Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.458
Peer-review started: July 19, 2021
First decision: October 16, 2021
Revised: October 20, 2021
Accepted: December 2, 2021
Article in press: December 2, 2021
Published online: January 14, 2022
Processing time: 176 Days and 15.8 Hours
Gastric leiomyomas and gastric stromal tumors are the most common types of gastric tumors encountered. In recent years, the incidence of the two types of tumors has been increasing, but the differential diagnosis is still a challenge in clinical work. However, as there are many reports on stromal tumors and inflammation-related indicators are gradually being paid attention to as important factors in predicting tumor prognosis, the two main purposes of this study were to explore the inflammation-related differences between the two types of tumors and to develop a nomogram as a predictive model.
To explore the differences in platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), lymphocyte mononuclear cell ratio (LMR), and SII between the two types of tumors, and simultaneously create the nomogram model.
This study enrolled 88 patients in the gastric stromal tumor group and 56 patients in the gastric leiomyoma group, and the relevant data of the two groups were entered into the system for an integrated analysis. The primary objective of this study was to identify the differences in the inflammation index between the two types of tumors.
There were statistically significant differences between the two groups in sex, age, and tumor location. In comparison, gastric leiomyomas seem to be more common in women, young patients, and gastric cardia, which is in line with our previous research; the groups showed the following statistical differences: PLR (158.2% vs 134.3%, P = 0.028), NLR (2.35 vs 1.68, P = 0.000), LMR (5.75 vs 10.8, P = 0.004), and SII (546.2 vs 384.3, P = 0.003). The results of the multivariate logistic regression analysis showed that sex, age, tumor location, and LMR were independent risk factors for the identification of the two types of tumors. After considering the risk factors selected by the above analysis into the predictive model, a predictive model for distinguishing gastrointestinal stromal tumors from gastric leiomyomas was established as the nomogram.
Gastric leiomyomas and gastric stromal tumors are not only different in factors such as age of the patient, but also in inflammatory indicators such as LMR and PLR. We have established a predictive model related to the laboratory indicators and are looking forward to further research conducted in this clinical area.
Core Tip: We found that inflammation-related factors such as platelet-lymphocyte ratio and neutrophil-lymphocyte ratio were different in patients with gastrointestinal stromal tumors and leiomyomas, which also reflected the different inflammatory status between them. Meanwhile, this study constructed a relevant differential diagnosis model through a nomogram and evaluated its accuracy, which may be helpful for future studies.
- Citation: Zhai YH, Zheng Z, Deng W, Yin J, Bai ZG, Liu XY, Zhang J, Zhang ZT. Inflammation-related indicators to distinguish between gastric stromal tumors and leiomyomas: A retrospective study. World J Clin Cases 2022; 10(2): 458-468
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/458.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.458
Gastric leiomyomas and gastrointestinal stromal tumors (GISTs) are common tumors of the gastric mucosa. It is difficult to differentiate between the two types of tumors when making the initial diagnosis. As there is at present is no method available to distinguish between the two types before surgery, it can only be determined by pathology after surgery.
GISTs are the most common type of mesenchymal tumors and are mainly characterized by the expression of CD117. Stromal tumors mainly occur in the gastroin
Gastric leiomyomas are also gastric tumors with spindle cell-like pathology, but unlike gastric stromal tumors, have no malignant potential and are a type of benign tumors. Studies have shown that gastric leiomyomas are more likely to occur in the gastric cardia—unlike GIST[6,7]. As the tumor grows, although there is no tendency to become malignant, bleeding and other symptoms occur (such as ulcers), all of which affect the patients’ quality of life. The same treatment methods are often used for gastric stromal tumors and for gastric leiomyomas, mainly because it is generally impossible to accurately distinguish the two types of tumors in the early stage. If a more accurate diagnosis could be made, the possibility of a more applicable treatment option could then be given to patients with gastric leiomyoma, and dynamic clinical observations will be more in line with current standardized diagnosis and treatment. Unlike gastric stromal tumors, leiomyomas do not have the possibility of receiving targeted therapy, and require no follow-up and observation. The quality of life and life expectancy of patients is better than that of patients diagnosed with GISTs.
