 | 0.966 | 0.335 |
| Gender, n (%) | 0.140 | 0.708 | ||
| Male | 67 (37.85) | 11 (34.38) | ||
| Female | 110 (62.15) | 21 (65.63) | ||
| Short diameter of lymph node (cm) | 0.62 ± 0.11 | 0.60 ± 0.08 | 0.982 | 0.327 |
| Long diameter of lymph node (cm) | 1.38 ± 0.20 | 1.29 ± 0.23 | 2.288 | 0.023 |
| Long diameter/short diameter, n (%) | 6.965 | 0.008 | ||
| < 2 | 88 (49.72) | 24 (75.00) | ||
| ≥ 2 | 89 (50.28) | 8 (25.00) | ||
| Number of collected cells, n (%) | 15.034 | 0.000 | ||
| Insufficient | 11 (6.21) | 9 (28.13) | ||
| Sufficient | 166 (93.79) | 23 (71.88) | ||
| Serum TSH (ng/mL) | 2.09 ± 0.39 | 2.31 ± 0.46 | -2.854 | 0.005 |
| Serum TgAb (IU/mL) | 20.83 ± 5.17 | 22.15 ± 5.83 | -1.303 | 0.194 |
| Serum Tg (ng/mL) | 18.94 ± 4.20 | 16.84 ± 4.00 | 2.621 | 0.009 |
| Number of cervical lymph node metastases | 3.41 ± 0.84 | 3.15 ± 0.76 | 1.634 | 0.104 |
| Characteristics of ultrasonic signs, n (%) | 4.885 | 0.027 | ||
| Signs of metastasis | 142 (80.23) | 20 (62.50) | ||
| No signs of metastasis | 35 (19.77) | 12 (37.50) |
The results of FNA-Tg differential diagnosis of cervical lymph node metastasis were adopted as dependent variables, and the statistically significant indexes, such as long diameter, long diameter/short diameter lymph node ratio, the number of collected cells, serum TSH, serum Tg, and characteristics of US signs, were adopted as independent variables to establish a logistic regression model. A long diameter/short diameter ratio < 2, insufficient number of acquired cells, low level of serum Tg, and absence of typical US signs of lymph node metastasis were found to increase the risk of cervical lymph node metastasis in patients with thyroid carcinoma misdiagnosed using FNA-Tg (P < 0.05) (Table 6).
| Factors | SE | Walds | P value | OR | 95%CI | ||
| Long diameter of lymph node | 0.611 | 0.412 | 2.199 | 0.138 | 1.842 | 0.822 | 4.131 |
| Long diameter/short diameter | 0.741 | 0.338 | 4.806 | 0.041 | 2.098 | 1.082 | 4.069 |
| Number of collected cells | -0.612 | 0.296 | 4.275 | 0.047 | 0.542 | 0.304 | 0.969 |
| Serum TSH | 0.285 | 0.217 | 1.725 | 0.216 | 1.330 | 0.869 | 2.035 |
| Serum Tg | -0.442 | 0.186 | 5.647 | 0.025 | 1.556 | 1.081 | 2.240 |
| Characteristics of ultrasonic signs | 0.804 | 0.358 | 5.044 | 0.037 | 2.234 | 1.108 | 4.507 |
| Constant term | 1.309 | 0.684 | 3.662 | 0.091 | 3.702 | 0.969 | 14.149 |
Our study showed that patients with positive cervical lymph node metastasis had significantly higher rates of cortical centripetal thickening, hypoechogenicity of the cortex and medulla, long diameter/short diameter ratio < 2, partial liquefaction or fusion of lymph nodes, abundant internal blood supply, and hilar absence than patients with negative lymph node metastasis (P < 0.05). These are the typical US characteristics of lymph node metastasis. The normal oval structure of the lymph nodes can be destroyed by the cancer cells; they have an irregular or round shape with a change in the vertical and horizontal diameter ratio. The internal structure can also be destroyed. In the case of lymph node metastasis, the lymphadenocortex involvement occurs first, leading to the loss of the cutaneous medulla structure. Moreover, the infiltration of cancer cells destroys the normal blood supply to the lymph nodes, and US usually reveals an uneven blood supply to the lymph nodes.