Gastric tumors are often found through gastroscopy and computed tomography (CT), and then judged by the benign or malignant characteristics based on its microscopic appearance and the CT imaging; however, this is not the gold standard for diagnosis. Despite this, the differential diagnosis of the two types of tumors often cannot be reached before the pathology result is obtained. The pathology sample obtained by an endoscopy is sometimes inferior, and the support of the immunohistochemical results is needed after obtaining a pathology sample[8], which also hinders the diagnosis and treatment process of patients. Endoscopic ultrasonography is a relatively reliable examination to clarify the origin and invasion of tumors. Studies done have proven that endoscopic ultrasonography has a high accuracy rate and guiding significance for clarifying the prognosis and assisting in the diagnosis of patients, which has provided clarification on their prognosis for many of them[9]. In addition, the application of multi-slice computed tomography (MSCT) in recent years has made the preoperative diagnosis of the two types of tumors clearer. The CT values of the tumors have been analyzed and it exposed the differences among them[10]; however, the application of these differences needs further research. The current prediction model is still flawed, and it is impossible to make an overall assessment of these tumors. Although endoscopic ultrasound can provide a hierarchical analysis, it is limited by the physician’s level of experience. The objectivity and accuracy still need to be improved via further research and clinical studies. Therefore, more objective and accessible laboratory indicators are needed to help distinguish the two types of tumors.
Inflammatory and nutritional indicators are often used to predict the prognosis of cancer patients. For malignant tumors, Balkwill et al[11] proposed the tumor inflammatory theory, linking the occurrence and development of tumors with inflammatory factors; advances in the field of tumor research are inseparable from the discussion of the influence of its inflammatory environment[11]. Other studies have also shown that the tumor-related inflammation index is related to the tumor microenvironment and affects the body's immune function to a certain extent. When discussing the inflammatory indicators, (in addition to the white blood cell value, neutrophil value, and C-reactive protein), other factors such as platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), and lymphocyte mononuclear cell ratio (LMR), can reflect more clearly the conditions surrounding the tumor[12-14], and these are also considered to be related to the prognosis of the patient. In both the studies of gastric cancer and gastric stromal tumors, this view that inflammatory markers have an important role to play has been confirmed. With regards to the research on the prognosis of tumors, our focus was more directed to the role of the tumor microenvironment and whether the two types can be differentiated. Gastric stromal tumors are prone to be malignant, but the questions are whether they have higher inflammatory indicators and whether they are different from gastric leiomyomas; none of which has been confirmed by previous studies.
In comparison, because it has no malignant potential, the prognosis of a patient with a gastric leiomyoma is better; therefore, a gastric leiomyoma may form a better microenvironmental basis that should be reflected in the inflammation indicators. Therefore, we suspected that the identification of the two types of benign tumors could also be carried out by comparing the levels of laboratory indicators such as PLR and NLR. At the same time, combined with imaging and other methods, there may be a higher accuracy, and it could also be used for treatment. However, there is currently no relevant research showing that PLR, NLR, and other inflammatory indicators are different in the two types of tumors, and there is no research on the notion of including them in the differential diagnosis. Therefore, we conducted a retrospective study on the patients who presented with the two types of tumors in our hospital, based on a multiple regression analysis of the influencing factors, and the total score can be calculated from a visual representation showing the probable risk of individuals with corresponding diseases[15]. In addition, it is easy to screen high-risk patients quickly and efficiently and make a timely and effective intervention[16,17]. This retrospective study aimed to explore the differences in PLR, NLR, LMR, and SII between the two types of tumors and simultaneously create a nomogram model, in order to provide a new method of differential diagnosis for clinical practice.
This study was a retrospective cohort study approved by the Ethics Committee of the Institute of Friendship Hospital, Capital Medical University, and was conducted in accordance with good clinical practice guidelines and the Helsinki Declaration.
This retrospective study included 144 patients who were diagnosed with gastric stromal tumors or gastric leiomyomas from the Beijing Friendship Hospital, Capital Medical University between December, 2016 and December, 2020. There were 88 patients with gastric stromal tumors and 56 patients with gastric leiomyomas. We divided the patients into a gastric stromal tumor group and a gastric leiomyoma group according to their pathology. The two groups of patients were grouped by the postoperative pathology, and the inflammation index was calculated based on the results of the preoperative examination and the laboratory tests after the patients were admitted to the hospital. The examination results were strictly controlled to ensure that the two groups had equal examination time before surgery, as well as a thorough analysis of the patient's medication and treatment to prevent antibiotics and other drugs from affecting the patient's examination results. There were also strict admission and exclusion standards.
The eligibility criteria were as follows: (1) Age between 18 and 75 years; (2) Histologically confirmed gastric stromal tumor or gastric leiomyoma; and (3) Eastern Cooperative Oncology Group (ECOG) between 0 and 2 grades.
The exclusion criteria were as follows: (1) An unclear pathological diagnosis; (2) A history of surgery in the past six months; and (3) A history of a malignant tumor.
The definition of the inflammation index had to be supported by reference ranges, and therefore we analyzed the values of PLR, NLR, LMR, and SII. We defined PLR as the ratio of platelets to lymphocytes, NLR as the ratio of neutrophils to lymphocytes, LMR was defined as the ratio of lymphocytes to monocytes, and SII was defined as the product of neutrophils and PLR. All values were based on the results of the patient's admission and preoperative inspections, and there were no patients included with acute stress disease or antibiotic administration within half a month to ensure the reliability of the values. All patients underwent surgical treatment after completing the preoperative examination, and follow-up treatment plans were determined based on the postoperative pathology (checked by an experienced pathologist). All the material were collected following a specific procedural protocol, the size of the tumor was clearly marked for ulcers and then verified by a formal pathology report. The units of various research indicators were unified, and planning in terms of the nomogram model was carried out according to relevant research.
The primary objective of this study was the difference in the inflammation index between the two types of tumors. We recorded a patient-related data baseline to eliminate interference from the two types of patients. We hypothesized that the gastric stromal tumor and gastric leiomyoma groups had differences in related inflammatory indicators, and that could be used as the basis for a differential diagnosis. This retrospective cohort study included 88 patients in the stromal tumor group and 56 patients in the gastric leiomyoma group. We then graded according to the pathological risk, resulting in 56 people in the low-risk stromal tumor group. A subgroup analysis of gastric stromal tumor patients was performed in this group to determine whether there would be relevant numerical differences in low-risk situations. Baseline patient data were also collected. The living ability score and tumor location of the two groups were recorded and compared in the related tables. The sex and age of the patients were also included.
We used a t-test to compare the inflammation index between the two treatment groups, which included all patients in this study, and statistical analyses were carried out using IBM SPSS 25.0 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.).
In the gastric stromal tumor group 88 were patients enrolled, and 56 patients in the gastric leiomyoma group; the relevant data of the two groups were recorded into the system for integrated analysis. Basic baseline information (sex, age, tumor location,
| Characteristics | GIST group (n = 88), (%) | Gastric leiomyoma group (n = 56), (%) | P value | ||
| Gender (male/female) | 47/41 | 53.4/46.6 | 18/38 | 32.1/67.9 | 0.012a |
| Age (median) | 61.1 | 50.1 | 0.00a | ||
| ECOG | 0.31 | ||||
| 0 | 86 | 97.7 | 52 | 92.8 | |
| 1 | 1 | 1.1 | 3 | 5.3 | |
| 2 | 1 | 1.1 | 1 | 1.7 | |
| Tumor location | 0.00a | ||||
| Cardia | 3 | 3.4 | 25 | 44.6 | |
| Fundus | 46 | 52.2 | 20 | 35.7 | |
| Body | 27 | 30.7 | 8 | 14.3 | |
| Antrum | 12 | 13.6 | 3 | 5.3 | |
| Diameter | 0.122 | ||||
| ≤ 2 cm | 11 | 12.5 | 14 | 25 | |
| 2-5 cm | 54 | 61.4 | 32 | 57.1 | |
| ≥ 5 cm | 23 | 26.1 | 10 | 17.9 | |
We then analyzed the inflammation indicators in the two groups. The two groups showed the following statistical differences: PLR (158.2 vs 134.3, P = 0.028), NLR (2.35 vs 1.68, P = 0.000), LMR (5.75 vs 10.8, P = 0.004), and SII (546.2 vs 384.3, P = 0.003) (Table 2). The differences in the inflammation indicators between the two groups were significant, which was in line with our conjecture before the study.