Tg is secreted by normal thyroid tissue and differentiated thyroid carcinoma and is a marker of tumor protein in peripheral blood[8,9]. Tg expression is negligible in normal lymph nodes; d, it can be expressed in differentiated thyroid carcinoma, and lymph node metastasis and its concentration in tissue puncture fluid are much higher than that in serum[10]. Detecting FNA-Tg levels in the eluent of needle biopsy samples can help reach the differential diagnosis of cervical lymph node metastasis in thyroid carcinoma. In this study, we adopted specific reference values for detecting positive lymph node metastasis using FNA-Tg. The FNA-Tg value was significantly higher in patients with positive lymph node metastasis than patients with negative lymph node metastasis (P < 0.05). This suggests that because the thyroid tissue has a secretory function in the lymph node tissue, it may appear as lymph node metastasis due to the biological characteristics of the cell. Currently, FNAC is considered the most direct method to diagnose lymph node properties, as it can directly obtain the cells of the lesion and its tissue. However, its smear can be affected by factors such as blood, glia, and cell count, leading to a low sensitivity[10-12]. When the lymph nodes are too small and the smear cells are insufficient, the sensitivity and specificity of FNAC diagnosis can be reduced, leading to an increase in false negatives, affecting the clinical diagnostic efficiency, and reducing the predictive accuracy[13].
Affected by many factors, the positive threshold of FNA-Tg remains controversial[14,15]. Although previous studies have reported that the diagnostic sensitivity of FNA-Tg was better than that of FNAC[16], our results showed no significant differ
In this study, a univariate analysis of the influence of FNA-Tg findings on the diagnosis of cervical lymph node metastasis revealed a significant difference between the two groups (P < 0.05) in terms of the rate of long diameter, the long diameter/ short diameter ratio of the lymph nodes, the number of collected cells, serum TSH level, serum Tg level, and US characteristics. The multivariate analysis showed that a long diameter/short diameter ratio of < 2, an insufficient amount of acquired cells, low level of serum Tg, and absence of typical US signs increased the risk of cervical lymph node metastasis in patients with thyroid carcinoma misdiagnosed by FNA-Tg (P < 0.05).
Some studies[17] have reported that the loss of thyroid tissue and inhibition of serum TSH after thyroidectomy may decrease serum Tg levels, and the levels of serum Tg can independently influence the diagnosis of FNA-Tg. Inhibition of serum TSH can reduce the serum Tg level, and a false negative FNA-Tg diagnosis is possible. In contrast, when serum Tg is not reduced, a false positive FNA-Tg diagnosis is possible[18]. Therefore, it is suggested that FNA-Tg should be tested after TSH stimulation. The diagnostic performance of the FNA-Tg diagnostic threshold varies with thyroid status and serum Tg concentration, but there is no doubt that FNA-Tg detection as an auxiliary diagnostic method can bring about all of its unique advantages.
In this study, patients with suspicious cervical lymph node findings on US were studied. FNAC and FNA-Tg were performed to determine whether or not the diagnosis was thyroid lymph node metastatic carcinoma. Compared with previous studies[19,20], in our study, univariate and multivariate analyses of factors affecting FNA-Tg diagnosis were conducted for the first time, and the results were highly reliable. However, there were also some limitations to our study. The detection process and threshold setting lacked unified standards. Moreover, the factors affecting the test results were numerous and unclear. Thyroid inflammation, autoimmune diseases, and endocrine system diseases can all affect the serum Tg determination to some extent, especially in patients with false-positive and false-negative results. Therefore, underlying diseases should also be considered.
In conclusion, when diagnosing thyroid carcinoma patients with cervical lymph node metastasis, FNA-Tg can be affected by various factors, and its diagnostic value alone is not high; however, combined with FNAC, the sensitivity and specificity of diagnosis are significantly improved, providing a significant reference value to guide the treatment.
Fine-needle aspiration cytology (FNAC) can offer further cytological diagnostic support for lymph nodes with suspicious ultrasound (US) results.
Fine-needle aspiration of thyroglobulin (FNA-Tg) reportedly has a relatively high diagnostic value in lymph node metastasis and recurrence of differentiated thyroid carcinoma.
We explore and describe the value of FNA-Tg combined with US-guided FNAC to diagnose cervical lymph node metastasis in patients with thyroid carcinoma.
A total of 209 pathologically diagnosed thyroid carcinoma patients who visited the Thyroid Surgery Department of the Hospital were selected.
The sensitivity and specificity of US-guided FNAC, FNA-Tg, and US-guided FNAC + FNA-Tg were 85.48% and 90.59%, 83.06% and 87.06%, and 96.77% and 91.76%, respectively.
Combined with US-guided FNAC, it is significantly improved.