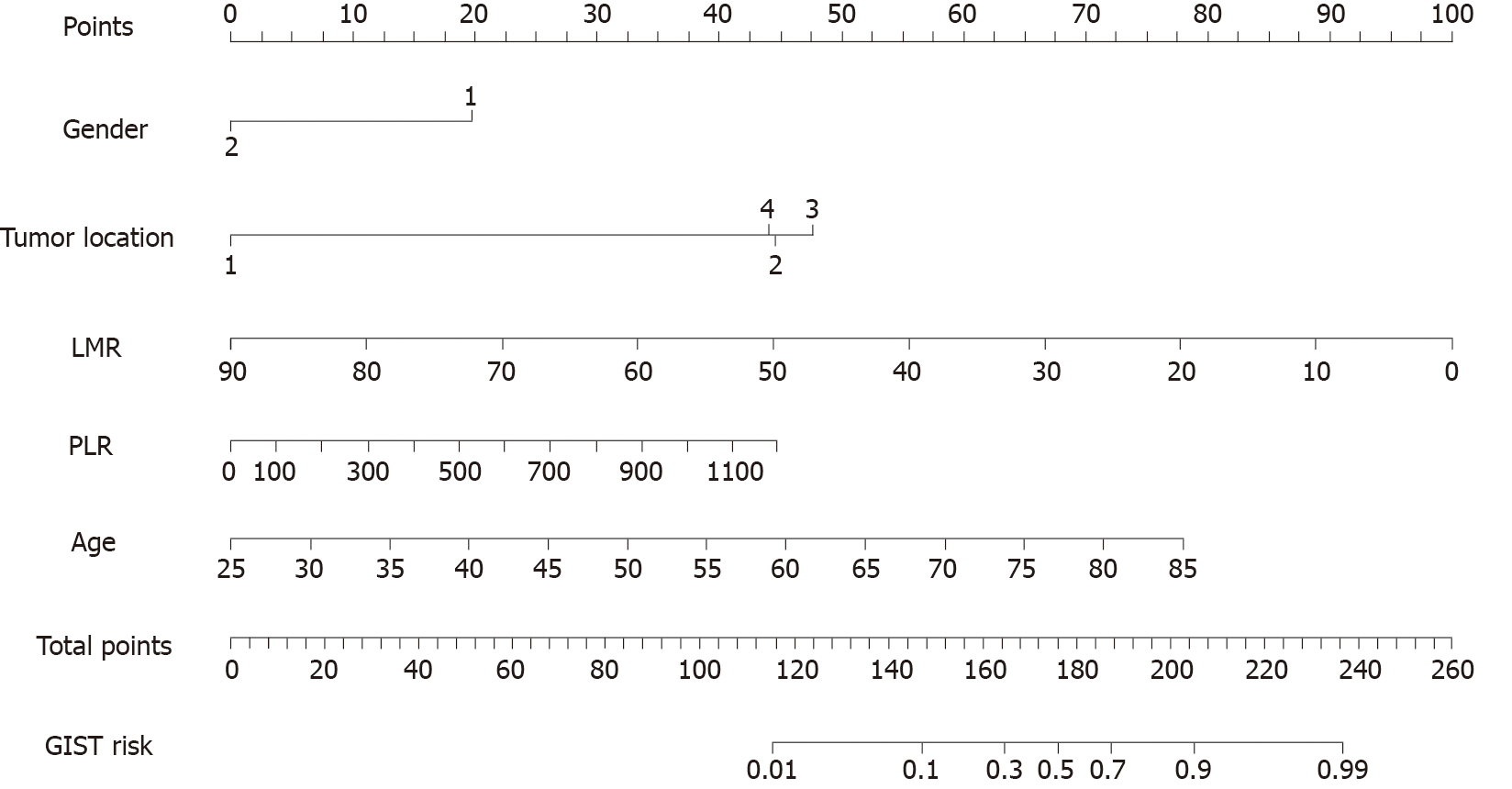
Based on multinomial logistic regression, we constructed predictive models of the GIST risk, and the results of the multivariate logistic regression analysis showed that sex, age, tumor location, and LMR were independent risk factors for the identification of the two types of tumors, and the difference was statistically significant (P < 0.05) (Table 3).
| Group | β | SE | Wald | P value | OR | 95%CI | |
| Gender | Male | - | - | - | - | 1 | - |
| Female | 1.548 | 0.548 | 7.97 | 0.005 | 4.7 | 1.605-13.762 | |
| Age | -0.097 | 0.024 | 16.939 | 0 | 0.907 | 0.866-0.950 | |
| Tumor locations | Cardia | - | - | - | - | 1 | - |
| Fundus | -3.501 | 0.804 | 18.948 | 0 | 0.03 | 0.006-0.146 | |
| Body | -3.572 | 0.854 | 17.482 | 0.001 | 0.028 | 0.005-0.150 | |
| Antrum | -3.315 | 1.039 | 10.19 | 0.001 | 0.036 | 0.005-0.278 | |
| LMR | 0.092 | 0.042 | 4.715 | 0.03 | 1.096 | 1.009-1.191 |
After incorporating the risk factors selected by the above analysis into the predictive model, a predictive model for distinguishing GISTs from gastric leiomyomas was established (Figure 1). The nomogram was applied as follows: The score value corresponding to each predictive index of the score was obtained, and the sum of these score values was recorded as the total score, and the predicted probability corresponding to the total score was the risk of the GIST onset.
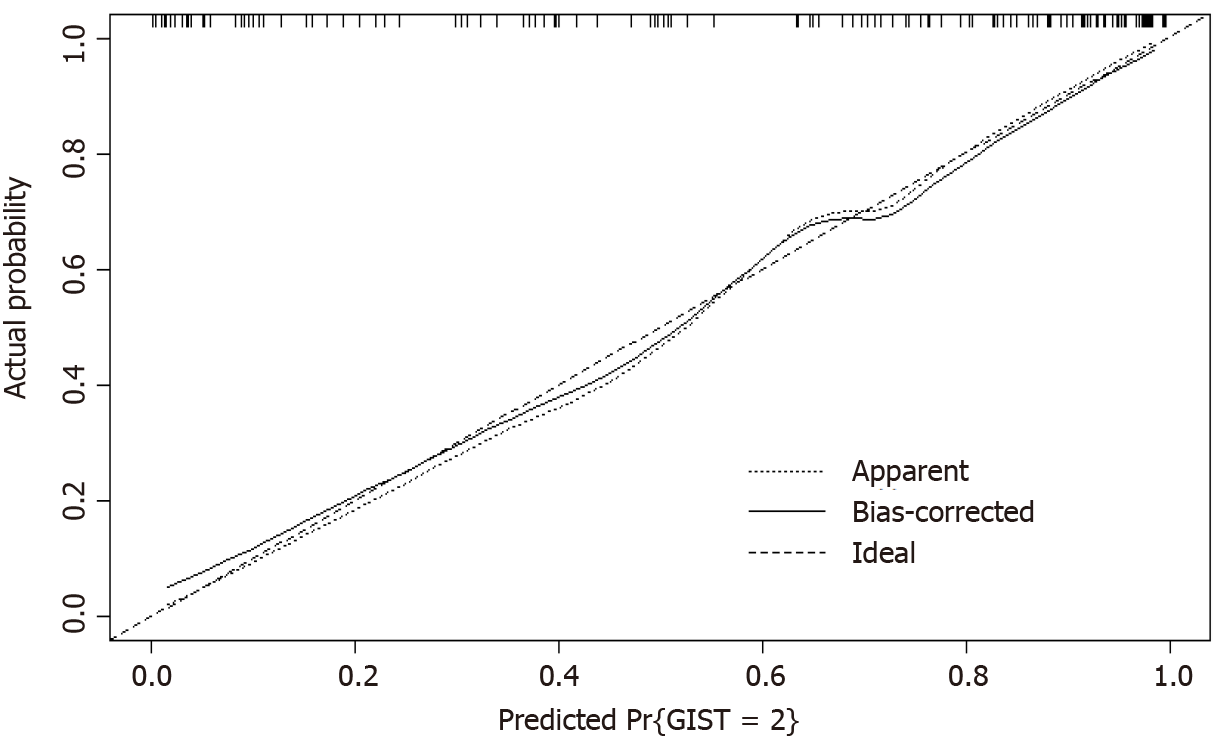
The calibration plot was used to explain the bias of a classifier, and the dashed line in the plot indicates the ideal model in which the predicted and actual probabilities are perfectly identical. The actual performance is indicated by a dotted line. The solid line shows the bootstrap-corrected performance. The bootstrap calibration plot (Figure 2) indicates good agreement between the nomogram and pathology results.
In order to clarify whether there was an increase in the inflammatory response level of stromal tumors due to high risk, and whether such differences remained in existence in the relatively lower-risk stromal tumor group, we conducted a subgroup analysis and selected the low-risk group and compared that with the leiomyoma group. There were also statistical differences in some inflammation indicators (LMR 5.42 to 10.82, P = 0.002; NLR 2.17 to 1.68, P = 0.016). We therefore inferred that LMR and NLR seemed more sensitive than PLR and SII in the subgroup analysis (Table 4).
Gastric stromal tumors and leiomyomas are both spindle cell stromal tumors, but the difference in source and malignancy should determine the type of treatment of the two types of tumors. In clinical practice, however, the two types of tumors are usually difficult to distinguish before surgical pathology is performed. For benign tumors–such as gastric leiomyomas–dynamic clinical observation is the course that should be taken, and the choice of surgery should be made with caution. Gastric leiomyoma is more likely to occur in the cardiac region, and the surgical difficulty and postoperative risk are greater in this region than in other locations. Thus, we would carefully choose the surgical option for gastric leiomyoma to avoid secondary harm to the patients. For gastric stromal tumors with malignant potential, surgical methods and surgical indications are determined according to symptoms and tumor size, and ultrasound gastroscopy is an important tool for the differentiation of the two types of tumors; however, it remains difficult to achieve a complete differentiation between the two types of tumors in clinical practice. The guidelines state that for stromal tumors with a diameter greater than 2 cm, surgical resection is recommended as treatment because of their tendency to be malignant. However, because gastric leiomyomas originate from Cajal cells and are benign tumors, it is very important to perform accurate evaluations before surgery. In order to achieve this, a more reliable diagnosis and improved treatment standards are needed; the main purpose of this study was to determine the difference between the two types of tumors on the basis of objective clinical indicators and consider it as a type of predictive indicator. The research our center has performed confirmed our conjecture, and further research should be conducted.
Inflammation indicators reflect the balance between systemic inflammation and the body's immune status, and compared with the white blood cell values, it tends to be more accurate. Virchow's research linked inflammation with tumors, and also brought inflammation into tumor research, which is also the basis of this study. The values of PLR, NLR, and other inflammation-related indicators in predicting the prognosis and invasion of malignant tumors have been studied in many cases and tumors, including gastric cancer[18,19]. In terms of stromal tumors, related studies have confirmed its value in predicting prognosis. This study focused on inflammatory factors and aimed to explore the difference in inflammation between the two types of tumors.
In the selected types of inflammatory factor indicators, we found differences between the two types of tumors. In this study, the inflammatory environment of gastric stromal tumors was more intricate than that of gastric leiomyomas, and the inflammatory conditions reflected by stromal tumors were more obvious. It is generally believed that tumors such as gastric stromal tumors are located in a relatively local environment and should not change the inflammatory index. However, these differences were observed in this study. We conducted a subgroup analysis of the stromal tumor group based on the risk grade, and compared the low-risk group with the leiomyoma group. After that, we could still find a difference in the values of inflammatory factors between the two groups. Based on this, we believe that gastric stromal tumors can indeed change the inflammatory environment in which they are located, resulting in a stronger inflammatory response in the body.
We then compared the type of inflammatory factor which had the better predictive ability based on logistic regression analysis. We analyzed its correlation, and the results showed that among these types of indicators, only LMR showed significance in the regression analysis. LMR indicators may therefore be advantageous and instructive. Compared with other indicators, LMR was more sensitive, which was a gratifying result. We then constructed a nomogram model based on the results of logistic regression analysis, in which we clarified the diagnostic value of sex, tumor location, LMR, and SII. The nomogram is a visual graphical tool for predicting the probability of individual clinical events–based on a multi-factor statistical model. It has been widely used in various prediction models to predict the risk of disease more accurately[20]. After the model is created (and through further improvement), it is expected to clarify the predictor effect of the differential diagnosis for these tumors.
The relevant analysis for expanation deserves to be conducted further. How to understand this change in inflammatory index change is a question that should be considered. Previous studies have focused on the impact the impact of this type of inflammatory index on the prognosis of gastric stromal tumors. Focal lesions of gastric stromal tumors are recognized, and the presence of immune cells in the microenvironment of gastric stromal tumors is also the basis for immunotherapy, especially the application of imatinib[21]. Various immune cells in the tumor microenvironment, such as tumor-associated macrophages, tumor-associated lymphocytes, and natural killer cells exhibit characteristics related to the progression of GIST[22]. Based on this view, gastric stromal tumors are related to changes in immunity and may influence the body’s inflammatory response such as with gastric cancer[23]. The same results were not found in the gastric leiomyoma group. The differences between the two types of tumors require further research.
This study had its limitations. First, this was a retrospective study; thus, the selection of samples and follow-up work were limited. Second, the sample size of this study was relatively small; therefore, the results could not be generalized, and relevant research results still need to be obtained to prove these outcomes using a large sample size and a cohort study design. Third, we have not yet determined the reasons for such differences, nor have we designed a corresponding scoring system based on this feature for the next step of verification. The identification of the two types of tumors also needs to be combined with endoscopic and imaging-related indicators, which is an aspect that should be considered in future research. If imaging and laboratory indicators can be combined, the prediction accuracy of the two types of tumors will definitely be improved.
We found that there were different inflammatory states in patients with gastric leiomyomas and gastric stromal tumors. This difference could assist clinicians to distinguish between the two types of tumors before surgery in order to choose more appropriate treatments. The reason for this difference is unknown, and whether a highly sensitive scoring system can be created based on these indicators is also worth researching. Overall, we believe that the differences between the two types of tumors will aid our future clinical work; however, there is also a need to conduct further research on this aspect.
Gastric leiomyoma and gastrointestinal stromal tumor are common tumors in the gastric mucosa. The two types of tumors are difficult to differentiate in the initial diagnosis. Inflammation-related indicators reflect the status of inflammation, which could distinguish two kinds of tumors.
A predictive model was constructed based on the nomogram to clarify the kind of tumors, which may help us figuring out tumor microenvironment of gastric stromal tumor. The paper will be the basis of the further research.
The purpose is to explore the differences in platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), lymphocyte mononuclear cell ratio (LMR), and SII between the two types of tumors, clarify the relationship and find which is the most important factor in that.
Of 88 patients in the gastric stromal tumor group and 56 patients in the gastric leiomyoma group were enrolled into this study, and the relevant data of the two groups were entered into the system for integrated analysis. Nomogram was used to create a predictive model for that. Subgroup analysis was carried out to prove the presence of difference in low-risk stromal tumors.
The two groups were in PLR (158.2 vs 134.3, P = 0.028), NLR (2.35 vs 1.68, P = 0.000), LMR (5.75 vs 10.8, P = 0.004), SII (546.2 vs 384.3, P = 0.003) showed statistical differences. The results of the subsequent multivariate Logistic regression analysis showed that gender, age, tumor location, and LMR were independent risk factors for the identification of the two types of tumors. Nomogram model and calibration plot was constructed and subgroup analysis showed that LMR and NLR seems more sensitive than PLR and SII.
It is the first time to find that inflammation-related indicators are different between gastric stromal tumors and leiomyomas, which provides us a new method to differentiate them.
Further research need to be conducted to explain the reason of the the difference, and combine other examinations, such as computed tomography, to create an appropriate model for that.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tolunay HE S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013;42:399-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (2)] |
| 2. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 3. | Sanchez-Hidalgo JM, Duran-Martinez M, Molero-Payan R, Rufian-Peña S, Arjona-Sanchez A, Casado-Adam A, Cosano-Alvarez A, Briceño-Delgado J. Gastrointestinal stromal tumors: A multidisciplinary challenge. World J Gastroenterol. 2018;24:1925-1941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Lopes CV, Hartmann AA, Artifon ELA. EUS-FNA WITH 19 OR 22 GAUGES NEEDLES FOR GASTRIC SUBEPITHELIAL LESIONS OF THE MUSCLE LAYER. Arq Bras Cir Dig. 2018;31:e1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Huang H, Liu YX, Zhan ZL, Liang H, Wang P, Ren XB. Different sites and prognoses of gastrointestinal stromal tumors of the stomach: report of 187 cases. World J Surg. 2010;34:1523-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Lin YM, Chiu NC, Li AF, Liu CA, Chou YH, Chiou YY. Unusual gastric tumors and tumor-like lesions: Radiological with pathological correlation and literature review. World J Gastroenterol. 2017;23:2493-2504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 7. | Cameron S, Beham A, Schildhaus HU. Current Standard and Future Perspectives in the Treatment of Gastrointestinal Stromal Tumors. Digestion. 2017;95:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Martin-Cardona A, Fernandez-Esparrach G, Subtil JC, Iglesias-Garcia J, Garcia-Guix M, Barturen Barroso A, Gimeno-Garcia AZ, Esteban JM, Pardo Balteiro A, Velasco-Guardado A, Vazquez-Sequeiros E, Loras C, Martinez-Moreno B, Castellot A, Huertas C, Martinez-Lapiedra M, Sanchez-Yague A, Teran A, Morales-Alvarado VJ, Betes M, de la Iglesia D, Sánchez-Montes C, Lozano MD, Lariño-Noia J, Gines A, Tebe C, Gornals JB; On belhaf of Spanish Group for EUS-Guided TA in the adrenal gland. EUS-guided tissue acquisition in the study of the adrenal glands: Results of a nationwide multicenter study. PLoS One. 2019;14:e0216658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Ji F, Wang ZW, Wang LJ, Ning JW, Xu GQ. Clinicopathological characteristics of gastrointestinal mesenchymal tumors and diagnostic value of endoscopic ultrasonography. J Gastroenterol Hepatol. 2008;23:e318-e324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Wang J, Zhou X, Xu F, Ao W, Hu H. Value of CT Imaging in the Differentiation of Gastric Leiomyoma From Gastric Stromal Tumor. Can Assoc Radiol J. 2021;72:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5762] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 12. | Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ. Clinical significance of preoperative neutrophil-lymphocyte vs platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 13. | Hutterer GC, Sobolev N, Ehrlich GC, Gutschi T, Stojakovic T, Mannweiler S, Pummer K, Zigeuner R, Pichler M, Dalpiaz O. Pretreatment lymphocyte-monocyte ratio as a potential prognostic factor in a cohort of patients with upper tract urothelial carcinoma. J Clin Pathol. 2015;68:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Cao X, Cui J, Yu T, Li Z, Zhao G. Fibrinogen/Albumin Ratio Index Is an Independent Prognosis Predictor of Recurrence-Free Survival in Patients After Surgical Resection of Gastrointestinal Stromal Tumors. Front Oncol. 2020;10:1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Hoshino N, Hida K, Sakai Y, Osada S, Idani H, Sato T, Takii Y, Bando H, Shiomi A, Saito N. Nomogram for predicting anastomotic leakage after low anterior resection for rectal cancer. Int J Colorectal Dis. 2018;33:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Park SY. Nomogram: An analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. 2018;155:1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 368] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 17. | Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 18. | Zhu GS, Tian SB, Wang H, Ma MG, Liu Y, Du HS, Long YP. Preoperative Neutrophil Lymphocyte Ratio and Platelet Lymphocyte Ratio Cannot Predict Lymph Node Metastasis and Prognosis in Patients with Early Gastric Cancer: a Single Institution Investigation in China. Curr Med Sci. 2018;38:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Lou N, Zhang L, Chen XD, Pang WY, Arvine C, Huang YP, Zhuang CL, Shen X. A novel scoring system associating with preoperative platelet/Lymphocyte and clinicopathologic features to predict lymph node metastasis in early gastric cancer. J Surg Res. 2017;209:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Xu X, Wang W, Zhang Q, Cai W, Wu M, Qin T, Liu H. A Generic Nomogram Predicting the Stage of Liver Fibrosis Based on Serum Biochemical Indicators Among Chronic Hepatitis B Patients. Front Med (Lausanne). 2021;8:669800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Cheng CT, Tsai CY, Yeh CN, Chiang KC, Chen YY, Wang SY, Chen TW, Tseng JH, Jung SM, Chen TC, Yeh TS. Clinical significance of pathological complete response in patients with metastatic gastrointestinal stromal tumors after imatinib mesylate treatment--lessons learned. Anticancer Res. 2014;34:6617-6625. [PubMed] |
| 22. | Tan Y, Garcia-Buitrago MT, Trent JC, Rosenberg AE. The immune system and gastrointestinal stromal tumor: a wealth of opportunities. Curr Opin Oncol. 2015;27:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, Bin J, Liao Y, Rao J, Liao W. Tumor Microenvironment Characterization in Gastric Cancer Identifies Prognostic and Immunotherapeutically Relevant Gene Signatures. Cancer Immunol Res. 2019;7:737-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 669] [Article Influence: 111.5] [Reference Citation Analysis (0)] |