The detection process and threshold setting lacked unified standards.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haymart MR, Spartalis E S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Jeon SJ, Kim E, Park JS, Son KR, Baek JH, Kim YS, Park DJ, Cho BY, Na DG. Diagnostic benefit of thyroglobulin measurement in fine-needle aspiration for diagnosing metastatic cervical lymph nodes from papillary thyroid cancer: correlations with US features. Korean J Radiol. 2009;10:106-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | He M, Lin C, Yin L, Lin Y, Zhang S, Ma M. Value of Dual-Energy Computed Tomography for Diagnosing Cervical Lymph Node Metastasis in Patients With Papillary Thyroid Cancer. J Comput Assist Tomogr. 2019;43:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Feldkamp J, Führer D, Luster M, Musholt TJ, Spitzweg C, Schott M. Fine Needle Aspiration in the Investigation of Thyroid Nodules. Dtsch Arztebl Int. 2016;113:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S; American Thyroid Association Guidelines Task Force. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2015;25:716-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 805] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 5. | Zhu XH, Zhou JN, Qian YY, Yang K, Wen QL, Zhang QH, Xia L, Ge MH, Sun CX. Diagnostic values of thyroglobulin in lymph node fine-needle aspiration washout: a systematic review and meta-analysis diagnostic values of FNA-Tg. Endocr J. 2020;67:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Sun R, Zhang J, Zhang F, Fan J, Yuan Y, Li C. Selectively predictive calcium supplementation using NCCN risk stratification system after thyroidectomy with differentiated thyroid cancer. Int J Clin Exp Med. 2015;8:21939-21946. [PubMed] |
| 7. | Moon JH, Kim YI, Lim JA, Choi HS, Cho SW, Kim KW, Park HJ, Paeng JC, Park YJ, Yi KH, Park DJ, Kim SE, Chung JK. Thyroglobulin in washout fluid from lymph node fine-needle aspiration biopsy in papillary thyroid cancer: large-scale validation of the cutoff value to determine malignancy and evaluation of discrepant results. J Clin Endocrinol Metab. 2013;98:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Xu Y, Wu D, Wu W, Jiang J, Xi C, Ye N, Wang Y, Xu X. Diagnostic value of cytology, thyroglobulin, and combination of them in fine-needle aspiration of metastatic lymph nodes in patients with differentiated thyroid cancer: A systematic review and network meta-analysis. Medicine (Baltimore). 2019;98:e17859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Liu C, Xiao C, Chen J, Li X, Feng Z, Gao Q, Liu Z. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer. 2019;19:622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | Lee K, Anastasopoulou C, Chandran C, Cassaro S. Thyroid Cancer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2021. [PubMed] |
| 11. | Gestrich C, Cowden D, Harbhajanka A. Cytomorphology of glioblastoma metastic to a cervical lymph node diagnosed by fine needle aspiration (FNA): A case report and review of literature. Diagn Cytopathol. 2020;48:567-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Wang J, Jiang X, Xiao G, Zhou W, Hu Y. Excellent diagnostic performance of FNA-Tg in detecting lymph nodes metastases from papillary thyroid cancer. Future Oncol. 2020;16:2735-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Uruno T, Miyauchi A, Shimizu K, Tomoda C, Takamura Y, Ito Y, Miya A, Kobayashi K, Matsuzuka F, Amino N, Kuma K. Usefulness of thyroglobulin measurement in fine-needle aspiration biopsy specimens for diagnosing cervical lymph node metastasis in patients with papillary thyroid cancer. World J Surg. 2005;29:483-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Wang M, Liu X, Wei B, Liu N, Li Q, Su X. Mucinous breast carcinoma metastatic to thyroid gland: Report of a case diagnosed by fine-needle aspiration cytology. Diagn Cytopathol. 2020;48:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Seneldir H, Kir G, Soylemez T, Girgin RB, Ozbay N, Ozen F, Ankarali H, Bas G, Alimoglu O. Diagnostic accuracy of molecular testing with three molecular markers on thyroid fine-needle aspiration cytology with abnormal category. Diagn Cytopathol. 2020;48:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Zhu Y, Song Y, Xu G, Fan Z, Ren W. Causes of misdiagnoses by thyroid fine-needle aspiration cytology (FNAC): our experience and a systematic review. Diagn Pathol. 2020;15:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Adhami M, Michail P, Rao A, Bhatt CR, Grodski S, Serpell JW, Lee JC. Anti-Thyroid Antibodies and TSH as Potential Markers of Thyroid Carcinoma and Aggressive Behavior in Patients with Indeterminate Fine-Needle Aspiration Cytology. World J Surg. 2020;44:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Giovanella L, Bongiovanni M, Trimboli P. Diagnostic value of thyroglobulin assay in cervical lymph node fine-needle aspirations for metastatic differentiated thyroid cancer. Curr Opin Oncol. 2013;25:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388:2783-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 1019] [Article Influence: 113.2] [Reference Citation Analysis (1)] |
| 20. | Kahramangil B, Kose E, Donmez M, Aydin H, Reynolds JP, Krishnamurthy V, Jin J, Shin J, Siperstein A, Berber E. Thyroglobulin washout from cervical lymph node fine needle aspiration biopsies in patients with differentiated thyroid cancer: an analysis of different expressions to use in post-total thyroidectomy follow-up. Surgery. 2020;167:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |